Abstract
In addition to archetypal cognitive defects, Down syndrome (DS) is characterized by altered lymphocyte development and function, including premature thymic involution and increased incidence of infections. However, the potential mechanisms for these changes have not been fully elucidated. The current study used the Ts65Dn mouse model of DS to assess deficiencies in T-cell development and possible molecular alterations. Ts65Dn mice exhibited premature thymic involution and a threefold to fourfold decrease in the number and proportion of immature, double-negative thymocyte progenitors. In addition, there were twofold fewer double-positive and CD4 single-positive thymocytes in Ts65Dn thymuses. Reflecting this deficient thymic function, there were fewer naive T cells in the spleen and polyclonal stimulation of peripheral T cells exhibited a marked reduction in proliferation, suggesting a senescent phenotype. In contrast, B-cell progenitors were unchanged in the bone marrow of Ts65Dn mice, but in the spleen, there were decreased transitional and follicular B cells and these cells proliferated less upon antigen receptor stimulus but not in response to lipopolysaccharide. As a potential mechanism for diminished thymic function, immature thymocyte populations expressed diminished levels of the cytokine receptor interleukin-7Rα, which was associated with decreased proliferation and increased apoptosis. Increased oxidative stress and inhibition of the Notch pathway were identified as possible mediators of decreased interleukin-7Rα expression in Ts65Dn mice. The data suggest that immature thymocyte defects underlie immune dysfunction in DS and that increased oxidative stress and reduced cytokine signalling may alter lymphocyte development in Ts65Dn mice.
Keywords: Down syndrome, interleukin-7 receptor, lymphocyte development, reactive oxygen species, thymus
Introduction
Numerous studies have indicated that the adaptive immune system is altered in individuals with Down syndrome (DS), with defects ranging from the level of immature haematopoietic progenitor cells to mature lymphocytes in the periphery.1 Since the 1970s, it has been observed that individuals with DS seemed to exhibit diseases arising from defects in the immune system, such as the increased frequency of respiratory infections, leukaemia, and autoimmune diseases such as diabetes. Significantly, these diseases, although not as commonly associated with DS as the deficiencies in cognitive function, are major causes of morbidity and mortality.2,3 For this reason, the hypothesis has been developed that the immune system is inherently defective in DS. However, the underlying mechanisms for these global defects in adaptive immune function are unclear, and the molecular mechanisms inducing these changes have not been examined in detail.
T-cell development occurs in the thymus, which does not contain its own self-renewing population of stem cells and must be continuously seeded by bone-marrow-derived haematopoietic progenitors that travel through the circulation.4,5 Previous studies have shown loss of bone marrow haematopoietic progenitor populations in Ts65Dn mice, a mouse model for Down syndrome with triplication of a region of mouse chromosome 16 that is syntenic to human chromosome 21.6,7 Significantly, there were defects in the common lymphoid progenitor and lymphoid-primed multipotent progenitor populations, which have been reported to have thymus-seeding potential.8,9 Previous studies of mechanisms for immune defects in individuals with DS have proposed deficits in the thymic stroma, which supports thymocyte development,10–12 and others have found decreased recent thymic emigrants to repopulate peripheral lymphocytes.13,14 Diminished thymic function has been proposed as a causative factor in altered lymphocyte function in DS, characterized by fewer naive T cells14 or decreased proliferative response to phytohaemagglutinin.1 However, to date, there has not been a detailed analysis of lymphocyte development in a mouse model of DS or analysis of T-cell function.
The interleukin-7 (IL-7)/IL-7Rα receptor system plays an essential role in lymphoid development and homeostasis by promoting proliferation and inhibiting apoptosis.15,16 Loss of IL-7 signalling results in the impairment of thymocyte development, thymic involution and severe lymphopenia.17,18 Interleukin-7Rα is expressed robustly during the DN2 and DN3 stages of thymocyte development until β-selection, is down-regulated during the ISP and DP stages, and is re-expressed again during the SP stage. Regulation of IL-7Rα expression is still relatively unclear, although it has been proposed that both T-cell receptor activation and concentrations of the ligand IL-7 can control IL-7Rα surface expression.19 In addition, a recent report suggested that Notch signalling controlled IL-7Rα transcription in T-lineage progenitors.20
The goal of this study was to determine how the previously described changes in bone marrow progenitors in the Ts65Dn mouse model of DS may affect T-cell development and function and determine possible mechanisms for changes in thymic and splenic T cells. Importantly, the current data indicate changes in composition and function of T-cell progenitors in the thymus ex vivo, especially within the immature, double-negative (DN) thymocyte populations. Decreased IL-7Rα expression in the DN thymocytes was identified as a potential mechanism for the defects observed in these populations. Furthermore, the changes in the thymic progenitors were reflected by significant decreases in T-cell function as measured by in vitro proliferation in response to polyclonal stimuli. Hence, the data indicate that loss of immature thymocyte function leads to changes in the adaptive immune system of Ts65Dn mice that may mirror some of the immune defects observed in individuals with DS.
Materials and methods
Mice
Female C57BL/6, male trisomic Ts65Dn mice (stock # 01924) and euploid littermates 4–8 weeks old were purchased from the Jackson Laboratory (Bar Harbor, ME). This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animal care was provided in accordance with protocols reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) in the Office of Animal Welfare Assurance at the University of Maryland, Baltimore (Assurance Number A3200-01).
Antibodies
CD4 biotin (GK1.5), CD5 biotin (Ly-1), CD8α biotin (53-6.7), CD11b biotin (M1/70), TER-119 biotin were purchased from BD Biosciences (San Jose, CA) and CD135 PE (A2F10.1) was purchased from BioLegend (San Diego, CA). All other antibodies were purchased from eBioscience (San Diego, CA): CD3ε biotin (145-2C11), CD8β biotin (H35-17.2), CD8α allophycocyanin (APC)/APC-Cy7 (53-6.7), CD48 FITC (HM 48.1), c-kit/CD117 APC-Cy7 (2B8), CD11c biotin (N418), CD19 biotin/APC-Cy7 (1D3), B220 biotin (RA3-6B2), Gr-1 biotin (RB6-8C5), NK1.1 biotin (PK136), T-cell receptor (TCR) γδ (UC7-13D5), TCR-β (H57-597), CD127 Alexa Fluor 647/phycoerythrin (PE) (A7R34), CD25 FITC/APC (PC61.5), Streptavidin efluor 450, CD16/32 PE-Cy7 (93), CD4 PE-Cy7 (GK1.5), CD44 PE-Cy7 (IM7), CD23 (B3B4), CD21 (8D9), CD80 (16-10A1), MHC II (M5/114.15.2), IgM (11/41), IgD (11-26), CD93 (AA4.1) and CD43 (R2/60).
Flow cytometric analysis of haematopoietic progenitor and mature lymphocyte phenotype
Immature, DN thymocytes were stained with a pool of antibodies recognizing lineage (Lin) markers. The lineage mix contained antibodies to B220, CD3ε, CD8β, CD8α, CD11b, Gr-1, CD11c, NK1.1, TCR-β, and TCR-γ as previously described.21 The DN thymocytes, after lineage gating, were further characterized into DN1 (CD44+ CD25−), DN2 (CD44+ CD25+). DN3 (CD44− CD25+), and DN4 (CD44− CD25−) populations.22 Early T-lineage progenitors (ETPs) after lineage gating, were defined as CD44+ CD25− c-Kithi IL-7R−/lo.21 Effector/effector memory splenic T cells were defined as CD44hi CD62Llo, and central memory T cells were defined as CD44hi CD62Lhi.23 Bone marrow B cells were defined based upon previously reported markers.24,25 Pre-pro B cells were defined as B220+ CD19− CD43+ IgM−, pro-B cells were defined as B220+ CD19+ CD43+ IgM−, pre-B cells were defined as B220+ CD19+ CD43− IgM−, immature B cells were defined as B220+ CD19+ CD43− IgM+, and mature B cells were defined as B220+ IgM+ IgD+. In the spleen, B-cell subsets were defined as described by Allman and Pillai.26 CD19+ B cells were defined as transitional (T) B-cell subsets; T1: B220+ AA4+ IgMhi CD23−; T2: B220+ AA4+ IgMhi CD23+; T3: B220+ AA4+ IgMlo CD23+ or marginal zone (MZ) B-cell subsets; MZ: B220+ AA4− IgMhi CD21hi CD23−; or marginal zone precursor (MZP): B220+ AA4− IgMhi CD21hi CD23+, or follicular (Fol) B-cell subsets were defined as Fol I: B220+ AA4− IgMlo CD21lo IgD+; or Fol II: B220+ AA4− IgMhi CD21lo IgD+. Compensation settings and lineage gates were based upon single colour controls. Analysis was performed with flowjo (Tree Star, Inc., Ashland, OR)
Intracellular reactive oxygen species were analysed in selected subsets by using the oxidation sensitive dye dichlorodihydrofluorescein diacetate (DCFDA) as previously described.6 Cells were incubated ex vivo with 2 μm DCFDA at 37° for 15 min, washed and surface stained. As a loading control, parallel samples were incubated with the oxidized control dye fluorescein diacetate (FDA) (0·01 μm) at 37° for 15 min, washed, and surface stained as described above. FACS analysis was performed immediately. DCFDA mean channel fluorescence was normalized to FDA uptake, and the data are shown as the per cent increase in DCFDA fluorescence in cells from Ts65Dn mice over euploid controls ± SEM.
Measurement of intracellular glutathione
Intracellular glutathione levels were measured in progenitor subsets by flow cytometry using monochlorobimane (MCB) essentially as previously described.6 Briefly, thymic cells were surface stained as described above and then incubated for 10 min at room temperature with 20 μm monochlorobimane. Cells were washed and analysed immediately by flow cytometry.
Bromodeoxyuridine cell proliferation analysis
Mice were injected intraperitomeally with 100 mg/kg bromodeoxyuridine (BrdU) twice a day for 2 days. BrdU incorporation was detected in defined subsets by intracellular staining using an FITC anti-BrdU antibody as suggested by the supplier (BD Biosciences). The expression of Bcl-2 was detected in defined thymic subsets by intracellular staining, as indicated by the supplier, using PE anti-Bcl-2 antibodies (BD Biosciences). Cells were analysed by flow cytometry.
CFSE cell proliferation analysis
Red blood cell-depleted splenocytes were washed in PBS by centrifugation at 200× g for 7 min, then resuspended in PBS at a final concentration of 10 × 106 cells/ml. Carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene OR) was added to the cell suspension at a final concentration of 0·25 μm, and the cells were incubated at 37° in a water bath for 15 min. The CFSE-labelled cells were then washed twice with complete media to quench residual CFSE, resuspended at 2 × 106 cells/ml, and cultured in plates coated with 0·5 μg/ml or 5 μg/ml anti-CD3 antibody (2C11). Alternatively, cells were incubated with the Toll-like receptor 4 agonist lipopolysaccharide (1, 0·1 or 0·01 ng/ml) or soluble anti-mouse IgM (1 or 10 mg/ml) in the presence or absence of IL-4 (10 ng/ml). Proliferation of T or B cells, as assessed by CFSE dilution in TCR+ or CD19+ cells, respectively, was measured after 48 and 72 hr and percentage of proliferating cells was calculated using flowjo.
Quantitative PCR
Total RNA was isolated from thymus or bone marrow cells using the Nucleospin kit (Macherey Nagel, Bethlehem, PA). Expression of mRNA was measured as indicated by the supplier using the following Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA) for IL-7Rα (Assay ID 00434295), IL-7 (Assay ID: 01295803), NQO1 (Assay ID 00500821) and Hes-1 (Assay ID: 01342805); HPRT (Assay ID 03024075) was used as a control. For microRNA (miRNA), total RNA was isolated by the miRNeasy kit (Qiagen, Valencia, CA) for miRNA detection. Expression of miRNA was measured as indicated by the supplier using the following Taqman microRNA assays (Applied Biosystems): miR-155 (Assay ID 002571) and miR-125b (Assay ID 000449); u6 rRNA (Assay ID 001973) was used as a control. The relative mRNA or miRNA expression levels were calculated based on the ΔCT method.27
Statistical analysis
Statistical significance was analysed by Student's t-test or Wilcoxon signed rank test using prism. Conditions were deemed significantly different if P < 0·05.
Results
T-cell progenitor populations in the thymus are altered in Ts65Dn mice
Previous data in Ts65Dn mice6 suggested defects in the common lymphoid progenitor (CLP) and lymphoid-primed multipotent progenitor populations (LMPP), which have been reported to have thymus-seeding potential, at 3–4 months of age.8,9 Furthermore, an earlier report indicated significant changes in Ts65Dn thymic ultrastructural morphology at 2–3 months.10 At 3–4 months of age, Ts65Dn mice exhibited a twofold decrease in thymic cellularity compared with euploid mice (Fig. 1a), and mice of this age were used for all subsequent studies.
Figure 1.
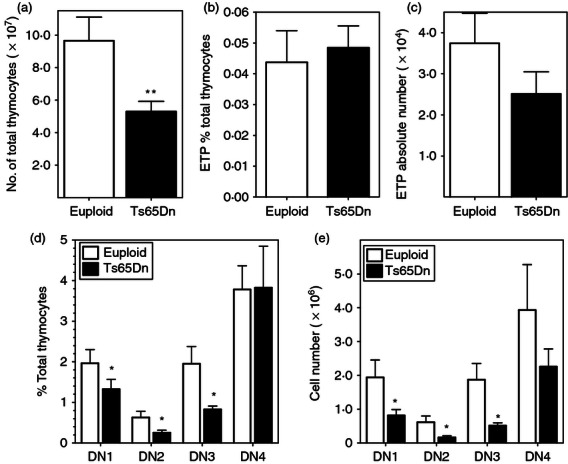
Thymocyte populations are altered in Ts65Dn mice. Thymocyte populations from Ts65Dn mice (closed bars) or euploid littermates (open bars) were assessed ex vivo by flow cytometry as defined in the Materials and methods. (a) Thymic cellularity (total viable, nucleated cells) is reduced in Ts65Dn mice (closed bars) in comparison to euploid mice (open bars). (n = 9). (b,c) Early T-cell progenitor (ETP) population as (b) a percentage of the entire thymocyte population and (c) absolute numbers of ETPs in the thymus. (d,e) Double-negative (DN) thymocytes, after lineage gating, were further characterized into DN1, DN2, DN3 and DN4 populations as described in the Materials and methods. (d) DN thymocyte populations as a percentage of the entire thymocyte population and (e) absolute numbers of DN thymocyte populations (n = 5; *P < 0·05; **P < 0·01 by Wilcoxon signed rank test).
Following seeding by the bone marrow lymphoid progenitors, T-cell commitment and development occurs in the DN thymocyte population (LMPP), which can be divided into subsets based on CD44 and CD25 expression, after excluding all lineage-positive cells. In the DN thymocyte population, the ETPs: Lin−, CD44+, CD25−, c-Kithi, IL-7Rα−/lo have been suggested to be the precursor for all thymocytes with T-lineage potential.21 Unlike the significantly lower percentages of the proposed bone marrow-derived precursors (CLP, LMPP)6 there was no significant difference in the ETP populations of Ts65Dn and euploid mice as a percentage of total thymocyte number, indicating that there was no preferential loss of ETP compared with other thymocyte populations (Fig. 1b). However, because of the thymic involution of the Ts65Dn mice, there were fewer ETPs in the thymus of Ts65Dn mice in comparison to euploid mice, although the differences were not significant (Fig. 1c).
Further analysis of the DN subsets indicated that Ts65Dn mice had a lower percentage of DN1 (CD44+ CD25−), DN2 (CD44+ CD25+) and DN3 (CD44− CD25+) thymocytes compared with euploid mice (Fig. 1d). There was no significant difference in the percentage of DN4 thymocytes. As a result of decreased thymic cellularity, Ts65Dn mice had approximately threefold to fourfold fewer total DN1, DN2 and DN3 thymocytes with no preferential loss of a single subset. The decreased number of DN4 thymocytes in the Ts65Dn mice was not significantly different (Fig. 1e). Similarly, there were significantly fewer mature thymocytes, with twofold decreases in the number of double-positive (DP) and CD4 single-positive (SP) thymocytes in Ts65Dn mice compared with euploid mice (see Supplementary material; Fig. S1a). However, there were not significant differences in percentage representation of the mature thymocyte populations in the Ts65Dn thymus in comparison to euploid mice (Fig. S1b) with the exception of an increased percentage of CD8 SP thymocytes. Hence, early thymocyte development during T-cell commitment is altered in Ts65Dn mice but more mature thymocyte populations are relatively unaffected.
Mature lymphocyte populations in the spleen are altered in Ts65Dn mice
To determine how the changes in the lymphoid progenitor cells in the bone marrow and thymus may affect mature lymphocyte homeostasis and function, the composition of the spleen was examined. In contrast to the thymus, there were no significant differences in splenic size and cellularity between Ts65Dn mice (10·9 ± 1·6 ×× 107 cells/spleen) and euploid mice (11·56 ± 1·56 × 107 cells/spleen; n = 10) or in the majority of the subsets. Similar to a previous report,7 there was a slight increase in the percentage of TCR+ T cells, and a slight decrease in the percentage of CD19+ cells, but these changes were not significant (not shown). Consistent with previous studies of individuals with DS,28 there was a significant decrease in the percentage of CD44lo CD62Lhi naive T cells, with a concurrent increase in the CD44hi CD62Llo effector/effector memory population and a small increase in the CD44hi CD62Lhi central memory population (Fig. 2a).
Figure 2.
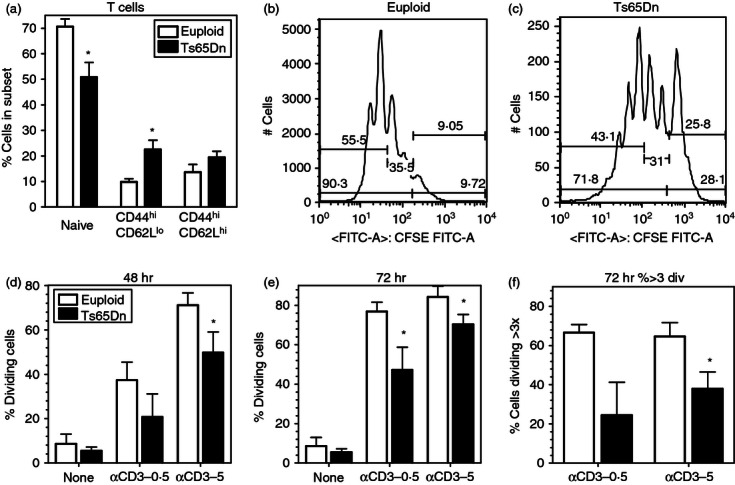
Diminished naive T cells and T-cell proliferation in Ts65Dn mice. (a) Naive (CD44lo, CD62Lhi), effector (CD44hi, CD62Llo), and memory (CD44hi, CD62Lhi) T-cell sub-populations (gated on TCR+ cells) in spleens from Ts65Dn mice (closed bars) and euploid mice (open bars) (n = 5,*P < 0·05 by paired t-test). (b,c) Representative examples of CFSE staining of T cells upon anti-CD3 stimulation in euploid (b) and Ts65Dn (c) splenocytes. (d–f) Analysis of CFSE staining in T cells stimulated on immobilized anti-CD3 at 0.5 or 5 μg/ml after 48 hr (b) and 72 hr (c,d) in Ts65Dn mice (closed bars) and euploid mice (open bars). The data represent the percentage of TCR+ cells undergoing greater than or equal to 1 division (b,c) or greater than 3 divisions (d) (n = 5, *P < 0·05 by paired t-test).
T-lymphocyte function in the periphery is altered in Ts65Dn mice
Previous reports on DS have suggested that limited thymic output would lead to decreased function and immunosenescence in peripheral immune cells.14 To assess lymphocyte functional capacity in Ts65Dn mice, whole splenocytes were stimulated with immobilized anti-CD3 antibody (at 0.5 or 5 μg/ml) for 48 and 72 hr in vitro. Proliferation of CFSE-labelled splenocytes was measured in TCR+ T cells by flow cytometry (representative flow data shown in Fig. 2b,c) as described in the Materials and methods. There was a significant decrease in the percentage of TCR+ cells that had undergone at least one division in cells from Ts65Dn mice compared with euploid controls after 48 hr (Fig. 2d) and 72 hr (Fig. 2e). There was also a significant decrease in the percentage of TCR+ cells that had undergone more than three divisions after 72 hr (Fig. 2f) in cells from Ts65Dn mice. Changes in proliferation were not the result of cell death because the percentage of viable cells was not different between euploid and Ts65Dn cells at both time-points (not shown). Hence, although the proportions of peripheral immune cells in Ts65Dn mice are relatively unchanged, there are significant defects in the function of peripheral T cells that suggest a senescent phenotype in this mouse model of DS.
Decreased expression of the IL-7Rα chain in the thymus
As a possible mechanism for thymic alterations in Ts65Dn mice, IL-7Rα was assessed because it plays a non-redundant role in thymic development, promoting proliferation and survival of immature, DN thymocytes.18 Consistent with our previous observations in bone marrow lymphoid progenitors,6 the percentage of specific thymocyte subsets that were IL-7Rα+ was decreased in the lineage-negative (total DN thymocytes), DN2 and DN3 populations (Fig. 3a). The absolute number of cells expressing the IL-7Rα chain was significantly decreased in the Lin− and all the DN thymocyte populations (Fig. 3b). Interleukin-7Rα is normally down-regulated in DP thymocytes and re-expressed in positively selected CD4 and CD8 SP thymocytes.15 In contrast to the data in DN thymocytes, when mature DP and SP thymocytes were analysed neither the percentage of IL-7Rα+ cells (Fig. S1c) nor the number of positive cells (not shown) was decreased.
Figure 3.
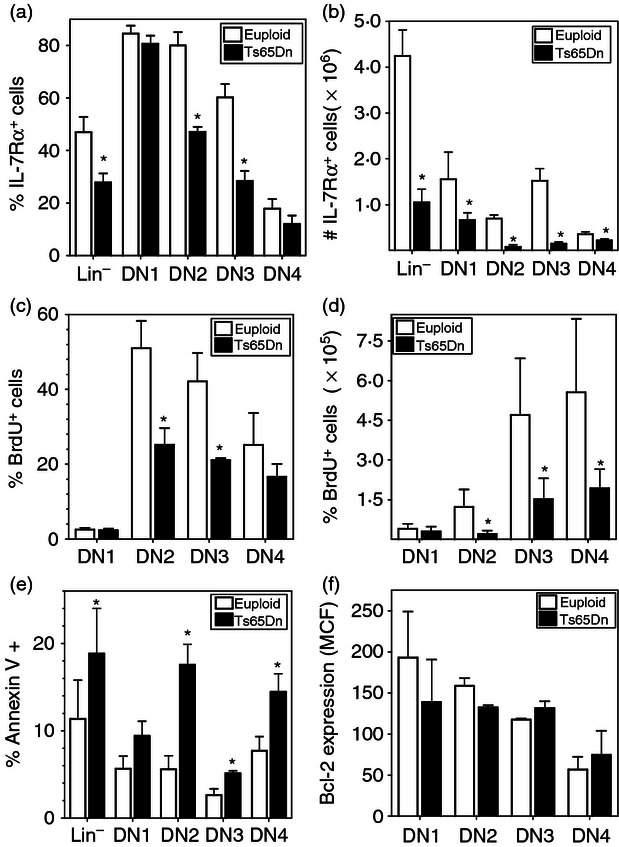
Interleukin-7 receptor α (IL-7Rα) expression and defects in proliferation and survival in the thymus of Ts65Dn mice. (a,b) IL-7Rα expression in double-negative (DN) thymocyte sub-populations from Ts65Dn mice (closed bars) or euploid littermates (open bars) was assessed by flow cytometry. The data are expressed as (a) the % IL-7Rα+ cells in each sub-population or (b) the number of IL-7Rα+ cells per thymus (n = 5,*P < 0·05 by Wilcoxon signed rank test). (c,d) Ts65Dn mice (closed bars) or euploid littermates (open bars) were injected with bromodeoxyuridine (BrdU) twice a day for 2 days as described in the Materials and methods. BrdU incorporation in DN thymocyte sub-populations was assessed by flow cytometry as described in the Materials and methods. The data are expressed as (c) the per cent BrdU+ cells in each sub-population or (d) the number of BrdU+ cells per thymus. (e) Apoptosis, as assessed by Annexin V binding, and (f) bcl-2 protein expression in DN thymocyte sub-populations from Ts65Dn mice (closed bars) or euploid littermates (open bars) was measured by flow cytometry as described in the Materials and methods. The data are expressed as the per cent AnnexinV+ cells or the mean fluorescence intensity (MFI) of bcl-2 staining in each sub-population (n = 4, *P < 0·05 by paired t-test).
To measure whether changes in IL-7Rα were associated with altered cell proliferation in the thymus, mice were injected with BrdU for 2 days and incorporation was assessed ex vivo. Consistent with those populations having decreased IL-7Rα expression, lower percentages of BrdU+ cells were found in the DN2 and DN3 populations (Fig. 3c), and significantly fewer BrdU+ cells were detected in the DN2, DN3 and DN4 populations of Ts65Dn mice in comparison to euploid mice (Fig. 3d), indicating defects in thymocyte proliferation. No significant changes were observed in the more mature DP and SP thymocyte populations (see Supplementary material, Fig. S2a,b). A previous report suggested increased apoptosis in Ts65Dn thymuses in situ10 and analysis of the thymocytes ex vivo by Annexin V staining also indicated increased apoptosis of thymocytes from Ts65Dn mice. Consistent with the role of IL-7Rα, increased apoptosis was only observed in the DN thymocyte populations in the Ts65Dn mice (Fig. 3e), whereas there were no differences in Annexin V staining in mature DP and SP thymocytes (Fig. S2c). One mechanism by which IL-7Rα regulates thymocyte survival, is through induction of the expression of the anti-apoptotic protein Bcl-2.17 However, no significant differences in Bcl2 expression were detected in all the thymocyte populations by intracellular staining (Fig. 3f, Fig. S2d).
Because of the role(s) of IL-7Rα in survival of peripheral T cells, especially CD8+ memory T cells, surface expression of IL-7Rα was also measured in the splenic T-cell subsets. A small decrease in IL-7Rα-positive cells was observed in both CD4+ (see Supplementary material, Fig. S3a) and CD8+ (Fig. S3b) subsets, although the magnitude of decrease was not commensurate with that observed in DN thymocytes. Hence, alterations in IL-7Rα expression appear to be limited to immature lymphocyte progenitors and not the more committed mature cells.
B-lymphocyte function and development in Ts65Dn mice
B-cell proliferative responses were also assessed in the Ts65Dn mice to determine whether immune dysfunction was limited to T cells. Total splenocytes were stimulated with varying concentrations of anti-IgM, anti-IgM in combination with IL-4, and Escherichia coli lipopolysaccharide, a known B-cell activator. Proliferation was then assessed in CD19+ B-cells by flow cytometry using CFSE dilution as in Fig. 2. Compared with cells from euploid control mice, there was a significant decrease in the percentage of Ts65Dn CD19+ cells that had undergone at least one division after 48 hr (Fig. 4a) and 72 hr (Fig. 4b) in response to stimulation by anti-IgM, and anti-IgM in combination with IL-4. In contrast, no significant difference was observed when cells were stimulated with various concentrations of lipopolysaccharide either at 48 hr (Fig. 4c) or 72 hr (Fig. 4d).
Figure 4.
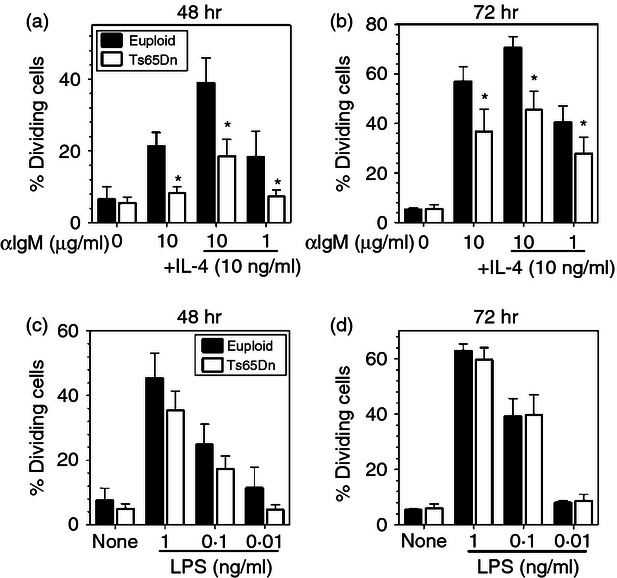
Decreased B-cell proliferation in Ts65Dn mice. CFSE labelled, total splenocytes from Ts65Dn mice (closed bars) and euploid mice (open bars) were stimulated with (a,b) indicated concentrations of anti-IgM in the absence or presence of 10 ng/ml interleukin-4 (IL-4) or (c,d) graded concentrations of lipopolysaccharide (LPS). The data represent the percentage of cells that had undergone at least one division as indicated by CFSE analysis after (a,c) 48 hr or (b,d) 72 hr incubation (n = 4; *P < 0·05 by paired t-test).
To determine whether changes in B-cell development in the Ts65Dn mice reflected the changes in B-cell function, peripheral B-cell subsets were defined by flow cytometry.26 Consistent with decreased proliferation of spleen B cells, there were small but significant decreases in the presence of both follicular (Fol I) and transitional (T1 and T3) B-cell subsets in the splenic B cells from Ts65Dn mice (Fig. 5a). Furthermore, there was an increased percentage of CD19+ cells expressing high levels of both MHC II and CD80, which has been proposed as markers of memory B cells29 (Fig. 5b).
Figure 5.
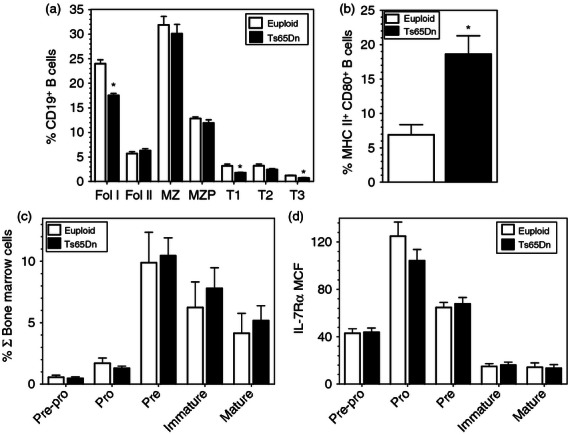
B-cell progenitors and mature subsets in spleen and bone marrow. (a,b) Spleen and (c,d) bone marrow cells from Ts65Dn mice (closed bars) and euploid mice (open bars) were surface stained and analysed by flow cytometry. (a,b) CD19+ B-cell subsets were analysed as described in the Materials and methods and (a) broken down into follicular (Fol I, Fol II), marginal zone subsets (MZ, MZP) and transitional (T1, T2, T3) B-cell subsets. The data are expressed as the percentage of CD19+ cells in the spleen ± SEM. (b) The percentages of CD19+ cells expressing MHC II and CD80 are shown. (c,d) Bone marrow B-cell subsets were defined as described in the Materials and methods. The data are expressed as (c) the percentage of total bone marrow cells or (d) the mean fluorescence intensity (MFI) of interleukin-7 receptor α (IL-7Rα) staining in the indicated subsets (n = 5; *P < 0·05 by paired t-test).
These data suggested alterations in peripheral B cells in Ts65Dn mice, but in contrast, B-cell progenitors in the bone marrow were not changed compared with euploid controls. There were no significant alterations in the percentage of pre-pro, pro-, pre-, immature and mature B-cell populations (Fig. 5c) based upon published cell surface markers.24,25 Furthermore, while B-cell development is also dependent upon IL-7 in the mouse,30 there were no differences in IL-7Rα expression in the bone marrow B-cell subsets (Fig. 5d).
Potential regulators of IL-7Rα expression
Down-regulation of IL-7Rα protein expression in the thymus was, at least in part, transcriptional because quantitative PCR analysis of total thymocytes indicated a nearly twofold decrease in IL-7Rα mRNA levels (Fig. 6a). Another potential mechanism for decreased IL-7Rα expression could be a result of the ‘altruistic’ down-regulation of the receptor by increased concentrations of the ligand IL-7 produced by thymic stromal cells.31 However, there was no increase in IL-7 mRNA expression in total thymus from Ts65Dn mice compared with euploid controls (Fig. 6b).
Figure 6.
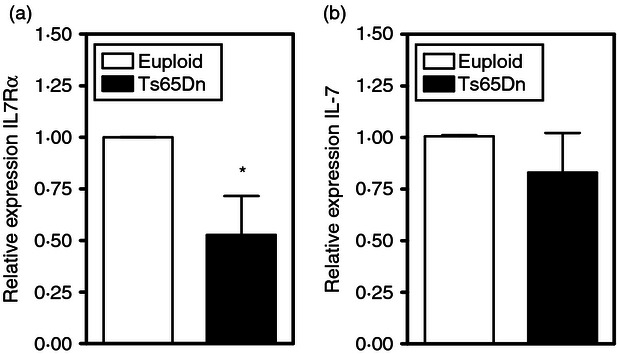
Interleukin-7 (IL-7)/IL-7Rα gene expression in Ts65Dn thymuses. IL-7Rα (a) and IL-7 (b) mRNA expression was assessed in total thymus by quantitative PCR in Ts65Dn (closed bar) and euploid controls (open bar). These data are presented as the expression of the indicated mRNA relative to control HPRT mRNA. (n = 5, *P < 0·05 by paired t-test).
Previous data have suggested that increased oxidative stress, potentially linked to decreased reduced glutathione levels, induced a loss of IL-7Rα expression in bone marrow haematopoietic progenitors.6 Consistent with this observation, reduced glutathione, measured with MCB, was significantly decreased in immature, DN Ts65Dn thymocytes, but not in the total thymocytes, in comparison to euploid controls (Fig. 7a). In addition, consistent with previous observations in haematopoietic stem cells and bone marrow lymphoid progenitors,6 DN thymocytes exhibited enhanced oxidation of the redox-sensitive dye DCFDA (Fig. 7b), whereas there was little increase in DP thymocytes and no significant increase in DCFDA oxidation in splenic T cells (not shown). Hence, increases in oxidative stress may be linked to decreased IL-7Rα expression and function in the thymus as well.
Figure 7.
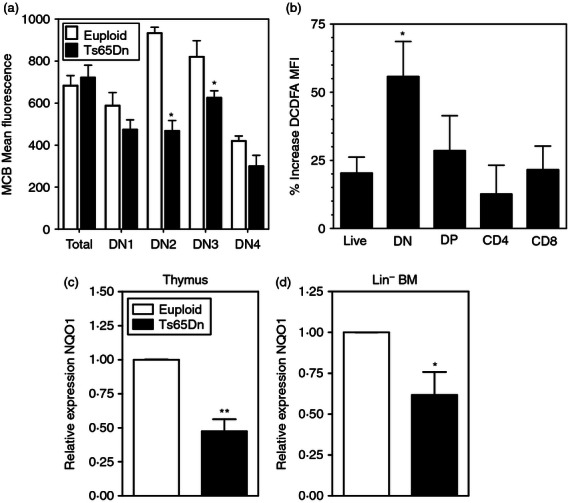
Increased oxidative stress in Ts65Dn haematopoietic progenitors. (a) Intracellular glutathione levels were measured in the indicated thymocyte sub-populations by flow cytometry using monochlorobimane (MCB) as described in the Materials and methods in euploid (open bars) or Ts65Dn (closed bars) mice (n = 4,*P < 0·05 by paired t-test). (b) DCFDA oxidation in the indicated thymic subsets was measured as described in the Materials and methods in cells from euploid (open bars) or Ts65Dn (closed bars) mice. The data are expressed as the per cent increased DCFDA fluorescence in Ts65Dn cells over euploid cells ± SEM (n = 4,*P < 0·05 by paired t-test). (c) NQO1 mRNA expression was assessed in whole thymus (c) and Lin− bone marrow (d) by quantitative PCR. These data are presented as the expression of the indicated mRNA relative to control HPRT mRNA (n = 5, *P < 0·05, **P < 0·01 by paired t-test).
One triplicated gene in DS potentially linked to oxidative stress is BACH1, and increased levels of BACH1 have been described in tissues from individuals with DS.32 BACH1, reported to be well expressed in thymus,33 inhibits Nrf2-mediated induction of antioxidant gene expression through antioxidant response elements (ARE). NAD(P)H:quinone oxidoreductase1 (NQO1) is an antioxidant flavoprotein that is a known target and established marker of Nrf-2 activation.34 NQO1 expression was decreased twofold in Ts65Dn thymuses (Fig. 7c) and Lin− bone marrow (Fig. 7d) in comparison with euploid controls. Deficient NQO1 induction is consistent with decreased Nrf2-mediated antioxidant response induction in Ts65Dn thymocytes and haematopoietic progenitors, which may cause increased oxidative stress and contribute to haematopoietic progenitor and thymic dysfunction.
It is unclear whether oxidative stress affects IL-7Rα transcription, but inhibition of the Notch signalling pathway was shown to down-regulate IL-7Rα expression in T-cell lineage, but not B-cell progenitors.20 Furthermore, a recent report suggested that decreased Nrf2 activation led to diminished Notch expression and signalling.35 To determine whether Notch activation was affected in Ts65Dn thymocytes, expression of the Notch target gene Hes-1 was measured in total thymus by quantitative PCR. Expression of Hes-1 was decreased 25% compared with euploid controls (Fig. 8a). Similar changes were also observed in Lin− bone marrow cells (Fig. 8b). As an additional potential mechanism to down-regulate IL-7Rα levels, changes in miRNA expression levels were measured in Ts65Dn mice. Tissue samples from individuals with Down syndrome have increased expression of miRNAs encoded by the triplicated chromosome36 and sequence analysis in the Ts65Dn mice indicated that the same miRNAs (miR-155, miR-125b, let-7c, miR-802 and miR-99a) are also encoded by the triplicated portion of MMU-16. Both miR-155 and miR-125b are known to be expressed in haematopoietic cells,37 and analysis of the 3′-untranslated region of the IL-7Rα gene using targetscan,38 indicated that it contains consensus recognition sites for both miR-155 and miR-125b. Furthermore, B cells from transgenic mice over-expressing miR-155 had down-regulated IL-7Rα mRNA levels.39 A significant increase in both miR-125b and miR-155 was observed in total thymocytes, as well as in immature, DN thymocytes from Ts65Dn mice (Fig. 8c). Expression of miR-125b and miR-155 was also analysed in the bone marrow. The miR-155 expression was increased in both lineage-negative and total bone marrow samples in Ts65Dn mice in comparison to euploid mice, whereas miR-125b expression was increased only in lineage-negative cells and not total bone marrow (Fig. 8d). Hence, decreased Notch activation and increases in miRNA may also contribute to the decreased levels of IL-7Rα expression in haematopoietic progenitors in the thymus and bone marrow.
Figure 8.
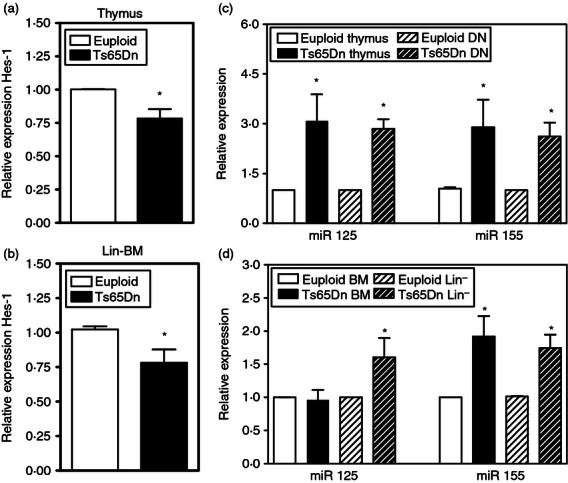
Notch activation and microRNA (miRNA) expression in Ts65Dn thymuses and bone marrow (BM). (a,b) Hes-1 mRNA expression was assessed in (a) total thymus or (b) lineage negative (Lin−) BM cells by quantitative PCR in Ts65Dn (closed bar) and euploid controls (open bar). These data are expressed as the normalized expression of the indicated mRNA relative to control HPRT mRNA (n = 5, *P < 0·05 by paired t-test). (c,d) miR125 and miR155 expression were assessed in (c) total thymus and double-negative (DN) thymocytes or (d) Lin− and total BM cells by quantitative PCR as described in the Materials and methods. These data are presented as the normalized expression of the indicated miRNA relative to control u6 rRNA (n = 5,*P < 0·05 by paired t-test).
Discussion
Although deficient immune responses and premature aging of the adaptive immune system has been reported for many years in DS, there is still controversy whether DS represents a model of immunosenescence or exhibits inherent immunodeficiency. Furthermore, underlying mechanisms that may affect lymphoid development and function have not been examined in depth. Older literature proposed changes in samples from individuals with DS, including altered thymic architecture and expression of adhesion molecules and inflammatory cytokines,11,40 whereas recent reports have focused upon defects in thymic gene expression41 and thymic emigrants in human DS.13,14 Using the Ts65Dn mouse model to further define the changes in T-cell lineage development in DS, the data suggest that decreases in IL-7Rα expression in immature lymphoid cells lead to impaired thymic development. These data are consistent with previous observations in bone marrow progenitors,12 and suggest a potential mechanism for immune alterations in DS that lead to a premature aging phenotype and senescence of peripheral lymphocytes.
Similar to data in humans12 and mice,10 the Ts65Dn thymus was significantly smaller and hypocellular. This hypocellularity seemed to be a result of selective loss of the immature DN populations because the relative proportions of the more mature DP and CD4 SP and CD8 SP thymocytes was not different in Ts65Dn mice. The loss of DN thymocytes was accompanied by a decrease in the proportion and absolute number of cells expressing IL-7Rα in the lineage negative and DN populations. This was also associated with decreased proliferation and increased apoptosis of the immature DN2 and DN3 populations. Interleukin-7 signalling has been shown to be essential for DN thymocyte proliferation and survival,18 and previous studies have shown that lack of IL-7 or IL-7Rα results in an overall decrease in thymic cellularity.17,42 Therefore, diminished IL-7Rα expression and/or IL-7 signalling may be causing proliferative and survival defects in the DN thymocyte populations and contributing to Ts65Dn thymic hypocellularity. The loss of IL-7Rα expression, however, was selective for T-cell progenitors rather than cells committed to the T-cell lineage. Cells that had already undergone β-selection had similar cell surface expression levels of IL-7Rα comparing Ts65Dn with euploid controls. This is also reflected in the periphery, where there were small decreases in IL-7Rα expression in the spleens of Ts65Dn mice. The IL-7 signalling pathway plays an essential role in peripheral T-cell homeostasis43,44 as well as the generation and maintenance of memory T cells.45 Previous reports indicated increased plasma IL-7 in individuals with DS,13 but although assay sensitivity precluded measuring IL-7 protein in Ts65Dn mice, IL-7 mRNA levels were not changed. Therefore, the modest changes in IL-7Rα in the periphery may result in the observed changes in naive and central memory T cells. It is unclear why there is decreased IL-7Rα expression selectively in immature lymphoid progenitors, but the current results have identified potential regulators of IL-7Rα expression.
One potential mechanism for regulation of IL-7Rα expression may be increases in oxidative stress. Previous data suggested that exposure of IL-7Rα+ cells to pro-oxidants in vitro decreased the percentage of IL-7Rα+ cells.6 Existing10,41 and current data suggest the presence of increased oxidative stress in Ts65Dn thymus, and the results suggest that decreased antioxidant defences, including glutathione and antioxidant enzymes, promote pro-oxidant conditions in Ts65Dn mice. Inefficient induction of antioxidant enzyme defences may also contribute to increased oxidative stress in Ts65Dn thymus. Decreased NQO1 expression reflects diminished signalling through Nrf2-antioxidant response element-dependent gene expression.34 Nrf2-antioxidant response element-induced expression of cytoprotective enzymes is a major mechanism for cellular defence against xenobiotics and oxidative stress. A possible mechanism for decreased NQO1 expression is the triplication of BACH1 on mouse chromosome 16 in the Ts65Dn mouse. Over-expression of BACH1 has been found in tissue from individuals with DS32 and is known to inhibit the Nrf-2-mediated induction of NQO1 expression.46 Conversely, BACH1-deficient mice show greatly enhanced expression of the Nrf2 target gene, haeme oxygenase-1 in the thymus.33 A recent study of human DS thymus also identified decreased expression of another Nrf2 target, peroxiredoxin 2 and decreased levels of this antioxidant enzyme may also promote increased oxidative stress in DS thymocytes.41 Insufficient antioxidant production in the Ts65Dn haematopoietic and lymphoid progenitor populations in the bone marrow and thymus may therefore be inducing a state of redox imbalance and affecting progenitor function, potentially through regulation of IL-7Rα levels.
Direct transcriptional regulation of IL-7Rα expression in Ts65Dn was implicated by the nearly twofold decrease in mRNA in total thymus. Notch signalling has been shown to regulate IL-7Rα expression in developing T cells but not B cells,20 and a small decrease in expression of the Notch signalling target Hes-1 was observed in whole thymuses and lineage-negative haematopoietic progenitors of Ts65Dn mice. Notch-mediated transcription could be down-regulated in Ts65Dn through decreased Nrf2-dependent control of Notch expression,35 in which down-regulation of Nrf2 function was shown to result in decreased Hes-1 expression. Hence, decreased Nrf2 activation in the Ts65Dn lymphocyte progenitors might be associated with inhibition of Notch-dependent IL-7Rα expression. Another possible mechanism of decreased IL-7Rα-expression is the increased expression of miRNAs that can potentially inhibit IL-7Rα mRNA expression. Mouse chromosome 16 and human chromosome 21 are known to encode five miRNA, including miR-99a, let-7c, miR-125b-2, miR-155 and miR-802 and previous studies found increased levels of miR-155 and miR-125b in tissues from individuals with DS.36 Sequence analysis indicated consensus binding sites for these miRs in the 3′-untranslated region of IL-7Rα transcripts and PCR analysis found increased expression of miR-125b and miR-155 in the thymus and bone marrow. This analysis is supported by the findings that transgenic mice over-expressing miR-155 in B cells exhibited decreased IL-7Rα mRNA expression.39 Hence, regulation of IL-7Rα expression by transcriptional activators and miRNA may contribute to changes in thymocyte function in DS and Ts65Dn mice.
In contrast to thymic progenitors, there were only minor differences in cellularity and subset composition of splenic leucocytes in Ts65Dn mice compared with euploid controls although further analysis of the CD4+ and CD8+ T-cell populations revealed an overall decrease in the percentage of naive cells and an increase in the effector/memory populations. Combined with the thymic involution, this increased proportion of memory cells suggests an aged, senescent immune system. Consistent with this hypothesis, a decreased percentage of recent thymic emigrants was observed in children with DS, and is postulated to contribute to the apparent immunosenescence of the T-lymphocyte population in the DS immune system.14 As a result of the decrease in recent thymic emigrants, it has been suggested that peripheral T cells in individuals with DS undergo increased homeostatic proliferation in comparison to the general population.14 Because of mixed genetic background in Ts65Dn mice, differences in recent thymic emigrants cannot be reliably measured. Nevertheless, the data are consistent with a loss of thymic precursors in the Ts65Dn mice leading to altered peripheral T-cell populations.
Defects in Ts65Dn peripheral T-cell function are most evident in the decreased proliferation in response to polyclonal stimulation. This loss of function may be consistent with immune dysfunction in DS, as lymphocytes from individuals with DS have also been shown to exhibit a decreased proliferative response to polyclonal stimuli such as phytohaemagglutinin,47,48 in addition to the documented decrease in responses in some individuals with DS to vaccinations.49,50 Vaccine studies have shown that IL-7 and TCR signalling can synergize to promote antigen-specific effector cell generation, especially when using subdominant antigens.51 Therefore decreased IL-7Rα expression as well as the deficient proliferation in response to TCR stimulation may contribute to the T-cell dysfunction observed in DS. It is tempting to speculate that the impaired proliferation in the immature thymocyte subsets as a consequence of decreased IL-7Rα expression may be one of the causes of accelerated thymic involution as well as decreased thymic output in DS. In turn, the increased, possibly excessive, homeostatic cycling of peripheral T cells in individuals with DS may result in premature senescence and impaired function.
The changes in lymphocyte responses were not limited to T cells as B-cell proliferation was also diminished in response to antigen receptor stimulation, but not lipopolysaccharide. This is consistent with an anergic/senescent phenotype in the peripheral lymphocyte pools. However, in contrast to thymic development, B-cell progenitors in the bone marrow and IL-7Rα expression on those cells were not altered in the Ts65Dn mice, suggesting a selective effect on T-lymphocyte precursors. It is interesting, but unclear, why the previously reported decrease in CLP in Ts65Dn bone marrow6 only results in diminished T-cell progenitors. One postulate is that decreases in Notch signalling, due to BACH1-mediated inhibition of Nrf2 or increased DYRK1a52 in DS, leads to impaired T-cell specification, but not B-cell development. The resultant changes in mature B-cell function and spleen subsets may be, as has been proposed previously,53 due to altered T-cell help. The data also suggest that oxidative stress may also be selective in nature because increased DCFDA oxidation and decreased reduced glutathione were limited to the DN thymocyte populations and the more mature thymocytes and peripheral T cells did not show these alterations. Hence, the defects in immunological development in the Ts65Dn mice seem to be limited to immature haematopoietic progenitors, particularly T-lineage precursors, although the mechanisms and potential biochemical effects in DS remain to be tested.
Hence, these data demonstrate significant defects in immature and mature T-lymphocyte populations of Ts65Dn mice, with changes in both the composition and function of the cells of the thymus and spleen. The data suggest that decreased IL-7Rα expression may underlie this dysfunction, causing decreased proliferation and function. Taken together with the haematopoietic stem and progenitor defects in previous studies,6 the data indicate an overall dysfunction of adaptive immune system development in Ts65Dn mice.
Acknowledgments
The authors wish to thank Ian M. Kaplan for helpful discussions and Regina Harley for expert assistance in cell sorting. This work was supported by funding from the US Public Health Service (AI070823) (MSW) and the LeJeune Foundation (PJY).
Glossary
- ARE
antioxidant response element
- DS
Down syndrome
- ETP
early thymic progenitor
- MCB
monochlorobimane
- NQO1
NAD(P)H:quinone oxidoreductase1
- RTE
recent thymic emigrants
Disclosure
The authors declare that they have no competing interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Mature thymocytes and interleukin-7 receptor α (IL-7Rα) expression are not altered in Ts65Dn mice.
Figure S2. Proliferation and apoptosis not affected in Ts65Dn mature thymocytes.
Figure S3. Interleukin-7 receptor α (IL-7Rα) expression in peripheral T-cell populations is not markedly altered in Ts65Dn mice.
References
- 1.Kusters MA, Verstegen RH, Gemen EF, de Vries E. Intrinsic defect of the immune system in children with Down syndrome: a review. Clin Exp Immunol. 2009;156:189–93. doi: 10.1111/j.1365-2249.2009.03890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nespoli L, Burgio GR, Ugazio AG, Maccario R. Immunological features of Down's syndrome: a review. J Intellect Disabil Res. 1993;37((Pt 6)):543–51. doi: 10.1111/j.1365-2788.1993.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 3.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359:1019–25. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- 4.Spangrude GJ, Scollay R. Differentiation of hematopoietic stem cells in irradiated mouse thymic lobes. Kinetics and phenotype of progeny. J Immunol. 1990;145:3661–8. [PubMed] [Google Scholar]
- 5.Donskoy E, Foss D, Goldschneider I. Gated importation of prothymocytes by adult mouse thymus is coordinated with their periodic mobilization from bone marrow. J Immunol. 2003;171:3568–75. doi: 10.4049/jimmunol.171.7.3568. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzo LP, Chen H, Shatynski KE, Clark S, Yuan R, Harrison DE, Yarowsky PJ, Williams MS. Defective hematopoietic stem cell and lymphoid progenitor development in the Ts65Dn mouse model of Down syndrome: potential role of oxidative stress. Antioxid Redox Signal. 2011;15:2083–94. doi: 10.1089/ars.2010.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirsammer G, Jilani S, Liu H, Davis E, Gurbuxani S, Le Beau MM, Crispino JD. Highly penetrant myeloproliferative disease in the Ts65Dn mouse model of Down syndrome. Blood. 2008;111:767–75. doi: 10.1182/blood-2007-04-085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serwold T, Ehrlich LI, Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2009;113:807–15. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sitnicka E, Buza-Vidas N, Ahlenius H, et al. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood. 2007;110:2955–64. doi: 10.1182/blood-2006-10-054726. [DOI] [PubMed] [Google Scholar]
- 10.Paz-Miguel JE, Flores R, Sanchez-Velasco P, Ocejo-Vinyals G, Escribano de Diego J, Lopez de Rego J, Leyva-Cobian F. Reactive oxygen intermediates during programmed cell death induced in the thymus of the Ts(1716)65Dn mouse, a murine model for human Down's syndrome. J Immunol. 1999;163:5399–410. [PubMed] [Google Scholar]
- 11.Murphy M, Friend DS, Pike-Nobile L, Epstein LB. Tumor necrosis factor-α and IFN-γ expression in human thymus. Localization and overexpression in Down syndrome (trisomy 21) J Immunol. 1992;149:2506–12. [PubMed] [Google Scholar]
- 12.Larocca LM, Lauriola L, Ranelletti FO, Piantelli M, Maggiano N, Ricci R, Capelli A. Morphological and immunohistochemical study of Down syndrome thymus. Am J Med Genet Suppl. 1990;7:225–30. doi: 10.1002/ajmg.1320370745. [DOI] [PubMed] [Google Scholar]
- 13.Roat E, Prada N, Lugli E, et al. Homeostatic cytokines and expansion of regulatory T cells accompany thymic impairment in children with Down syndrome. Rejuvenation Res. 2008;11:573–83. doi: 10.1089/rej.2007.0648. [DOI] [PubMed] [Google Scholar]
- 14.Bloemers BL, Bont L, de Weger RA, Otto SA, Borghans JA, Tesselaar K. Decreased thymic output accounts for decreased naive T cell numbers in children with Down syndrome. J Immunol. 2011;186:4500–7. doi: 10.4049/jimmunol.1001700. [DOI] [PubMed] [Google Scholar]
- 15.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 16.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–54. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 17.von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–54. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 18.Peschon JJ, Morrissey PJ, Grabstein KH, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–60. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–32. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Garcia S, Garcia-Peydro M, Martin-Gayo E, et al. CSL-MAML-dependent Notch1 signaling controls T lineage-specific IL-7Rα gene expression in early human thymopoiesis and leukemia. J Exp Med. 2009;206:779–91. doi: 10.1084/jem.20081922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allman D, Sambandam A, Kim S, Miller JP, Pagan A, Well D, Meraz A, Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–74. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 22.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3– CD4– CD8– triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–52. [PubMed] [Google Scholar]
- 23.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 24.Matthias P, Rolink AG. Transcriptional networks in developing and mature B cells. Nat Rev Immunol. 2005;5:497–508. doi: 10.1038/nri1633. [DOI] [PubMed] [Google Scholar]
- 25.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6:107–16. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 26.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–57. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–ΔΔ C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Barrena MJ, Echaniz P, Garcia-Serrano C, Cuadrado E. Imbalance of the CD4+ subpopulations expressing CD45RA and CD29 antigens in the peripheral blood of adults and children with Down syndrome. Scand J Immunol. 1993;38:323–6. doi: 10.1111/j.1365-3083.1993.tb01733.x. [DOI] [PubMed] [Google Scholar]
- 29.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–14. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Namen AE, Lupton S, Hjerrild K, et al. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–3. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 31.Munitic I, Williams JA, Yang Y, Dong B, Lucas PJ, El Kassar N, Gress RE, Ashwell JD. Dynamic regulation of IL-7 receptor expression is required for normal thymopoiesis. Blood. 2004;104:4165–72. doi: 10.1182/blood-2004-06-2484. [DOI] [PubMed] [Google Scholar]
- 32.Ferrando-Miguel R, Cheon MS, Yang JW, Lubec G. Overexpression of transcription factor BACH1 in fetal Down syndrome brain. J Neural Transm Suppl. 2003;67:193–205. doi: 10.1007/978-3-7091-6721-2_17. [DOI] [PubMed] [Google Scholar]
- 33.Sun J, Hoshino H, Takaku K, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–24. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci USA. 1996;93:14960–5. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakabayashi N, Shin S, Slocum SL, et al. Regulation of notch1 signaling by nrf2: implications for tissue regeneration. Sci Signal. 2010;3:ra52. doi: 10.1126/scisignal.2000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn DE, Nuovo GJ, Terry AV, Jr, et al. Chromosome 21-derived microRNAs provide an etiological basis for aberrant protein expression in human Down syndrome brains. J Biol Chem. 2010;285:1529–43. doi: 10.1074/jbc.M109.033407. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Baltimore D, Boldin MP, O'Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008;9:839–45. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- 38.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 39.Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, Croce CM. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in Eµ-miR155 transgenic mice. Proc Natl Acad Sci USA. 2006;103:7024–9. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy M, Epstein LB. Down syndrome (DS) peripheral blood contains phenotypically mature CD3+ TCRαβ+ cells but abnormal proportions of TCR αβ+, TCR γδ+, and CD4+ CD45RA+ cells: evidence for an inefficient release of mature T cells by the DS thymus. Clin Immunol Immunopathol. 1992;62:245–51. doi: 10.1016/0090-1229(92)90079-4. [DOI] [PubMed] [Google Scholar]
- 41.Lima FA, Moreira-Filho CA, Ramos PL, et al. Decreased AIRE expression and global thymic hypofunction in Down syndrome. J Immunol. 2011;187:3422–30. doi: 10.4049/jimmunol.1003053. [DOI] [PubMed] [Google Scholar]
- 42.Grabstein KH, Waldschmidt TJ, Finkelman FD, Hess BW, Alpert AR, Boiani NE, Namen AE, Morrissey PJ. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993;178:257–64. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vella A, Teague TK, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J Exp Med. 1997;186:325–30. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–76. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 45.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 46.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 47.Burgio GR, Ugazio AG, Nespoli L, Marcioni AF, Bottelli AM, Pasquali F. Derangements of immunoglobulin levels, phytohemagglutinin responsiveness and T and B cell markers in Down's syndrome at different ages. Eur J Immunol. 1975;5:600–3. doi: 10.1002/eji.1830050904. [DOI] [PubMed] [Google Scholar]
- 48.Rigas DA, Elsasser P, Hecht F. Impaired in vitro response of circulating lymphocytes to phytohemagglutinin in Down's syndrome: dose– and time–response curves and relation to cellular immunity. Int Arch Allergy Appl Immunol. 1970;39:587–608. doi: 10.1159/000230384. [DOI] [PubMed] [Google Scholar]
- 49.Costa-Carvalho BT, Martinez RM, Dias AT, et al. Antibody response to pneumococcal capsular polysaccharide vaccine in Down syndrome patients. Braz J Med Biol Res. 2006;39:1587–92. doi: 10.1590/s0100-879x2006001200010. [DOI] [PubMed] [Google Scholar]
- 50.Epstein LB, Philip R. Abnormalities of the immune response to influenza antigen in Down syndrome (trisomy 21) Prog Clin Biol Res. 1987;246:163–82. [PubMed] [Google Scholar]
- 51.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–87. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Martinez J, Vela EM, Tora-Ponsioen M, Ocana OH, Nieto MA, Galceran J. Attenuation of Notch signalling by the Down-syndrome-associated kinase DYRK1A. J Cell Sci. 2009;122:1574–83. doi: 10.1242/jcs.044354. [DOI] [PubMed] [Google Scholar]
- 53.Verstegen RH, Kusters MA, Gemen EF, de Vries E. Down syndrome B-lymphocyte subpopulations, intrinsic defect or decreased T-lymphocyte help. Pediatr Res. 2010;67:563–9. doi: 10.1203/PDR.0b013e3181d4ecc1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


