Abstract
ADP-ribosylation is the addition of one or more (up to some hundreds) ADP-ribose moieties to acceptor proteins. There are two major families of enzymes that catalyse this reaction: extracellular ADP-ribosyl-transferases (ARTs), which are bound to the cell membrane by a glycosylphosphatidylinositol anchor or are secreted, and poly(ADP-ribose)-polymerases (PARPs), which are present in the cell nucleus and/or cytoplasm. Recent findings revealed a wide immunological role for ADP-ribosylating enzymes. ARTs, by sensing extracellular NAD concentration, can act as danger detectors. PARP-1, the prototypical representative of the PARP family, known to protect cells from genomic instability, is involved in the development of inflammatory responses and several forms of cell death. PARP-1 also plays a role in adaptive immunity by modulating the ability of dendritic cells to stimulate T cells or by directly affecting the differentiation and functions of T and B cells. Both PARP-1 and PARP-14 (CoaSt6) knockout mice were described to display reduced T helper type 2 cell differentiation and allergic responses. Our recent findings showed that PARP-1 is involved in the differentiation of Foxp3+ regulatory T (Treg) cells, suggesting a role for PARP-1 in tolerance induction. Also ARTs regulate Treg cell homeostasis by promoting Treg cell apoptosis during inflammatory responses. PARP inhibitors ameliorate immune-mediated diseases in several experimental models, including rheumatoid arthritis, colitis, experimental autoimmune encephalomyelitis and allergy. Together these findings show that ADP-ribosylating enzymes, in particular PARP-1, play a pivotal role in the regulation of immune responses and may represent a good target for new therapeutic approaches in immune-mediated diseases.
Keywords: autoimmunity, immunotherapeutics, inflammation
Introduction
Post-translational modifications largely contribute to cell physiology by regulating protein stability, localization and functions. Many of these modifications occur in response to and regulate the interaction with environmental cues, these features being particularly relevant to immune cell functions.
ADP-ribosylation is the post-translational addition of ADP-ribose moieties from NAD to target proteins. ADP-ribosylating enzymes control a wide array of cellular processes, including DNA repair, chromatin structure, transcriptional regulation, mitochondrial functions, apoptosis, necrosis, cell division and differentiation. In the last decade, a wealth of findings have highlighted the role of ADP-ribosylating enzymes in inflammation and immune functions.1
The first molecule shown to have ADP-ribosylating activity was the diphtheria toxin which, by targeting the elongation factor-2 in eukaryotic cells, inhibits protein synthesis.2 However, ADP-ribosylating enzymes are present in almost all organisms, indicating that this reaction is a phylogenetically ancient post-translational modification. ADP-ribosylation is catalysed by extracellular ADP-ribosyl-transferases (ARTs) or by nuclear/cytoplasmic poly(ADP-ribose)-polymerases (PARPs). Based on sequence, structural homologies and similarity of reactions catalysed, a unification of all ADP-ribose transferases (ARTs and PARPs) in one large protein family has been recently proposed.3
Among the ARTs (four expressed in humans; six in mice), ART1 (or ARTC1, ADP-ribosyl-transferase C2 and C3-like 1) and ART2 (ARTC2) are the best characterized family members. These cell membrane glycosylphosphatidylinositol-anchored receptors covalently bind single ADP-ribose moieties to extracellular or cell surface proteins using the extracellular NAD as substrate. As discussed below, by sensing NAD extracellular concentration, the level of which is very low under physiological conditions,4 ARTs act as danger sensors and play a relevant role in inflammatory/immune responses.
PARPs (or ARTDs, ADP-ribosyl-transferase diphtheria toxin-like) constitute a large group of proteins (17 in humans; 16 in mice) sharing a highly conserved sequence within the active site, defined as the PARP signature. PARPs synthesize and bind branched polymers of ADP-ribose to acceptor proteins, such as PARPs themselves, histones, DNA repair proteins, transcription factors and chromatin regulators.1 Actually, not all PARPs synthesize polymers.5 PARP-1 accounts for the majority of the poly(ADP-ribose) (PAR) polymer synthesis. Although characterized for a long time as a DNA damage sensor and as a key factor in DNA repair systems, the structural basis for DNA damage-dependent PARylation by PARP-1 has been elucidated only recently.6 PARylation also plays an important role in the regulation of gene transcription independently from DNA damage.5,7,8 The activation of PARP-1 and other family members is regulated in response to signal pathways and modulates the activity of many transcription factors, as already reviewed.7,9,10
Due to its negative charge, the ADP-ribose polymer drastically affects protein functions. As also DNA is negatively charged, ADP-ribosylated histones are repelled by DNA, relaxing nucleosomal structure and allowing DNA repair enzymes as well as transcription factors to access DNA.11 The half-life of ADP-ribose polymers is very short (minutes) because they are quickly detached from acceptor proteins and/or hydrolysed to mono(ADP-ribose) by poly(ADP-ribose)-glycohydrolase (PARG) and PAR hydrolase (ARH).12 Free or protein-bound ADP-ribose polymers also work as signal transducers by interacting with other proteins through their PAR recognition motifs.13 The dynamic equilibrium between polymer synthesis and degradation and the role of PARs in signal transduction are particularly important during stress responses requiring fast adaptation to environmental changes.14 PARylation is therefore a post-translational modification that plays an important role in epigenetic regulation and gene expression under physiological conditions, also when DNA integrity is maintained.
Recently, several aspects of PARylation and PARP enzymes, with particular emphasis on PARP1, have been reviewed: members and family organization,15 new nomenclature,3 nuclear functions and molecular mechanisms,1 chromatin structure and gene expression,7,11 stress responses,14 role in cellular signalling,9,16 therapeutic potential of PARP inhibitors.17,18
Here, we summarize and discuss the recently uncovered immunological role of ADP-ribosylating enzymes from the PARP and ART families.
ADP-ribosylation at the crossroads of inflammation, cell death and innate immunity
Both ARTs and PARPs are involved in several inflammatory processes. The role of PARPs in inflammation has been investigated with pharmacological inhibitors and confirmed and/or better focused with gene-specific deficient mice.19–22 As shown in several experimental models, PARP inhibitors display protective effects in acute and chronic inflammatory diseases (see below).23–27
PARP-1 sustains the expression of cytokines, chemokines and other inflammatory mediators such as tumour necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6, interferon-γ (IFN-γ), CCL3 and inducible nitric-oxide synthase (iNOS). PARP-1 is required for or increases the expression of several adhesion molecules (intercellular adhesion molecule 1, vascular cell adhesion molecule, P-selectin, E-selectin and mucosal addressin cell adhesion molecule 1), chemoattractant chemokines (IL-8, macrophage inflammatory proteins 1 and 2, monocyte chemoattractant protein 1) and matrix metalloproteinase 9. Consistently, PARP enzymatic inhibition or PARP-1 gene knock down results in inhibition of cell migration to inflammatory sites.28–30
A common set of inflammatory mediators (including iNOS, IL-1β, TNF-α) is regulated by both PARP-1 and PARP-2, indicating that these two enzymes modulate inflammation through partially overlapping mechanisms.31 As many of the enzymatic inhibitors are not acting exclusively on PARP-1 or PARP-2, caution should be applied when attributing the effects of PARP enzymatic inhibition to either family member. PARP-1 is involved in gene expression and activation of neutrophils, macrophages, dendritic cells, microglia and other cell types.19,20,32 PARP-1/2-mediated pro-inflammatory responses are not limited to cells of the innate immune system but play a relevant role also in other cell types such as endothelial cells and fibroblasts.28,33,34 PARP-1, -2 and -3 cooperatively regulate the activation (IL-1, TNF-α and CCL2 expression) of astrocytes, cells contributing not only to central nervous system homeostasis but also to the local innate immune responses 35 (Fig. 1).
Figure 1.
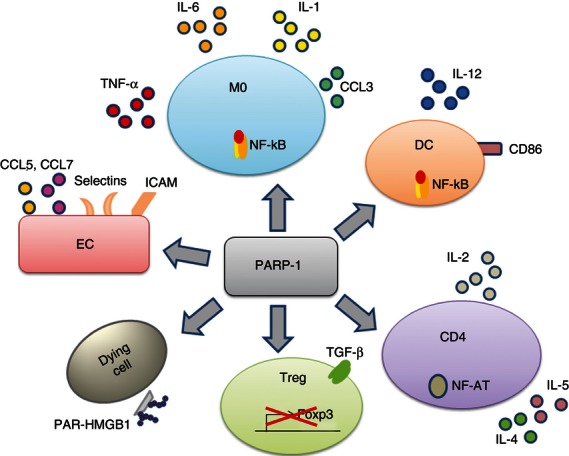
Poly(ADP-ribose)-polymerase 1 (PARP-1) is expressed in almost all cells and plays a central role in inflammation and immunity. PARP-1 regulates gene transcription through several mechanisms including epigenetic modifications and interactions with and modification of transcription factors. PARP-1 activates nuclear factor-κB (NF-κB; as shown in macrophages and dendritic cells) and NF-AT (T cells), increases the expression of co-stimulatory molecules (CD86), pro-inflammatory cytokines, chemokines and adhesion molecules in several cell types. In T cells, PARP-1 increases the expression of interleukin-2 (IL-2) and T helper type 2 (Th2) cytokines as well as cell proliferation, whereas it negatively controls the expression of Foxp3. PARP-1 by poly-ADP-ribosylating HMGB1 strengthens the danger signals associated with cell death. CD4, CD4 T cells; DC, dendritic cells; EC, endothelial cells; M0, macrophages; PAR-HMGB1, poly-ADP-ribosylated-HMGB1; Treg, regulatory T cells.
As PARG degrades the PAR polymers synthesized by PARPs, one could expect that PARG activity might result in anti-inflammatory effects. Conversely, as PARP-1 activity is blocked by extensive auto-PARylation, PARG inhibition results in PARP-1 hindrance. Indeed PARG inhibition, with gallotannin or with more recently developed non-tannin small molecules, protects astrocytes from oxidative stress and reduces infarct volume in brain ischaemia/reperfusion injury models.12,36 A control of the inflammatory response with PARG inhibitors was obtained also in an experimental spinal cord trauma model, resulting in neuroprotection.37
Central to the role of PARP-1 in inflammation is its involvement in nuclear factor-κB (NF-κB) activation. Two recent studies demonstrated that PARP-1 is required to trigger IKKγ (NEMO, NF-κB essential modulator) SUMOylation and monoubiquitination, which in turn allow IKK and NF-κB activation.38,39 Previous findings showed that PARP-1 interacts with elements of the NF-κB family and, acting as a docking molecule, favours the formation of the transcription complex, independently of its enzymatic activity.40 Another study demonstrated that PARylation sustains p65 NF-κB nuclear retention by decreasing its interaction with Crm1 (a nuclear exporting protein).41 The importance of these pathways is further strengthened by the resistance of PARP-1 knockout mice to lipopolysaccharide-induced septic shock. In macrophages from these animals, NF-κB-dependent TNF-α and iNOS expressions are compromised and the positive loop between oxidative stress and sustained PARP-1/PARP-2/NF-κB activation is compromised. As a consequence, the cytokine storm that would lead to death by septic shock is strongly reduced.42
PARP-1 promotes inflammation also by PARylating the high mobility group box 1 protein (HMGB1) and consequently inducing its translocation to the cytosol, where HMGB1 may leak out of necrotic cells and act as a danger pro-inflammatory factor.43 PARylated HMGB1 released by dying cells inhibits efferocytosis, reducing the clearance of apoptotic cells from inflammatory foci and further increasing inflammation.44 Interestingly, such a mechanism is targeted by a protease of the intracellular pathogen Chlamydia, which, by degrading HMGB1 and PARP-1, reduces the inflammatory response to membrane-damaged cells and prevents pathogen clearance.45
PARP-1 plays a relevant role in different forms of cell death.46 PARP-1 over-activation induced by severe genomic stress or intense inflammation results in intracellular NAD and ATP depletion and consequent necrosis due to energy failure.47 PARP-1 is also involved in apoptosis-inducing factor-mediated caspase-independent and caspase-dependent apoptosis.48–50 Recent findings revealed that also the PAR polymer itself can act as a cell death effector downstream of PARP-1, activating a form of cell death named parthanatos.46 Hence, PARP-1 is involved in the induction of several forms of cell death, and the prevailing pathway depends on the balance among the degree of PARP-1 activation, the metabolic status of the cell and other intervening factors.
Tissue damage due to direct injury or inflammation induces cell death and increases NAD+ concentration in extracellular compartments. A rise in extracellular NAD activates ART2, which ADP-ribosylates the P2X7 purinergic receptor leading to Ca2+ flux, pore formation and cell death. Hence, ARTs can function as danger sensors and regulate the response by amplifying the inflammatory process.
All of these findings show that (poly)ADP-ribosylation, as catalysed by ARTs and PARPs, is involved in several aspects of inflammation and contributes to the up-regulation of danger signals, creating the conditions to initiate and sustain the (innate) immune response.
(Poly)-ADP-ribosylation in T-cell development, differentiation and activation
T-cell development and function are regulated by PARylation. PARP-1 and PARP-2 expression are particularly high in the cortex and sub-capsular areas of the thymus, where immature lymphocytes actively proliferate. Parp-2 gene inactivation (not Parp-1) drastically reduces the thymus size and the number of CD4+ CD8+ double-positive (DP) thymocytes. Parp-2-deficient DP thymocytes display increased apoptosis, reduced T-cell receptor (TCR) expression and skewed TCR-α chain repertoire toward 50 Jα segments.51 Apoptosis can be reversed by the removal of p53,52 suggesting that cell death is induced by unresolved DNA damage. These findings indicate that PARP-2 is important for T-cell survival during thymopoiesis by preventing the activation of DNA damage-dependent apoptotic response during TCR-α rearrangements.
PARP-1 plays a role also in peripheral T cells. PARP-1 knockout T cells stimulated in the absence of antigen-presenting cells display a lower cell proliferation and a similar susceptibility to apoptosis compared with wild-type cells.53 A reduced proliferation, associated with an increased frequency of CD25/Foxp3 Treg cells, was also observed in purified PARP-1 knockout CD4 cells. Indeed, CD25-depleted CD4 cells from PARP-1 knockout mice proliferate at a rate comparable with or slightly higher than wild-type cells.54
During T-cell activation several transcription factors are activated through different signal pathways. PARP-1 contributes to this process by binding to and ADP-ribosylating NF-ATc1 and NF-ATc2.55,56 According to Valdor et al.,56 PARP-1 negatively regulates NF-AT as PARP inhibition increases the NF-AT-dependent trans-activation and delays NF-AT nuclear export through an unidentified mechanism. At variance, according to Olabisi et al. 55 PARylation increases NF-AT DNA binding and PARP-1 knockout T cells exhibit reduced expression of IL-2 and IL-4. Further studies will allow us to better understand how PARP-1 modulates NF-AT activation and function.
In T cells, PARP-1 regulates positively or negatively the expression of a large number of genes including genes coding for chemokines and cytokines.53 In PARP-1 knockout T cells, the expression of T helper type 1 (Th1) cytokine (IFN-γ) and chemokines (Xcl1, Ccl4 and Ccl9) are increased while IL-4 production is reduced.53 Our group found that naive CD4 cells from PARP-1 knockout mice generate less IL-4- and IL-5-secreting cells and express GATA-3 mRNA at lower levels than wild-type cells, even when stimulated under Th2-polarizing conditions (unpublished observations). Lack or enzymatic inhibition of PARP-1 exposes signal transducer and activator of transcription 6 (STAT6), which is required for IL-4 signalling, to calpain-mediated degradation, with consequent reduction in GATA-3 and IL-5 mRNA expression.57 Interestingly, STAT6-dependent activity is regulated also by PARP-14 (CoaSt6). Upon IL-4 stimulation, PARP-14 ADP-ribosylates the histone deacetylases HDACs present at the Gata3 promoter and favours STAT6 binding.58,59 Hence, GATA-3 mRNA expression and therefore Th2 cell differentiation are regulated at different levels by multiple members of the PARP family.
Recently, our group described an increased number but normal phenotype and function of Foxp3+ Treg cells in central (thymus) and peripheral lymphatic organs from PARP-1 knockout mice.54 PARP-1 knockout naive CD4 cells stimulated in the presence of transforming growth factor-β displayed a higher rate of conversion to inducible Foxp3+ Treg cells, associated with a higher Foxp3 mRNA expression. No differences in apoptosis susceptibility were observed under Treg-inducing conditions 54 (Table 1).
Table 1.
Immunological characteristics of poly(ADP-ribose)-polymerase-deficient mice
| Knock down gene | General features | Cytokine/immunoglobulin production | T-cell and B-cell compartments |
|---|---|---|---|
| PARP-1 | Knockout mice resistant to: Lipopolysaccharide-induced septic shock Streptozotomycin-induced diabetes Peroxynitrite-induced arthritis Oxazolone-induced contact hypersensitivity Reduced airway inflammation | ↓ NF-κB, NF-AT and AP-1 activation ↓ IL-1β and TNF-α production ↓ iNOS expression ↓ selectins, integrins and ICAMs expression ↓ IL-2 production ↓ Th2 cytokines (IL-4/IL-5/IL-13) production ↓ T-cell proliferation ↑/= IFN-γ production = IL-17 production ↑ Xcl1, Ccl4 and Ccl9 expression ↓ Immunoglobulin gene conversion ↓ IgG2a ↑ IgA ↑ IgG2b | Cell number in lymphatic organs: Normal/small increase in T-cell number, CD4, CD8 cells; Normal B-cell number; Increased Foxp3+ Treg cell number |
| PARP-2 | Genetic deletion or silencing provides protection in: Cerebral ischaemia Colitis Doxorubicin-induced vascular smooth muscle damage Impaired astrocyte activation | ↓ IL-1β production ↓ TNF-α production ↓ iNOS expression | Altered thymopoiesis: Reduced thymus size Increased Noxa expression and apoptosis in DP thymocytes Reduced TCR expression and skewed TCR-α chain repertoire |
| PARP-14 | Reduced airway inflammation | ↓ Th2 cell responses and IgE production ↓ IgA production in antigen-specific response ↓ IL-4-induced B-cell survival | Normal overall cell numbers in thymus, spleen, and lymph nodes Fewer marginal zone B cells More follicular B cells |
AP-1, activator protein 1; DP, double positive (CD4+ CD8+); Foxp3, forkhead box P3; ICAM, intercellular cell adhesion molecule; Ig, immunoglobulin; IL, interleukin; iNOS, inducible nitric oxide synthase; KO, knock out; LPS, lipopolysaccharide; PARP, poly(ADP-ribose)-polymerase; NF-AT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; Noxa, Latin for damage; TCR, antigen-specific T cell receptor; TNF, tumour necrosis factor; ↓, inhibition; ↑, upregulation.
Also, ARTs regulate Treg cell homeostasis. Treg cells express P2X7 at high levels rendering them more suscepto NAD-induced ART2·2-mediated cell death compared with other T cells.60 CD38 is an NAD-glycohydrolase that, by hydrolysing extracellular NAD+, limits its availability for ART2.61 Consistently, lower Treg cell numbers are found in mice deficient for CD38 indicating that extracellular NAD+ regulates Treg cell homeostasis.62 In the context of tissue damage and inflammation, when effector cells have to carry out their functions, the number of Treg cells could be limited by the ART2–P2X7 pathway 62 while differentiation of naive CD4 cells to inducible Treg cells could be inhibited by PARP-1.54 These findings suggest that NAD and ADP-ribosylation play an important role in (local) inflammatory/immune reactions.4
Although Treg and Th17 cells represent two developmentally related populations of CD4 T cells with opposite functions, naive CD4 from PARP-1 knockout mice generate similar numbers of IL-17-producing retinoic acid receptor-related orphan receptor γt-expressing CD4 cells when stimulated with transforming growth factor-β and IL-6.54 Consistently, the beneficial effects of PARP inhibitors observed in experimental autoimmune encephalomyelitis (EAE) models are not associated with a reduction in Th17 cells.32
PARP-1 was also described as being involved in CD8 T-cell homeostasis. In particular oxidative stress can induce apoptosis in CD8 T (and natural killer) cells through PARP-1 activation,63 this mechanism being exploited by tumour cells to evade the immune response.64
Roles of PARP in B-cell development
Some critical events in B-cell life require DNA resolution and rejoining, pointing to possible roles of PARP enzymes in B-cell development.65 Although B-cell numbers are normal in peripheral lymphatic organs from PARP-1 knockout mice 66 (and our unpublished observations), several studies showed that PARP-1 is involved in B-cell differentiation and functions. Mutations in the DNA-PK gene causes defective V(D)J recombination with arrest of B- and T-lymphocyte development, resulting in the severe combined immune deficiency. In T cells the deficient V(D)J recombination can be partially rescued by knocking out PARP-1, indicating that PARP-1 exerts an anti-recombinogenic function during T-cell maturation.67 Indeed, V(D)J recombination induces a DNA damage response in severe combined immune deficiency cells and PARylation supports coding end resolution, as shown in a B-cell precursor-derived cell line.68
Upon antigen stimulation, mature B cells undergo affinity maturation through the generation of immunoglobulin variants by somatic hypermutation or by gene conversion, followed by selection of the B-cell clones producing high-affinity antibody variants. Somatic hypermutation and gene conversion require the introduction of DNA lesions by the activation-induced cytidine deaminase (AID), followed by fixation via a mutagenic DNA repair mechanism.69,70 PARP-1 is highly expressed in germinal centres and promotes (unexpectedly) mutagenic rather than conservative repair mechanisms through its BRCT domain.71 Indeed, PARP-1 deficiency results in reduced gene conversion and in increased fidelity repair of AID-induced lesions in immunoglobulin chains.71
PARP-1 is also involved in the other AID-mediated process, that is class switch recombination.69 Indeed, depending on the stimuli provided to B cells, PARP-1 inhibition or gene knock down increases the frequency of immunoglobulin class recombination to IgA, IgG1 or IgG2b whereas it decreases switching to IgG2a, facilitating alternative end-joining.66,72,73 At variance, a selective impairment in IgA production associated with reduced marginal zone and increased follicular B-cell numbers was observed in PARP-14 knockout mice.74 Interestingly, during class switch recombination, PARP-2 acts as a suppressor that prevents illegitimate recombination between the immunoglobulin heavy chain locus and other genes (in particular c-myc).73
PARP-1 binds in a sequence-specific way to the first intron of Bcl-6, where it acts as a transcriptional repressor, and its enzymatic inhibition or silencing by small interfering RNA induces Bcl-6 mRNA expression.75 As down-regulation of Bcl-6 expression occurs during differentiation to plasma cells, PARP-1, by inhibiting Bcl-6 transcription, could sustain this process.
While PARPs take part in many critical events in B-cell maturation, there is only one study showing that ART7.1 is expressed in chicken B cells within the bursa of Fabricius where it is involved in B-cell signalling.76
Poly-ADP-ribosylation in immune-mediated diseases: a new therapeutic target?
The involvement of PARP enzymes in several immune-mediated diseases has been demonstrated in pre-clinical models using pharmacological inhibitors or gene-specific knockout mice 18,27 (Table 2). As revealed more recently, many of the inhibitors commonly used in PARP research bind to and dampen the enzymatic activity of more than one family member.77 Although the beneficial effects of PARP pharmacological inhibition cannot be ascribed to the inhibition of a unique PARP, some of the effects were confirmed with knockout mice, lacking only one functional PARP family member (Table 1).
Table 2.
Synopsis on poly(ADP-ribose)-polymerase inhibitors use in immune-mediated disease models
| Disease model | Inhibitor(s) used | Findings | Notes |
|---|---|---|---|
| Rheumatoid arthritis | AIQ | Reduced expression of inflammatory cytokines (TNF-α, IFN-γ, IL-6, IL-1β and IL-12), and chemokines (RANTES and MIP-2) in the joints of arthritic mice Increased IL-10 production Decreased collagen-specific Th1-cell expansion79 | PARP-1KO mice display reduced RA when immunized with collagen or treated with peroxynitrite78 |
| Experimental autoimmune encephalomyelitis | AIQ32 PJ3482 PHE83 PJ34 and PHE84 | Reduced NF-κB and AP-1 activation, inflammatory gene transcription (TNF-α, IFN-γ, iNOS), adhesion molecule expression, cell migration Reduced activation of macrophages, microglia, dendritic cells and astrocytes Reduced demyelination, better clinical score32,82–84 | AIQ targets PARP-1, -2, -377; PJ34 targets PARP-1 and PARP2 and less efficiently PARP-377; PHE binds to PARP-1, -2 and -3 and Tankyrases-1 and -277 In contrast to the results obtained with the inhibitors, PARP-1KO mice display more severe EAE compared with wild type mice85 |
| Colitis | GPI615027 Nicotinamide86 DiQ (1,5-dihydroxyisoquinoline)86 3-aminobenzamide89 | In DNBS/TNBS model: reduced AP-1 and NF-κB activation, inflammatory cytokine production, adhesion molecules, COX2, PGE2, apoptosis, with consequent reduced colon damage27,86 In IL-10-deficient mice: reduced TNF-α, IFN-γ, iNOS, normalized colonic permeability89 | DiQ targets PARP-1, -2, -377 PARP-1KO mice displayed reduced colon damage, apoptosis, JNK and AP-1 activation, and increased Bcl-2 expression87 |
| Allergic airway inflammation | TIQ-A94 PJ3459 | Reduced production of IL-5, IL-10, IL-13, and GM-CSF Reduced eosinophil recruitment94 | TIQ-A targets PARP-1, -3, and other family members including tankyrase-177 PJ34 targets PARP-1, -2 and, to a lesser extent other members, including tankyrase-1, -2 and PARP-1477 PARP-1KO94 and PARP-14KO59 mice show reduced Th2 cytokine production and allergic airway inflammation |
AIQ, 5-aminoisoquinolinone; COX2, cyclooxygenase-2; DNBS/TNBS, dinitrobenzene/trinitrobenzene sulphonic acid; DiQ, 1,5-dihydroxyisoquinoline; EAE, experimental autoimmune encephalomyelitis; GM-CSF, Granulocyte-macrophage colony-stimulating factor; GPI6150, 1,11b-dihydro-[2H]benzopyrano [4,3,2-de]isoquinolin-3-one; JNK, c-Jun N-terminal kinases; MIP-2, macrophage inflammatory protein-2 (CXCL2); PGE2, prostaglandin E2; PJ34, N-(6-Oxo-5,6-dihydrophenanthridin-2-yl)-(N,N-dimethylamino)acetamide; PHE, 6(5H)-phenanthridinone; RA, rheumatoid arthritis; Rantes, regulated on activation, normal T cell expressed and secreted (CCL5); TIQ-A, 4H-Thieno[2,3-c]isoquinolin-5-one; see also footnote in Table 1.
The first evidence showing that PARP-1 is involved in immune-mediated diseases was obtained in a model of rheumatoid arthritis. PARP-1 knockout mice display a less severe rheumatoid arthritis with reduced destruction of bone and cartilage associated with a lower IL-1β and monocyte chemoattractant protein 1 expression in arthritic joints compared with wild-type mice.78 When given to mice affected by collagen-induced arthritis, AIQ, an enzymatic inhibitor that targets PARP-1, -2 and -3,77 down-regulates the production of various inflammatory cytokines and chemokines, decreases the antigen-specific Th1-cell expansion, and induces the production of IL-10.79 In humans, specific PARP-1 promoter haplotypes seem to be associated with susceptibility to RA 80 while a single nucleotide polymorphism in exon 17 is not.81
Several studies investigated the effects of PARP inhibition in EAE. Pharmacological inhibitors dampened NF-κB and AP-1 activation, inflammatory gene transcription, adhesion molecule expression and cell migration resulting in a reduced demyelination and in a better clinical score.82–84 In secondary progressive multiple sclerosis/EAE, PARP inhibition impaired the expression of inflammatory factors and prevented new relapses, by interfering with the activation of microglia, macrophages and astrocytes induced by 15a-hydroxicholestene.32 In contrast with these findings Selvaraj et al. 85 reported that, upon immunization with myelin oligodendrocyte glycoprotein, PARP-1 knockout mice had an earlier onset and developed a more severe EAE compared with wild-type mice. In EAE spinal cords from wild-type mice, PARP-1 was down-regulated and PARP-3 (but not PARP-2) was drastically up-regulated in both PARP-1 knockout and wild-type mice.85 The PARP-1 inhibitors that induced the beneficial effects described above 32,82–84 are not exclusive to PARP-1 and target other members of the family.77 Further studies are therefore needed to clarify whether different PARPs could play distinct roles in central nervous system immune-mediated diseases and how the use of pharmacological inhibitors could be exploited for therapeutic purposes.
Several studies showed that PARP-1 deficiency and PARP inhibition confer resistance to inflammatory bowel disease in rodent models of dinitrobenzene/trinitrobenzene sulphonic acid (DNBS/TNBS)-induced colitis by dampening AP-1 and NF-κB activation, inflammatory cytokine production and apoptosis, with consequent reduced colon damage.26,86,87 Noteworthy, also PARG inhibitors, which hamper PAR degradation and induce PARP-1 inhibition by stabilizing its PARylated status, exert therapeutic effects in DNBS-induced inflammatory bowel disease.88 Therapeutic effects were also obtained in IL-10-deficient mice (which develop spontaneous colitis) using PARP inhibitors 89 or targeting PARP-2 with antisense oligonucleotides.90
During infection of gastric epithelial cells, H. pylori activates intracellular PARP-1, a process associated with the development of peptic ulcer disease and gastric cancer in human chronic infection.91 In mouse models, PARP inhibitors reduce gastric inflammation, prevent the T-cell-driven immunopathology and the formation of gastric precancerous lesions, and revert pre-existing lesions.92
The role of PARylation was also investigated in allergic airway inflammation. Following allergen challenge PARP-1 protein expression and activity are greatly increased in murine lungs.93 Even if with some specific differences, both PARP-1 knockout and PARP-14 knockout mice show reduced IL-5 and IL-13 production (also IL-4 in PARP-14 knockout), eosinophilic recruitment, allergic airway inflammation, and IgE levels compared with wild-type animals.59,94 Similar results were obtained using TIQ-A and PJ34 inhibitors,59,94 both of which target PARP-1 and to a lesser extent other family members.77 As discussed above, both PARP-1 and PARP-14 play a role in Th2 cell differentiation through different mechanisms, having an impact in allergic airway inflammation.57–59
Altogether the findings discussed above show that PARP pharmacological inhibition exerts protective effects in a variety of immune-mediated disease models affecting both innate and adaptive components of the immune system.
Conclusions and perspectives
Several PARP-1 inhibitors are undergoing clinical trials (at different stages) for cancer therapy with promising results.17 Their use is supported by the role PARP-1 plays in DNA damage detection and in base excision repair. As BRCA1 and BRCA2 proteins are critical for homologous recombination repair, the use of PARP inhibitors in BRCA-defective cancer cells is believed to result in unsustainable DNA damage and consequent tumour cell death. Pre-clinical data gave promising results also for the treatment of haematopoietic malignancies associated with ATM mutations.95 However, some setbacks96 should provoke reconsideration of our understanding of the mechanisms underlying the effects of PARP inhibitors, which might involve pathways other than DNA repair. Indeed, recent evidence revealed that PARP-1 inhibition limits tumour growth and development by affecting tumour-related gene expression and by inhibiting inflammatory signals and angiogenesis.97–100 This should not be surprising as inflammatory mediators are known to play critical roles in both tumour induction and tumour growth.
As shown in the pre-clinical models discussed above, almost all acute and chronic inflammatory conditions, having different aetiopathogenesis and occurring in different specific organs or being systemic, can be attenuated by PARP inhibition, pointing to a central role of these enzymes in immune-mediated diseases and making them a suitable therapeutic target. Yet few studies have been conducted in humans to explore this possibility. Considering the role of reactive oxygen species in the activation of PARP-1, its inhibition (or the inhibition of PARG) to limit tissue damage during reperfusion in acute events, such as myocardial infarction or brain stroke, might represent one of the prime indications to develop. Infarct and brain stroke treatment could require a few days of therapy limiting the risk of potential side effects. However, despite many promising pre-clinical studies,23,24 the beneficial effects of PARP inhibition in heart and cerebral ischaemia have been studied in only a small number of trials. In a phase I trial, aimed at the evaluation of safety, and pharmacokinetics/dynamics, a PARP inhibitor (INO-1001) reduced the levels of IL-6 and C-reactive protein in patients with myocardial infarction.101 Pre-clinical studies also suggest a possible use of PARP-1 inhibitors in chronic inflammatory diseases, such as multiple sclerosis102 and allergy/asthma,103 but no clinical studies are available. However, because of the long treatments needed for these diseases, caution should be used in considering therapeutic effects versus side effects.
As summarized in this review, the available findings point to a relevant pro-response/danger/activating role of PARylation in response to external cues. Many of the pro-inflammatory effects of PARPs are mediated by PARP-1 and PARP-2, which display partially overlapping functions, PARylate each other, and can form hetero-dimers. Both PARP-1 and PARP-2 activate inflammatory gene expression in several cell types but they also appear to play distinct roles in lymphocyte development. The available PARP inhibitors dampen the enzymatic activity of both PARP-1 and PARP-2. Also mono-ADP-ribosylation, as catalysed by ARTs in response to cell damage occurring during inflammation, acts as an amplifying danger signal. Remarkably, some PARP inhibitors can also inhibit other NAD-using enzymes including ARTs and sirtuins.18 Further research on poorly studied PARPs is needed to assess whether they also play an immunological role, and how different PARPs (for instance PARP-3 and PARP-1) interact and at which levels they regulate immune functions. In this context, as the specific inhibition of single PARPs is still an unmet issue, studies performed using pharmacological inhibitors should include confirmation experiments with PARP-deficient cells or small interfering RNA. Nevertheless, pluri-PARP inhibition might be useful in the treatment of certain diseases, whereas single PARP inhibition could be suitable in many others, especially to reduce side effects. The recently solved crystal structures of some PARPs in complex with inhibitors and a large study on the interactions between PARPs and a wide array of inhibitors open new possibilities in the development of inhibitors specific for a determined family member.77,104,105 In this context, the development of inhibitors targeting sites other than the catalytic domain and interfering with the interaction between a specific PARP and other molecules should also be considered, as they could be useful for therapeutic purposes in both inflammation and cancer.
Therapeutic targeting of PARPs in human immune-mediated diseases is a potentially fruitful field deserving further and specific studies.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honjo T, Nishizuka Y, Hayaishi O. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem. 1968;243:3553–5. [PubMed] [Google Scholar]
- 3.Hottiger MO, Hassa PO, Luscher B, Schuler H, Koch-Nolte F. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends Biochem Sci. 2010;35:208–19. doi: 10.1016/j.tibs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Grahnert A, Klein C, Schilling E, Wehrhahn J, Hauschildt S. NAD+: a modulator of immune functions. Innate Immun. 2011;17:212–33. doi: 10.1177/1753425910361989. [DOI] [PubMed] [Google Scholar]
- 5.Loseva O, Jemth AS, Bryant HE, Schuler H, Lehtio L, Karlberg T, Helleday T. PARP-3 is a mono-ADP-ribosylase that activates PARP-1 in the absence of DNA. J Biol Chem. 2010;285:8054–60. doi: 10.1074/jbc.M109.077834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langelier MF, Planck JL, Roy S, Pascal JM. Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science. 2012;336:728–32. doi: 10.1126/science.1216338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraus WL. Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr Opin Cell Biol. 2008;20:294–302. doi: 10.1016/j.ceb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potaman VN, Shlyakhtenko LS, Oussatcheva EA, Lyubchenko YL, Soldatenkov VA. Specific binding of poly(ADP-ribose) polymerase-1 to cruciform hairpins. J Mol Biol. 2005;348:609–15. doi: 10.1016/j.jmb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Armon M. PARP-1 activation in the ERK signaling pathway. Trends Pharmacol Sci. 2007;28:556–60. doi: 10.1016/j.tips.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Krishnakumar R, Gamble MJ, Frizzell KM, Berrocal JG, Kininis M, Kraus WL. Reciprocal binding of PARP-1 and histone H1 at promoters specifies transcriptional outcomes. Science. 2008;319:819–21. doi: 10.1126/science.1149250. [DOI] [PubMed] [Google Scholar]
- 11.Rouleau M, Aubin RA, Poirier GG. Poly(ADP-ribosyl)ated chromatin domains: access granted. J Cell Sci. 2004;117:815–25. doi: 10.1242/jcs.01080. [DOI] [PubMed] [Google Scholar]
- 12.Min W, Wang ZQ. Poly (ADP-ribose) glycohydrolase (PARG) and its therapeutic potential. Front Biosci. 2009;14:1619–26. doi: 10.2741/3329. [DOI] [PubMed] [Google Scholar]
- 13.Kraus WL. New functions for an ancient domain. Nat Struct Mol Biol. 2009;16:904–7. doi: 10.1038/nsmb0909-904. [DOI] [PubMed] [Google Scholar]
- 14.Luo X, Kraus WL. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 2012;26:417–32. doi: 10.1101/gad.183509.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassa PO, Hottiger MO. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci. 2008;13:3046–82. doi: 10.2741/2909. [DOI] [PubMed] [Google Scholar]
- 16.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–67. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 17.Megnin-Chanet F, Bollet MA, Hall J. Targeting poly(ADP-ribose) polymerase activity for cancer therapy. Cell Mol Life Sci. 2010;67:3649–62. doi: 10.1007/s00018-010-0490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giansanti V, Dona F, Tillhon M, Scovassi AI. PARP inhibitors: new tools to protect from inflammation. Biochem Pharmacol. 2010;80:1869–77. doi: 10.1016/j.bcp.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Hauschildt S, Scheipers P, Bessler W, Schwarz K, Ullmer A, Flad HD, Heine H. Role of ADP-ribosylation in activated monocytes/macrophages. Adv Exp Med Biol. 1997;419:249–52. doi: 10.1007/978-1-4419-8632-0_31. [DOI] [PubMed] [Google Scholar]
- 20.Szabo C, Lim LH, Cuzzocrea S, Getting SJ, Zingarelli B, Flower RJ, Salzman AL, Perretti M. Inhibition of poly (ADP-ribose) synthetase attenuates neutrophil recruitment and exerts antiinflammatory effects. J Exp Med. 1997;186:1041–9. doi: 10.1084/jem.186.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shall S, de Murcia G. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat Res. 2000;460:1–15. doi: 10.1016/s0921-8777(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 22.Laudisi F, Sambucci M, Pioli C. Poly (ADP-ribose) polymerase-1 (PARP-1) as immune regulator. Endocr Metab Immune Disord Drug Targets. 2011;11:326–33. doi: 10.2174/187153011797881184. [DOI] [PubMed] [Google Scholar]
- 23.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–60. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moroni F, Cozzi A, Chiarugi A, et al. Long-lasting neuroprotection and neurological improvement in stroke models with new, potent and brain permeable inhibitors of poly(ADP-ribose) polymerase. Br J Pharmacol. 2012;165:1487–500. doi: 10.1111/j.1476-5381.2011.01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabo C. Roles of poly(ADP-ribose) polymerase activation in the pathogenesis of diabetes mellitus and its complications. Pharmacol Res. 2005;52:60–71. doi: 10.1016/j.phrs.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Mazzon E, Dugo L, Li JH, Di Paola R, Genovese T, Caputi AP, Zhang J, Cuzzocrea S. GPI 6150, a PARP inhibitor, reduces the colon injury caused by dinitrobenzene sulfonic acid in the rat. Biochem Pharmacol. 2002;64:327–37. doi: 10.1016/s0006-2952(02)01075-4. [DOI] [PubMed] [Google Scholar]
- 27.Bai P, Virag L. Role of poly(ADP-ribose) polymerases in the regulation of inflammatory processes. FEBS Lett. 2012;586:3771–7. doi: 10.1016/j.febslet.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Zingarelli B, Salzman AL, Szabo C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res. 1998;83:85–94. doi: 10.1161/01.res.83.1.85. [DOI] [PubMed] [Google Scholar]
- 29.Sharp C, Warren A, Oshima T, Williams L, Li JH, Alexander JS. Poly ADP ribose-polymerase inhibitors prevent the upregulation of ICAM-1 and E-selectin in response to Th1 cytokine stimulation. Inflammation. 2001;25:157–63. doi: 10.1023/a:1011032313445. [DOI] [PubMed] [Google Scholar]
- 30.Ullrich O, Diestel A, Eyupoglu IY, Nitsch R. Regulation of microglial expression of integrins by poly(ADP-ribose) polymerase-1. Nat Cell Biol. 2001;3:1035–42. doi: 10.1038/ncb1201-1035. [DOI] [PubMed] [Google Scholar]
- 31.Szanto M, Brunyanszki A, Kiss B, Nagy L, Gergely P, Virag L, Bai P. Poly(ADP-ribose) polymerase-2: emerging transcriptional roles of a DNA-repair protein. Cell Mol Life Sci. 2012;69:4079–92. doi: 10.1007/s00018-012-1003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farez MF, Quintana FJ, Gandhi R, Izquierdo G, Lucas M, Weiner HL. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol. 2009;10:958–64. doi: 10.1038/ni.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrillo A, Monreal Y, Ramirez P, Marin L, Parrilla P, Oliver FJ, Yelamos J. Transcription regulation of TNF-α-early response genes by poly(ADP-ribose) polymerase-1 in murine heart endothelial cells. Nucleic Acids Res. 2004;32:757–66. doi: 10.1093/nar/gkh239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreone TL, O'Connor M, Denenberg A, Hake PW, Zingarelli B. Poly(ADP-ribose) polymerase-1 regulates activation of activator protein-1 in murine fibroblasts. J Immunol. 2003;170:2113–20. doi: 10.4049/jimmunol.170.4.2113. [DOI] [PubMed] [Google Scholar]
- 35.Phulwani NK, Kielian T. Poly (ADP-ribose) polymerases (PARPs) 1–3 regulate astrocyte activation. J Neurochem. 2008;106:578–90. doi: 10.1111/j.1471-4159.2008.05403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ying W, Swanson RA. The poly(ADP-ribose) glycohydrolase inhibitor gallotannin blocks oxidative astrocyte death. NeuroReport. 2000;11:1385–8. doi: 10.1097/00001756-200005150-00007. [DOI] [PubMed] [Google Scholar]
- 37.Cuzzocrea S, Genovese T, Mazzon E, et al. Poly(ADP-ribose) glycohydrolase activity mediates post-traumatic inflammatory reaction after experimental spinal cord trauma. J Pharmacol Exp Ther. 2006;319:127–38. doi: 10.1124/jpet.106.108076. [DOI] [PubMed] [Google Scholar]
- 38.Stilmann M, Hinz M, Arslan SC, Zimmer A, Schreiber V, Scheidereit C. A nuclear poly(ADP-ribose)-dependent signalosome confers DNA damage-induced IκB kinase activation. Mol Cell. 2009;36:365–78. doi: 10.1016/j.molcel.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 39.Hinz M, Stilmann M, Arslan SC, Khanna KK, Dittmar G, Scheidereit C. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-κB activation. Mol Cell. 2010;40:63–74. doi: 10.1016/j.molcel.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Hassa PO, Covic M, Hasan S, Imhof R, Hottiger MO. The enzymatic and DNA binding activity of PARP-1 are not required for NF-κB coactivator function. J Biol Chem. 2001;276:45588–97. doi: 10.1074/jbc.M106528200. [DOI] [PubMed] [Google Scholar]
- 41.Zerfaoui M, Errami Y, Naura AS, et al. Poly(ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-κB upon TLR4 stimulation. J Immunol. 2010;185:1894–902. doi: 10.4049/jimmunol.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliver FJ, Menissier-de Murcia J, Nacci C, et al. Resistance to endotoxic shock as a consequence of defective NF-κB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J. 1999;18:4446–54. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 44.Davis K, Banerjee S, Friggeri A, Bell C, Abraham E, Zerfaoui M. Poly(ADP-ribosyl)ation of high mobility group box 1 (HMGB1) protein enhances inhibition of efferocytosis. Mol Med. 2012;18:359–69. doi: 10.2119/molmed.2011.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu H, Schwarzer K, Forster M, Kniemeyer O, Forsbach-Birk V, Straube E, Rodel J. Role of high-mobility group box 1 protein and poly(ADP-ribose) polymerase 1 degradation in Chlamydia trachomatis-induced cytopathicity. Infect Immun. 2010;78:3288–97. doi: 10.1128/IAI.01404-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.David KK, Andrabi SA, Dawson TM, Dawson VL. Parthanatos, a messenger of death. Front Biosci. 2009;14:1116–28. doi: 10.2741/3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA. 1999;96:13978–82. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu SW, Wang H, Poitras MF, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–63. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 49.Halappanavar SS, Rhun YL, Mounir S, Martins LM, Huot J, Earnshaw WC, Shah GM. Survival and proliferation of cells expressing caspase-uncleavable Poly(ADP-ribose) polymerase in response to death-inducing DNA damage by an alkylating agent. J Biol Chem. 1999;274:37097–104. doi: 10.1074/jbc.274.52.37097. [DOI] [PubMed] [Google Scholar]
- 50.Malireddi RK, Ippagunta S, Lamkanfi M, Kanneganti TD. Cutting edge: proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J Immunol. 2010;185:3127–30. doi: 10.4049/jimmunol.1001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yelamos J, Monreal Y, Saenz L, et al. PARP-2 deficiency affects the survival of CD4+ CD8+ double-positive thymocytes. EMBO J. 2006;25:4350–60. doi: 10.1038/sj.emboj.7601301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicolas L, Martinez C, Baro C, et al. Loss of poly(ADP-ribose) polymerase-2 leads to rapid development of spontaneous T-cell lymphomas in p53-deficient mice. Oncogene. 2010;29:2877–83. doi: 10.1038/onc.2010.11. [DOI] [PubMed] [Google Scholar]
- 53.Saenz L, Lozano JJ, Valdor R, et al. Transcriptional regulation by poly(ADP-ribose) polymerase-1 during T cell activation. BMC Genomics. 2008;9:171. doi: 10.1186/1471-2164-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasta F, Laudisi F, Sambucci M, Rosado MM, Pioli C. Increased Foxp3+ regulatory T cells in poly(ADP-Ribose) polymerase-1 deficiency. J Immunol. 2010;184:3470–7. doi: 10.4049/jimmunol.0901568. [DOI] [PubMed] [Google Scholar]
- 55.Olabisi OA, Soto-Nieves N, Nieves E, et al. Regulation of transcription factor NFAT by ADP-ribosylation. Mol Cell Biol. 2008;28:2860–71. doi: 10.1128/MCB.01746-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valdor R, Schreiber V, Saenz L, et al. Regulation of NFAT by poly(ADP-ribose) polymerase activity in T cells. Mol Immunol. 2008;45:1863–71. doi: 10.1016/j.molimm.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 57.Datta R, Naura AS, Zerfaoui M, et al. PARP-1 deficiency blocks IL-5 expression through calpain-dependent degradation of STAT-6 in a murine asthma model. Allergy. 2011;66:853–61. doi: 10.1111/j.1398-9995.2011.02549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goenka S, Cho SH, Boothby M. Collaborator of Stat6 (CoaSt6)-associated poly(ADP-ribose) polymerase activity modulates Stat6-dependent gene transcription. J Biol Chem. 2007;282:18732–9. doi: 10.1074/jbc.M611283200. [DOI] [PubMed] [Google Scholar]
- 59.Mehrotra P, Hollenbeck A, Riley JP, Li F, Patel RJ, Akhtar N, Goenka S. Poly (ADP-ribose) polymerase 14 and its enzyme activity regulates TH2 differentiation and allergic airway disease. J Allergy Clin Immunol. 2012;131:521–31. doi: 10.1016/j.jaci.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+ CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol. 2005;175:3075–83. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- 61.Krebs C, Adriouch S, Braasch F, et al. CD38 controls ADP-ribosyltransferase-2-catalyzed ADP-ribosylation of T cell surface proteins. J Immunol. 2005;174:3298–305. doi: 10.4049/jimmunol.174.6.3298. [DOI] [PubMed] [Google Scholar]
- 62.Hubert S, Rissiek B, Klages K, et al. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J Exp Med. 2010;207:2561–8. doi: 10.1084/jem.20091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thoren FB, Romero AI, Hellstrand K. Oxygen radicals induce poly(ADP-ribose) polymerase-dependent cell death in cytotoxic lymphocytes. J Immunol. 2006;176:7301–7. doi: 10.4049/jimmunol.176.12.7301. [DOI] [PubMed] [Google Scholar]
- 64.Aurelius J, Thoren FB, Akhiani AA, Brune M, Palmqvist L, Hansson M, Hellstrand K, Martner A. Monocytic AML cells inactivate antileukemic lymphocytes: role of NADPH oxidase/gp91(phox) expression and the PARP-1/PAR pathway of apoptosis. Blood. 2012;119:5832–7. doi: 10.1182/blood-2011-11-391722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taccioli GE, Rathbun G, Oltz E, Stamato T, Jeggo PA, Alt FW. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993;260:207–10. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- 66.Ambrose HE, Willimott S, Beswick RW, Dantzer F, de Murcia JM, Yelamos J, Wagner SD. Poly(ADP-ribose) polymerase-1 (Parp-1)-deficient mice demonstrate abnormal antibody responses. Immunology. 2009;127:178–86. doi: 10.1111/j.1365-2567.2008.02921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrison C, Smith GC, Stingl L, Jackson SP, Wagner EF, Wang ZQ. Genetic interaction between PARP and DNA-PK in V(D)J recombination and tumorigenesis. Nat Genet. 1997;17:479–82. doi: 10.1038/ng1297-479. [DOI] [PubMed] [Google Scholar]
- 68.Brown ML, Franco D, Burkle A, Chang Y. Role of poly(ADP-ribosyl)ation in DNA-PKcs-independent V(D)J recombination. Proc Natl Acad Sci USA. 2002;99:4532–7. doi: 10.1073/pnas.072495299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 70.Arakawa H, Hauschild J, Buerstedde JM. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science. 2002;295:1301–6. doi: 10.1126/science.1067308. [DOI] [PubMed] [Google Scholar]
- 71.Paddock MN, Buelow BD, Takeda S, Scharenberg AM. The BRCT domain of PARP-1 is required for immunoglobulin gene conversion. PLoS Biol. 2010;8:e1000428. doi: 10.1371/journal.pbio.1000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shockett P, Stavnezer J. Inhibitors of poly(ADP-ribose) polymerase increase antibody class switching. J Immunol. 1993;151:6962–76. [PubMed] [Google Scholar]
- 73.Robert I, Dantzer F, Reina-San-Martin B. Parp1 facilitates alternative NHEJ, whereas Parp2 suppresses IgH/c-myc translocations during immunoglobulin class switch recombination. J Exp Med. 2009;206:1047–56. doi: 10.1084/jem.20082468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho SH, Goenka S, Henttinen T, Gudapati P, Reinikainen A, Eischen CM, Lahesmaa R, Boothby M. PARP-14, a member of the B aggressive lymphoma family, transduces survival signals in primary B cells. Blood. 2009;113:2416–25. doi: 10.1182/blood-2008-03-144121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ambrose HE, Papadopoulou V, Beswick RW, Wagner SD. Poly-(ADP-ribose) polymerase-1 (Parp-1) binds in a sequence-specific manner at the Bcl-6 locus and contributes to the regulation of Bcl-6 transcription. Oncogene. 2007;26:6244–52. doi: 10.1038/sj.onc.1210434. [DOI] [PubMed] [Google Scholar]
- 76.Terashima M, Takahashi M, Shimoyama M, Tanigawa Y, Urano T, Tsuchiya M. Glycosylphosphatidylinositol-anchored arginine-specific ADP-ribosyltransferase7.1 (Art7.1) on chicken B cells: the possible role of Art7 in B cell receptor signalling and proliferation. Mol Cell Biochem. 2009;320:93–100. doi: 10.1007/s11010-008-9902-6. [DOI] [PubMed] [Google Scholar]
- 77.Wahlberg E, Karlberg T, Kouznetsova E, et al. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nat Biotechnol. 2012;30:283–8. doi: 10.1038/nbt.2121. [DOI] [PubMed] [Google Scholar]
- 78.Garcia S, Bodano A, Gonzalez A, Forteza J, Gomez-Reino JJ, Conde C. Partial protection against collagen antibody-induced arthritis in PARP-1 deficient mice. Arthritis Res Ther. 2006;8:R14. doi: 10.1186/ar1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzalez-Rey E, Martinez-Romero R, O'Valle F, Aguilar-Quesada R, Conde C, Delgado M, Oliver FJ. Therapeutic effect of a poly(ADP-ribose) polymerase-1 inhibitor on experimental arthritis by downregulating inflammation and Th1 response. PLoS ONE. 2007;2:e1071. doi: 10.1371/journal.pone.0001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pascual M, Lopez-Nevot MA, Caliz R, Ferrer MA, Balsa A, Pascual-Salcedo D, Martin J. A poly(ADP-ribose) polymerase haplotype spanning the promoter region confers susceptibility to rheumatoid arthritis. Arthritis Rheum. 2003;48:638–41. doi: 10.1002/art.10864. [DOI] [PubMed] [Google Scholar]
- 81.Onaran I, Tezcan G, Ozgonenel L, Cetin E, Ozdemir AT, Kanigur-Sultuybek G. The Val762Ala polymorphism in the poly(ADP-ribose) polymerase-1 gene is not associated with susceptibility in Turkish rheumatoid arthritis patients. Rheumatol Int. 2009;29:797–800. doi: 10.1007/s00296-008-0772-8. [DOI] [PubMed] [Google Scholar]
- 82.Scott GS, Kean RB, Mikheeva T, Fabis MJ, Mabley JG, Szabo C, Hooper DC. The therapeutic effects of PJ34 [N-(6-oxo-5,6-dihydrophenanthridin-2-yl)-N, N-dimethylacetamide.HCl], a selective inhibitor of poly(ADP-ribose) polymerase, in experimental allergic encephalomyelitis are associated with immunomodulation. J Pharmacol Exp Ther. 2004;310:1053–61. doi: 10.1124/jpet.103.063214. [DOI] [PubMed] [Google Scholar]
- 83.Chiarugi A. Inhibitors of poly(ADP-ribose) polymerase-1 suppress transcriptional activation in lymphocytes and ameliorate autoimmune encephalomyelitis in rats. Br J Pharmacol. 2002;137:761–70. doi: 10.1038/sj.bjp.0704934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cavone L, Aldinucci A, Ballerini C, Biagioli T, Moroni F, Chiarugi A. PARP-1 inhibition prevents CNS migration of dendritic cells during EAE, suppressing the encephalitogenic response and relapse severity. Mult Scler. 2011;17:794–807. doi: 10.1177/1352458511399113. [DOI] [PubMed] [Google Scholar]
- 85.Selvaraj V, Soundarapandian MM, Chechneva O, Williams AJ, Sidorov MK, Soulika AM, Pleasure DE, Deng W. PARP-1 deficiency increases the severity of disease in a mouse model of multiple sclerosis. J Biol Chem. 2009;284:26070–84. doi: 10.1074/jbc.M109.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanchez-Fidalgo S, Villegas I, Martin A, Sanchez-Hidalgo M, Alarcon de la Lastra C. PARP inhibition reduces acute colonic inflammation in rats. Eur J Pharmacol. 2007;563:216–23. doi: 10.1016/j.ejphar.2007.01.070. [DOI] [PubMed] [Google Scholar]
- 87.Zingarelli B, Hake PW, Burroughs TJ, Piraino G, O'Connor M, Denenberg A. Activator protein-1 signalling pathway and apoptosis are modulated by poly(ADP-ribose) polymerase-1 in experimental colitis. Immunology. 2004;113:509–17. doi: 10.1111/j.1365-2567.2004.01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cuzzocrea S, Mazzon E, Genovese T, et al. Role of poly(ADP-ribose) glycohydrolase in the development of inflammatory bowel disease in mice. Free Radic Biol Med. 2007;42:90–105. doi: 10.1016/j.freeradbiomed.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 89.Jijon HB, Churchill T, Malfair D, Wessler A, Jewell LD, Parsons HG, Madsen KL. Inhibition of poly(ADP-ribose) polymerase attenuates inflammation in a model of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G641–51. doi: 10.1152/ajpgi.2000.279.3.G641. [DOI] [PubMed] [Google Scholar]
- 90.Popoff I, Jijon H, Monia B, Tavernini M, Ma M, McKay R, Madsen K. Antisense oligonucleotides to poly(ADP-ribose) polymerase-2 ameliorate colitis in interleukin-10-deficient mice. J Pharmacol Exp Ther. 2002;303:1145–54. doi: 10.1124/jpet.102.039768. [DOI] [PubMed] [Google Scholar]
- 91.Nossa CW, Jain P, Tamilselvam B, Gupta VR, Chen LF, Schreiber V, Desnoyers S, Blanke SR. Activation of the abundant nuclear factor poly(ADP-ribose) polymerase-1 by Helicobacter pylori. Proc Natl Acad Sci U S A. 2009;106:19998–20003. doi: 10.1073/pnas.0906753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Toller IM, Altmeyer M, Kohler E, Hottiger MO, Muller A. Inhibition of ADP ribosylation prevents and cures Helicobacter-induced gastric preneoplasia. Cancer Res. 2010;70:5912–22. doi: 10.1158/0008-5472.CAN-10-0528. [DOI] [PubMed] [Google Scholar]
- 93.Havranek T, Aujla PK, Nickola TJ, Rose MC, Scavo LM. Increased poly(ADP-ribose) polymerase (PARP)-1 expression and activity are associated with inflammation but not goblet cell metaplasia in murine models of allergen-induced airway inflammation. Exp Lung Res. 2010;36:381–9. doi: 10.3109/01902141003663360. [DOI] [PubMed] [Google Scholar]
- 94.Oumouna M, Datta R, Oumouna-Benachour K, Suzuki Y, Hans C, Matthews K, Fallon K, Boulares H. Poly(ADP-ribose) polymerase-1 inhibition prevents eosinophil recruitment by modulating Th2 cytokines in a murine model of allergic airway inflammation: a potential specific effect on IL-5. J Immunol. 2006;177:6489–96. doi: 10.4049/jimmunol.177.9.6489. [DOI] [PubMed] [Google Scholar]
- 95.Weston VJ, Oldreive CE, Skowronska A, et al. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood. 2010;116:4578–87. doi: 10.1182/blood-2010-01-265769. [DOI] [PubMed] [Google Scholar]
- 96.Guha M. PARP inhibitors stumble in breast cancer. Nat Biotechnol. 2011;29:373–4. doi: 10.1038/nbt0511-373. [DOI] [PubMed] [Google Scholar]
- 97.Quiles-Perez R, Munoz-Gamez JA, Ruiz-Extremera A, et al. Inhibition of poly adenosine diphosphate-ribose polymerase decreases hepatocellular carcinoma growth by modulation of tumor-related gene expression. Hepatology. 2010;51:255–66. doi: 10.1002/hep.23249. [DOI] [PubMed] [Google Scholar]
- 98.Martin-Oliva D, O'Valle F, Munoz-Gamez JA, et al. Crosstalk between PARP-1 and NF-κB modulates the promotion of skin neoplasia. Oncogene. 2004;23:5275–83. doi: 10.1038/sj.onc.1207696. [DOI] [PubMed] [Google Scholar]
- 99.Martin-Oliva D, Aguilar-Quesada R, O'Valle F, et al. Inhibition of poly(ADP-ribose) polymerase modulates tumor-related gene expression, including hypoxia-inducible factor-1 activation, during skin carcinogenesis. Cancer Res. 2006;66:5744–56. doi: 10.1158/0008-5472.CAN-05-3050. [DOI] [PubMed] [Google Scholar]
- 100.Rajesh M, Mukhopadhyay P, Godlewski G, Batkai S, Hasko G, Liaudet L, Pacher P. Poly(ADP-ribose)polymerase inhibition decreases angiogenesis. Biochem Biophys Res Commun. 2006;350:1056–62. doi: 10.1016/j.bbrc.2006.09.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morrow DA, Brickman CM, Murphy SA, et al. A randomized, placebo-controlled trial to evaluate the tolerability, safety, pharmacokinetics, and pharmacodynamics of a potent inhibitor of poly(ADP-ribose) polymerase (INO-1001) in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of the TIMI 37 trial. J Thromb Thrombolysis. 2009;27:359–64. doi: 10.1007/s11239-008-0230-1. [DOI] [PubMed] [Google Scholar]
- 102.Cavone L, Chiarugi A. Targeting poly(ADP-ribose) polymerase-1 as a promising approach for immunomodulation in multiple sclerosis? Trends Mol Med. 2012;18:92–100. doi: 10.1016/j.molmed.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 103.Szabo E, Kovacs I, Grune T, Haczku A, Virag L. PARP-1: a new player in the asthma field? Allergy. 2011;66:811–4. doi: 10.1111/j.1398-9995.2011.02551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karlberg T, Hammarstrom M, Schutz P, Svensson L, Schuler H. Crystal structure of the catalytic domain of human PARP2 in complex with PARP inhibitor ABT-888. Biochemistry. 2010;49:1056–8. doi: 10.1021/bi902079y. [DOI] [PubMed] [Google Scholar]
- 105.Lehtio L, Jemth AS, Collins R, et al. Structural basis for inhibitor specificity in human poly(ADP-ribose) polymerase-3. J Med Chem. 2009;52:3108–11. doi: 10.1021/jm900052j. [DOI] [PubMed] [Google Scholar]


