Abstract
Microbial infections are a major cause of infant mortality worldwide because of impaired immune defences in this population. The nature of this work was to further understand the mechanistic limitations of the neonatal and infant immune response. Interleukin-27 (IL-27) is a heterodimeric cytokine of the IL-12 family that is produced primarily by antigen-presenting cells and is immunosuppressive toward a variety of immune cell types. We show that IL-27 gene expression is elevated in cord blood-derived macrophages relative to macrophages originating from healthy adults. We also evaluated the duration over which elevated IL-27 gene expression may impact immune responses in mice. Age-dependent analysis of IL-27 gene expression indicated that levels of IL-27 remained significantly elevated throughout infancy and then declined in adult mice. Flow cytometric analysis of intracellular cytokine-stained splenocytes further confirmed these results. Interleukin-27 may be induced during pregnancy to contribute to the immunosuppressive environment at the fetal–maternal interface because we demonstrate dose-responsive gene expression to progesterone in macrophages. Neutralization of IL-27 in neonatal macrophages improved the ability of these cells to limit bacterial replication. Moreover, neutralization of IL-27 during incubation with the Mycobacterium bovis bacillus Calmette–Guérin vaccine augmented the level of interferon-γ elicited from allogeneic CD4+ T lymphocytes. This suggests that blocking IL-27 during vaccination and infection may improve immune responses in newborn and infant populations. Furthermore, mice will be a suitable model system to further address these possibilities.
Keywords: interferon-γ, interleukin-27, macrophages, neonate, T cells
Introduction
Microbial infections remain a major cause of mortality in early life, accounting for millions of infant deaths each year.1 This is further complicated by impaired humoral and cell-mediated adaptive responses to vaccines compared with adults.2 Impaired immunity in infants may be a remnant of the uterine environment where immunity is suppressed to promote successful pregnancy, the product of an immature immune system, or a combination of both. New studies that compare primary cells from infants and adults with a focus on individual cell types and specific mechanisms are needed to further dissect immune defects. This may lead to the development of targeted interventions to improve defence against microbial invasion and augment immune function.
Discrepancies in cytokine production between adult and neonatal immune cells have been widely reported and are generally biased toward T helper type 2 (Th2) responses. Tumour necrosis factor-α production in response to a subset of toll-like receptor ligands was substantially reduced in neonatal cord blood monocytes compared with cells taken from adults.3 Neonatal innate cells produced significantly less interferon (IFN-α), interleukin-12 (IL-12), and IFN-γ, but more IL-23, IL-6, IL-1β and IL-10 than their adult counterparts in response to an array of toll-like receptor ligands.4 Consistent with this study and with low levels of inflammatory cytokines, cord blood mononuclear cells produce enhanced levels of IL-10 compared with adult peripheral blood mononuclear cells in response to lipopolysaccharide.5
Induction of Th1 responses is also impaired in neonates.6 This notion is reinforced by studies showing that newborns mount lower Th1 responses than adults to commonly administered viral vaccines while producing elevated levels of Th2 cytokines.7–9 Similar outcomes are extensively reported in response to allogeneic stimulation during mixed leucocyte reactions.10–13 However, adult antigen-presenting cells are able to promote Th1 responses from cord blood lymphocytes.10 This suggests that the deficiency is not completely intrinsic to neonatal lymphocytes; the neonatal antigen-presenting cells probably play a significant role in influencing the nature of the T-lymphocyte response. While deficiency in IL-12 production by cord blood mononuclear cells may contribute, other factors may also be important.
Interleukin-27 is a heterodimeric cytokine of the IL-12 family that consists of the Epstein–Barr virus-induced gene 3 (EBI3) and IL-27p28 proteins.14 The predominant producers of IL-27 are antigen-presenting cells. Interleukin-27 signals through a receptor that is a heterodimer of WSX-1 and gp130 expressed on a number of cells of immunological nature.15 The IL-27 is unique in that it can both activate and suppress immune responses. It was originally described as a pro-inflammatory cytokine that promotes the differentiation of Th1 cells and production of IFN-γ.16,17 However, WSX-1-deficient animals mount Th1 responses, suggesting that IL-27 is compensated for in this regard.18–21 Since that time, the anti-inflammatory nature of IL-27 has become more widely documented. Interleukin-27 inhibits Th1, Th2 and Th17 lymphocytes in both mice and humans.19,22–24 It is also involved with the generation of anti-inflammatory T cells (Tr1) that produce large amounts of the immune suppressive cytokine IL-10.25 In addition, anti-inflammatory activity of IL-27 toward murine and human macrophages has been shown.21,26–28 Interleukin-27 limits inflammatory signalling and cytokine production with consequences of reducing control of bacterial growth.26,28 Increased WSX-1 expression has been associated with immunosuppressive macrophage phenotypes.29
Given the immunosuppressive nature of IL-27, we explored a potential involvement of this cytokine in neonatal immune responses. Human cord blood-derived macrophages express IL-27 at elevated levels compared with adult counterparts. In mice, this elevated expression is maintained through infancy. In vitro neutralization of IL-27 in neonatal macrophages improved control of bacteria and augmented the level of IFN-γ elicited from CD4+ T lymphocytes. This suggests that blocking IL-27 during vaccination and infection may improve immune responses in newborn and infant populations. Furthermore, the results here also demonstrate that age-dependent mouse studies will be a useful model to further evaluate the immunobiology of IL-27 in paediatric populations.
Materials and methods
Mycobacterium bovis BCG culture
Mycobacterium bovis bacillus Calmette–Guérin (BCG) was purchased from the American Type Culture Collection (ATCC, Manassas, VA). The bacteria were maintained in Middlebrook 7H9 broth supplemented with ADC enrichment media (Albumin, Dextrose, Catalase) at 37° with 5% CO2. For infections, mycobacteria were pelleted, diluted in Dulbecco's modified Eagle's medium (DMEM), and passed through a 27-gauge needle to disrupt clumped bacteria.
Cell culture
Human umbilical cord blood was obtained under Institutional Review Board approval from the Department of Obstetrics and Gynecology at the University of South Carolina and Palmetto Health Richland Hospital or Magee Women's Hospital in Pittsburgh, PA. All donations were from healthy infants of gestational age ≥ 37 weeks. Adult peripheral blood mononuclear cells were isolated from human buffy coats purchased from the New York Blood Center (New York, NY). Eligible donors were 16 years of age or older, at least 50 kg, and in good physical health. All blood donors were anonymous and de-identified. The cord blood was centrifuged at 1500 g for 15 min to isolate the buffy coat. Adult or cord blood-derived buffy coats were subjected to Ficoll (GE Healthcare, Waukesha, WI) density gradient centrifugation to isolate peripheral blood mononuclear cells. Monocytes were subsequently isolated from mononuclear cells by OptiPrep (Axis-Shield, Dundee, Scotland) density gradient centrifugation as described previously.26 Monocytes were allowed to adhere to plastic culture dishes for 1 hr in serum-free DMEM supplemented with 2 mm glutamine and 25 mm HEPES. Non-adherent cells were removed and the medium was replaced with DMEM supplemented with 2 mm glutamine, 25 mm HEPES, 20% fetal calf serum and 10% human serum. For experiments, the medium was replaced with DMEM supplemented with 2 mm glutamine, 25 mm HEPES and 1% human serum.
Macrophage infection and enumeration of BCG
Human macrophages cultivated in 96-well dishes (5 × 104/well) were treated with medium alone or soluble receptor to neutralize IL-27 (sIL-27R, 10 μg/ml) and infected with BCG (∼ multiplicity of infection 1). The sIL-27R was purchased from R&D Systems (Minneapolis, MN) and contains amino acids 34–516 of the IL-27Ra (WSX-1) chain fused through a linker domain with amino acids 100–330 of human IgG1 (50% neutralization dose = 1–4 μg/ml). Infected cultures were incubated for 5 days at 37° with 5% CO2. Culture supernatants were removed and macrophages were permeabilized with 0·05% saponin to release bacteria. Ten-fold serial dilutions were plated on Middlebrook 7H10 agar supplemented with OADC (Oleic Acid, Albumin, Dextrose, Catalase) enrichment and incubated 15 days at 37° with 5% CO2.
Age-dependent mouse experiments
Breeding colonies of C57BL/6 mice were maintained at the University of South Carolina Center for Colon Cancer Research Mouse Experimentation Core Facility. Male and female mice were used for experiments. Consistent with previous literature, mice in this study are defined as neonates until 8 days of life, infants up to 2 weeks, and are adults at 6 weeks.30 Animals were identified at the appropriate age (n = 6 for gene expression analysis, n = 4 for cytokine staining) for the study and killed using humane procedures to harvest the spleen. All procedures were carried out with the approval of the University of South Carolina's Institutional Animal Care and Use Committee. The spleen was placed in RNA stabilization solution (RNAlater™, QIAGEN, Germantown, MD) and stored at −80°.
RNA isolation and quantitative PCR
Human macrophages or mouse spleens were lysed or homogenized in PureZOL™ (Bio-Rad, Hercules, CA). RNA was isolated according to commercial product protocol. First-strand cDNA synthesis was performed using iScript (Bio-Rad) cDNA synthesis reagents according to protocol. Real-time cycling of reactions that included cDNA diluted 20-fold, gene-specific primer-probe sets (Life Technologies, Carlsbad, CA), and iQ™ Supermix (Bio-Rad) was performed in duplicate using an iQ5™ cycler (Bio-Rad). WSX-1 expression assays were performed with unlabelled forward and reverse primers described previously in a reaction similar to above with SsoFast™ EvaGreen® Supermix (Bio-Rad).31 Glyceraldehyde phosphate dehydrogenase (GAPDH) and β-actin were used as internal reference genes for human and mouse assays, respectively. For IL-27 and WSX-1 comparison between human neonates and adults, all donors were normalized relative to the lowest expresser across each age group for p28 or EBI3. For mouse gene expression, all age groups were normalized to the mean data obtained from mice at 4 days.
Immunolabelling and flow cytometry
To label murine splenocytes, single-cell suspensions were prepared by crushing the spleens in mesh baskets (40-µm pore size). The splenocytes were collected with PBS and pelleted by centrifugation at 400 g for 5 min. The cell pellet was suspended in 5 ml ACK red blood cell lysis buffer (Quality Biologicals, Gaithersburg, MD) and incubated at room temperature for 5 min. The splenocytes were subsequently washed with PBS, pelleted and suspended in serum-free DMEM. The splenocytes were enriched for loosely adherent cells by panning in 24-well plates for 4 hr with serum-free DMEM that contained monensin (10 μm). Splenocytes were harvested, washed with PBS, and suspended in flow stain buffer (PBS supplemented with 0·5% BSA and 0·1% sodium azide). All subsequent steps were performed at room temperature. Splenocytes were mixed for 15 min with unlabelled mouse IgG (0·5 μg/ml) to block Fc receptors and then surface-labelled with 1 μg rat anti-mouse F4/80 conjugated with phycoerythrin (clone BM8; eBioscience, San Diego, CA) or rat IgG2a isotype control in a volume of 100 μl for 1 hr with gentle rocking. Unbound antibody was removed by washing with flow stain buffer. Splenocytes were then fixed with 4% PBS-buffered paraformaldehyde for 20 min. Following a wash step, the cells were perforated with permeabilization buffer (flow stain buffer that contained 0·5% saponin) for 10 min. Splenocytes were then labelled with 1 μg rat anti-mouse IL-27 conjugated with fluorescein (clone 234205; R&D Systems) or IgG2b isotype control in a volume of 100 μl for 1 hr with gentle rocking. Unbound antibody was removed by washing with permeabilization buffer. Splenocytes were again fixed with 4% paraformaldehyde. Cells were collected with a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). A minimum of 15 000 gated events were collected for each sample group. To label human macrophages, cells cultured as described above were removed from culture dishes with PBS that contained 5 mm EDTA and 17 mm lidocaine. The cells were washed with PBS and pelleted by centrifugation at 400 g for 5 min. Fc receptors (FcR) were blocked with human FcR blocking reagent (20 μl/107 cells; Miltenyi, Bergisch Gladbach, Germany) diluted in flow stain buffer for 15 min with gentle rocking. Macrophages were then labelled with 1 μg anti-human CD14 conjugated with FITC (eBiosciences) or IgG1 isotype control in a volume of 100 μl for 1 hr with gentle rocking. Unbound antibody was removed by washing with flow stain buffer. Macrophages were fixed with 4% paraformaldehyde. A minimum of 5000 events were collected.
Allogeneic mixed leucocyte reactions and CD4+ T-cell stimulations
These assays were performed in 96-well plates. For mixed leucocyte reactions, macrophages (4 × 104/well) were treated for 24 hr with medium alone or sIL-27R (10 μg/ml) and then infected with M. bovis BCG (multiplicity of infection ∼1) for an additional 24 hr. CD4+ T cells isolated from cord blood mononuclear cells by negative antibody selection (Human CD4 Subset columns; R&D Systems) were then overlaid onto macrophage cultures. Five days following mixed culture, the supernatants were collected and IFN-γ concentration was measured by ELISA.
ELISA
Supernatants were collected from mixed leucocyte reactions and T-cell stimulation assays described above. Analysis of IFN-γ concentrations was carried out using the human IFN-γ DuoSet (R&D Systems) according to the manufacturer's instructions.
Statistical analysis
Statistical comparisons were performed using an unpaired samples t-test, Mann–Whitney U-test, paired sample t-test, or Friedman test of repeated measures depending on the nature and distribution of the data set. The appropriate test is indicated in the figure legend. The P values ≤ 0·05 were considered statistically significant.
Results
IL-27 gene expression is elevated in cord blood-derived macrophages
Our laboratory has demonstrated anti-inflammatory activity of IL-27 toward primary human adult macrophages during mycobacterial infection.26–28 Interleukin-27 limits inflammatory cytokine production and inflammatory cytokine receptor expression.26–28 Cumulatively, these activities compromise control of M. tuberculosis in human macrophages.26,28 Hence, we examined whether IL-27 may contribute to suboptimal immune responses in neonatal macrophages. Because the level of circulating macrophages was not sufficient to perform these experiments, monocytes were isolated from individual human umbilical cord or adult blood donors and differentiated to macrophages. To confirm the cellular phenotype, cord blood and adult cells were labelled for CD14, a marker of human monocytes/macrophages. As shown in Fig. 1, the labelling of cord blood and adult cells is comparable and a nearly homogeneous population of macrophages is found. These profiles are consistent with our previous report.26 RNA was isolated from steady-state macrophages and IL-27 gene expression was measured by real-time PCR. Expression of p28 and EBI3 was significantly elevated in the cord blood population (Fig. 2a,b). WSX-1 is the ligand-binding chain of the IL-27 receptor and is associated with immunosuppressive macrophage populations.29 The cord blood population did not exhibit a meaningful increase in WSX-1 gene expression compared with adults (Fig. 2c).
Figure 1.
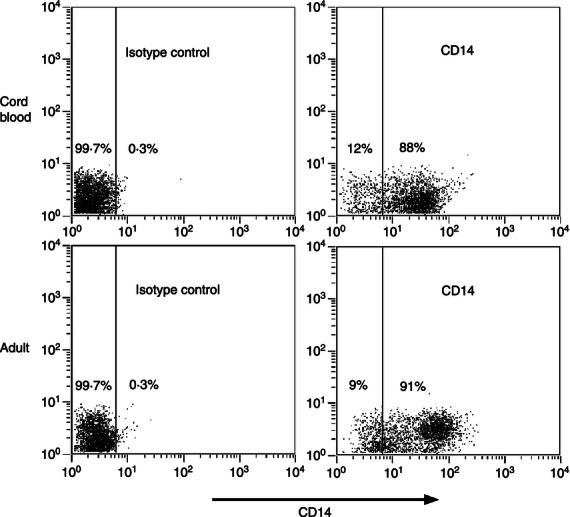
CD14 labelling of human macrophages. The cord blood or adult cells examined were labelled with FITC-conjugated anti-CD14 or isotype control as described in the Materials and methods. Dot plots for representative donors are shown; an equivalent analysis was carried out on separate donors at least three times.
Figure 2.
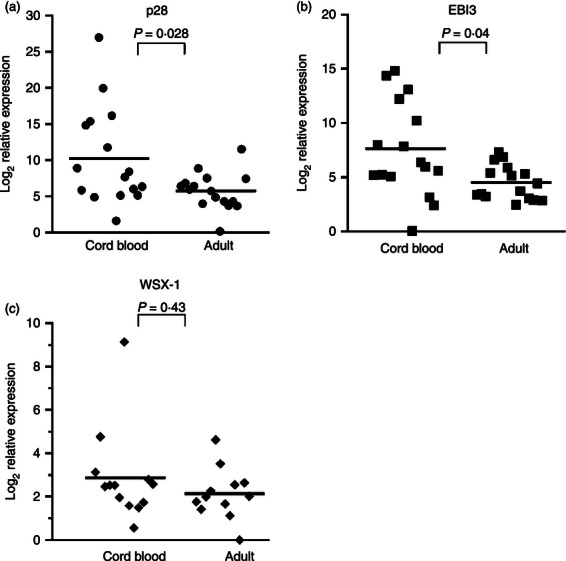
Interleukin-27 (IL-27) gene expression is elevated in cord blood macrophages. Monocytes were isolated from umbilical cord blood or adult buffy coats (n = 16) and differentiated to macrophages as described in the Materials and methods. Steady-state 7-day macrophages were harvested for RNA. Quantitative analysis of p28 (n = 16), Epstein–Barr virus-induced gene 3 (EBI3; n = 16), and WSX-1 (n = 14 cord blood, n = 12 adult) transcripts is presented as the log2 change in gene expression relative to GAPDH. Each symbol represents an individual donor. A Mann–Whitney U-test was used to establish statistical significance between donor groups in the 95% confidence interval; the corresponding P-values are indicated.
Interleukin-27 expression is elevated throughout infancy
Elevated levels of IL-27 may impact immune responses in the newborn population. We therefore wanted to determine whether the elevated expression of IL-27 in cord blood macrophages was a transient event in the transition of the fetus from the uterine environment, as suggested by the above results, or a more prolonged aspect of the infant immune response. To address this question in a controlled study where IL-27 expression would not be influenced by infection or other confounding conditions, we used an age-dependent mouse model. Spleens were harvested from C57BL/6 mice at various increments of age from the neonatal period through adulthood (n = 6 per age group) and RNA was isolated for quantitative gene expression analysis. Data were expressed relative to the mean gene expression of 4-day-old pups; however, it is important to indicate that IL-27 transcripts were detected at this age. An overall change in expression for both EBI3 and p28 was observed with the age of mice (P = 0·038 and 0·026, respectively). EBI3 and p28 gene expression increased sharply over the first 8 days of life (Fig. 3). Expression of EBI3 continued to increase modestly for 3 weeks and then decreased sharply as the mice transitioned to adults (Fig. 3). In contrast, p28 expression increased again from 8 to 12 days and then was maintained into adulthood (Fig. 3).
Figure 3.
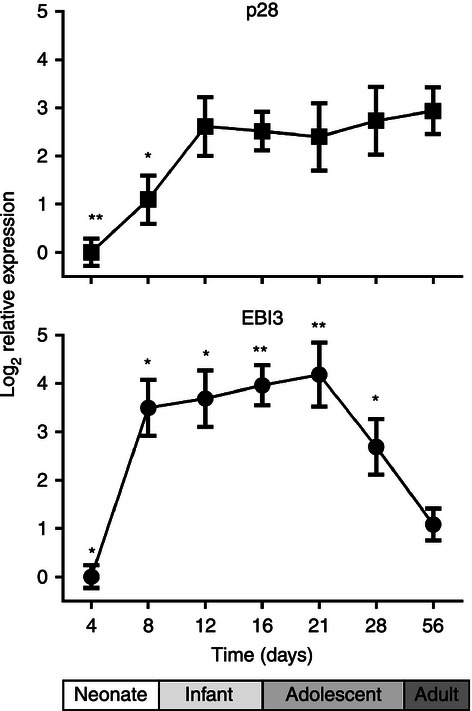
Interleukin-27 (IL-27) gene expression is elevated throughout infancy in mice. C57BL/6 mice (n = 6) were killed at the indicated time in days following birth. The spleens were removed and RNA was isolated as described in the Materials and methods. Quantitative analysis of p28 and Epstein–Barr virus-induced gene 3 (EBI3) transcripts is presented as the log2 change in gene expression. Expression levels within a sample were normalized to β-actin and expressed relative to the mean expression at 4 days. A t-test was used to establish statistical significance for the normally distributed data set in the 95% confidence interval. Comparisons were made between the indicated age group and adult mice within the p28 or EBI3 data series (*P < 0·05, **P < 0·005).
The pattern of IL-27 gene expression in mice was comparable to that of the human system for EBI3 but not p28. However, if EBI3 expression becomes a limiting factor for the assembly of IL-27, then functional IL-27 may be reduced in adult mice. We tested this hypothesis by immunolabelling splenocytes from different age mice for IL-27 followed by flow cytometric analysis. Additionally, the splenocytes were labelled for F4/80 to determine the level of macrophage-specific expression. Consistent with the gene expression analysis, the number of macrophages producing IL-27 (Fig. 4a,b) as well as the overall protein level (Fig. 4a,c), was increased in neonatal mice compared with adults and peaked toward the end of infancy. Importantly, a striking number of splenocytes that were negative for F4/80 also produced IL-27 in neonatal and infant mice (Fig. 4a,d). Some of these cells may be populations of macrophages that do not label for F4/80 or dendritic cells; this is later discussed in more detail. Together, these data demonstrate that although the murine system is not a precise replicate of the human primary macrophages, neonatal mice do produce greater levels of IL-27 that are maintained throughout infancy.
Figure 4.
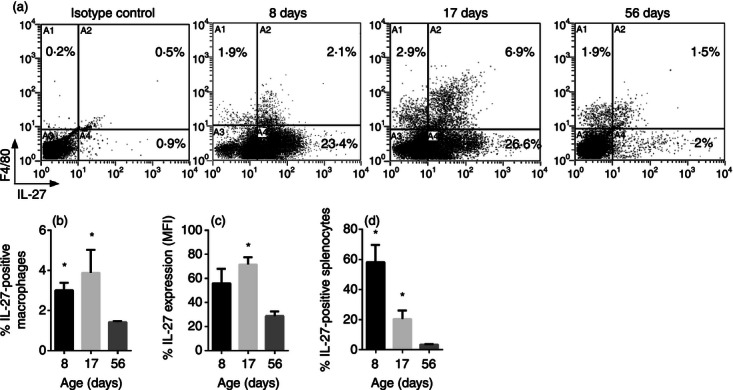
Interleukin-27 (IL-27) protein is elevated throughout infancy in mice. C57BL/6 mice (n = 4) were killed at the indicated time in days following birth. The spleens were removed and single cell suspensions were prepared. Splenocytes were labelled with phycoerythrin-conjugated anti-F4/80 and fluorescein-conjugated anti-IL-27 heterodimer as described in the Materials and methods. (a) Two-colour dot plots for a representative mouse at each age are shown. (b) The double-positive population (A2 quadrant) is represented at as the mean % positive IL-27-producing macrophages ± SE for four combined mice at each age. (c) The mean level of IL-27 expression as indicated by mean fluorescence intensity (MFI) of the double-positive (A2 quadrant) population ± SE is represented for four combined mice at each age. (d) The total IL-27-positive population (A2 + A4 quadrant) is represented at as the mean % positive IL-27-producing splenocytes ± SE for four combined mice at each age. The indicated statistical comparisons were made using the Mann–Whitney U-test (b, d) or unpaired samples t-test (c).
Progesterone induces IL-27 expression
During pregnancy, fetal trophoblasts must invade deep into the uterine lining without activating the maternal immune system. Meanwhile the decidual tissue of the uterus contains mononuclear cells capable of responding to the semi-allogeneic fetus throughout pregnancy. Hence, successful human pregnancy is a situation in which immunological tolerance must be initiated and maintained at the fetal–maternal interface. Progesterone levels that increase throughout pregnancy have been shown to skew immune responses in the Th2 direction and decidual tissue in successful pregnancy has been well characterized as immunosuppressive.32,33 We considered the possibility that IL-27 may be regulated by progesterone and expressed at elevated levels in the neonatal population as a consequence of the maternal environment. To investigate IL-27 expression in response to progesterone, we used macrophages from adult donors that were considered naive to this hormone. If progesterone regulates IL-27, the neonatal population may be insensitive to added levels. Progesterone levels are highest during pregnancy in the third trimester.34 Pregnant women were excluded from the donor pool. In these macrophage donors (n = 4), exposure to progesterone for 72-hr induced expression of both p28 and EBI3 in a dose-responsive manner (Fig. 5). These results suggest that IL-27 may be expressed at elevated levels in neonates as a consequence of the progesterone-rich environment during pregnancy.
Figure 5.
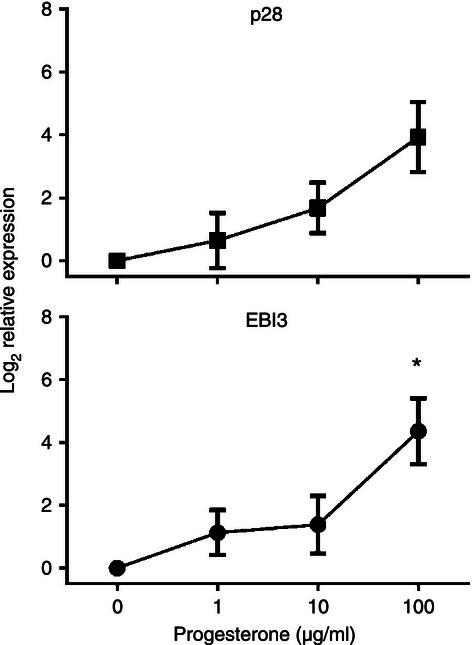
Progesterone induces interleukin-27 (IL-27) gene expression. Adult macrophages were cultured with or without progesterone at the indicated concentrations for 72 hr. Quantitative analysis of p28 or Epstein–Barr virus-induced gene 3 (EBI3) transcripts is presented as the mean log2 change in gene expression of duplicate samples ± SE for four combined donors. Values were normalized to the mean expression of GAPDH and expressed relative to that of medium alone (no progesterone). Significant changes in gene expression were established as determined by the non-parametric Friedman test of repeated measures; an asterisk indicates significance.
Interleukin-27 influences human macrophages to limit CD4+ T-cell proliferation and IFN-γ production
Interleukin-27 is a paradoxical cytokine that has been shown to contribute to differentiation of naive T cells toward a Th1 phenotype and promotion of IFN-γ.17 At the same time, IL-27 has been demonstrated to limit inflammatory responses associated with both T cells and macrophages.35 To begin to understand how IL-27 may influence neonatal and infant immune responses, we investigated the biology of elevated IL-27 in the context of both cell types. For these experiments, M. bovis BCG, the live vaccine strain for M. tuberculosis, was chosen as a model for both infection- and vaccination-induced responses. Cord blood macrophages were left untreated or treated with sIL-27R for 4 hr and then infected with BCG for an additional 5 days. The macrophages were then permeabilized and BCG was enumerated by standard plate counts. As shown in Fig. 6(a), neutralization of IL-27 before and during infection reduced the mycobacterial recovery. There were no substantial differences in macrophage viability between treatment groups as measured by an MTT assay (data not shown). In addition the uptake of BCG is similar in macrophages under each treatment condition (data not shown). These results are consistent with published studies that show IL-27 opposes innate immunity and control of bacteria.26,28
Figure 6.
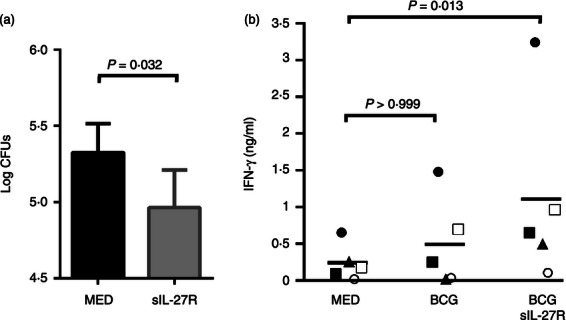
Neutralization of interleukin-27 (IL-27) improves neonatal innate and adaptive immune responses. (a) Cord blood macrophages were treated with sIL-27R or medium alone for 4 hr and then infected with bacillus Calmette–Guérin (BCG; multiplicity of infection ∼ 1). Data are represented as the mean colony-forming units (CFUs) recovered from infected macrophages ± SE at 5 days for four independent experiments. (b) Cord blood macrophages were cultured with sIL-27R or medium alone for 24 hr before infection with BCG (multiplicity of infection ∼ 1). Following infection for 24 hr CD4+ T cells from heterologous donors were added at a fivefold ratio. After 5 days, the IFN-γ concentration in culture supernatants was measured by ELISA. Data shown are for five independent experiments; each symbol represents a separate experiment performed with different macrophage and T-cell donors. A paired samples t-test (a) or Friedman test of repeated measures (b) was used to establish statistical significance in the 95% confidence interval.
Cord blood mononuclear cells have been shown to be impaired in the ability to stimulate IFN-γ from neonatal or adult lymphocytes.6 Hence, we evaluated the allogeneic IFN-γ response in the presence of a Th1 stimulus. BCG has become one of the most used of all vaccines and is administered to approximately 100 million newborns worldwide each year.36 These numbers rank BCG among the most prevalent vaccines given to newborns and children.36,37 Moreover, in South Africa > 95% of infants are vaccinated with BCG shortly after birth.38 Therefore, BCG serves as a relevant model for vaccination and a stimulus to drive a Th1 response. Cord blood macrophages were left untreated or were treated with sIL-27R for 24 hr. The macrophages were then left uninfected or infected with M. bovis BCG for 24 hr to activate them as an antigen-presenting cell. Following this initial period of infection, heterologous CD4+ T cells were isolated and overlaid onto macrophages for an additional 5 days. Supernatants were then collected and the levels of IFN-γ were measured by ELISA. The allogeneic response alone was minimal, as reported previously by many groups (Fig. 6b). Infection by BCG modestly increased the level of IFN-γ produced by CD4+ T cells (Fig. 6b). However, neutralization of IL-27 led to an increase in IFN-γ in every donor, as well as a greater than fivefold mean increase in cytokine compared with control (Fig. 6b).
Discussion
Newborns and infants exhibit increased susceptibility to infectious agents. To improve immune responses to infectious agents and vaccines in the newborn and infant population, it is important to fully understand the mechanistic nature of the immunological limitations in specific cell types. Interleukin-27 has emerged as a cytokine that can limit inflammatory responses by macrophages and lymphocytes.35 It could contribute to limitations in the immune response of newborns and infants. However, this possibility was not previously investigated.
We show here that human cord blood-derived macrophages express genes for both subunits of IL-27 at an elevated level compared with adult counterparts. Human cord blood-derived dendritic cells do not express IL-27 genes without stimulation; however, they exhibit elevated IL-27 gene expression compared with adults in response to the combination of lipopolysaccharide and IFN-γ.31 Although IL-27 gene expression was increased in the neonatal cohort, WSX-1 gene expression was not significantly different from that in adult cells. This is in contrast to neonatal dendritic cells and T cells, which exhibited a substantial increase in WSX-1 transcripts compared with cells from adults.31 This may suggest that neonatal macrophages are programmed differently for IL-27 expression, and neonatal dendritic cells and T cells are equipped for a high-efficiency response to IL-27. This further highlights the need for cell-type-specific comparisons to understand how immunological findings may influence the complete immune response.
The significance of elevated neonatal IL-27 expression is impacted by the duration of time in which a high level of expression is maintained. Obtaining a sufficient sample size from healthy paediatric volunteers to perform a reliable analysis is not feasible. Expression of IL-27 may be skewed in paediatric patients seeking medical care for a wide variety of conditions. We therefore used a mouse model to address age-dependent IL-27 expression in a controlled population. The expression of p28 increased following birth, peaked during infancy, and was maintained through adulthood. Similarly EBI3 expression increased following birth, was significantly higher in neonatal mice than adults, but continued to increase with age, peaking during late infancy. Despite the absence of a decline in p28 transcripts in adult mice, reduced EBI3 gene expression suggested the possibility that EBI3 may be a limiting factor in the assembly of IL-27. Therefore, we measured the levels of IL-27 protein by immunolabelling in splenocytes. The number of IL-27-positive macrophages was significantly increased in neonatal and infant mice compared with adults. Likewise, the level of protein expression (mean fluorescence intensity) was also increased in this population of cells from neonatal and infant mice. It is further evident that large numbers of IL-27-producing cells that do not label with F4/80 are present in neonatal and infant spleens. F4/80 is a common marker of mature macrophages but does not label all populations.39 Splenic red pulp macrophages highly express F4/80, but expression is more limited or absent on marginal zone macrophages.39,40 It is, however, worth noting that eosinophils can express F4/80; although this becomes more pronounced during parasitic infection and tissue remodelling, factors not relevant here.41,42 Therefore, the F4/80-negative population may include some other populations of macrophages; there is no single marker that can be used to uniformly identify this cell type. However, it seems likely that dendritic cells and other cell types are represented. Future experiments will further explore the contribution to IL-27 production by individual cell types. It will be important to do so as this population also exhibits age-dependent IL-27 expression. The mouse will be a useful model system for these studies, and to explore the complete impact of IL-27 in newborn and infant response to vaccines and infection.
One explanation for the elevated IL-27 expression in neonates would be transition from the immunosuppressive environment in utero. Indeed progesterone, which peaks during the third trimester of pregnancy,34 increased IL-27 gene expression in a dose-dependent fashion. Therefore, it was surprising that expression of IL-27 genes increased in the week following birth. However, these results do not contrast with the data observed from the human macrophages; cord blood monocytes are cultured for 7 days for differentiation to macrophages. We acknowledge that this does not exactly simulate in vivo parameters. Limitations exist in what can be done with each model. We are unable to obtain sufficient quantities of blood from 1-day-old murine neonates to differentiate monocyte-derived macrophages as with human cells. Conversely, the numbers of circulating macrophages in human cord blood are not sufficient for these studies and spleen specimens are not readily available. Nonetheless, it is possible that a cue in the first week of life outside the uterine environment stimulates IL-27 expression. This, however, does not preclude progesterone as a stimulus for IL-27 expression in utero. This is supported by IL-27 detection in placental tissues and in the vicinity of the trophoblast in pregnant mice.43,44 Neutralization of IL-27 during murine pregnancy promoted abortion.43,44 The expression of IL-27 may quickly subside when the neonate transitions out of the progesterone-rich environment but increases again over the first week of life. Clearly, some signal must be responsible for the maintenance of high IL-27 levels through infancy. Tissues from fetal and neonatal donors of various ages will be necessary to fully address these possibilities.
We wanted to determine the influence of IL-27 on neonatal macrophages. Interleukin-27 alone has not been shown to drive an alternatively activated (M2)/immunosuppressive macrophage phenotype in human adult cells.28 A gene expression profile indicates that the same is true for neonatal macrophages (data not shown). To begin to determine whether IL-27 opposes more optimal responses in neonates, we neutralized IL-27 before and during infection with a soluble receptor. BCG was used as a dual model for infection and vaccination. Fewer bacteria were recovered in macrophage cultures when IL-27 signalling was blocked. This is consistent with other reports regarding the influence of IL-27 on the control of mycobacteria in adult mice and human cells.20,21,26,28 Mixed leucocyte reactions with neonatal cells have yielded only limited IFN-γ production.6,10 Those findings are repeated here. Further stimulation of the macrophages by BCG, particularly relevant to infant vaccination, modestly increased IFN-γ output. However, this was increased more significantly by neutralization before and during co-culture with CD4+ T cells. Cumulatively, these data demonstrate that IL-27 impedes innate immune function to control BCG, and restricts the production of IFN-γ during allogeneic stimulation in the presence of a Th1 polarizing stimulus. The latter is independent of an antigen-specific response, but does have important implications in vaccination approaches. Combining improved microbial control with heightened T-cell activity could also markedly augment protective responses during infection. The nature of the effect of IL-27 on neonatal T cells in this context remains to be defined. However, future experiments in mice for which IL-27 receptor-deficient animals and T-cell receptor transgenic mice are available, will help to provide an increased understanding of the impact of IL-27 in newborn and infant immunology.
Acknowledgments
We would like to extend a very special thanks to the Department of Obstetrics and Gynecology at the University of South Carolina School of Medicine and Palmetto Health Richland Hospital, as well as to Rob McCorckle at Magee Women's Hospital (Pittsburgh, PA) for their gracious help in providing umbilical cord blood.
This work was supported by institutional funds supplied by the University of South Carolina School of Medicine and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103434.
We would also like to acknowledge grant support for the University of South Carolina Center for Colon Cancer Research Mouse Experimentation Core Facility from the NIH/NCRR COBRE grant P20RR17698.
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–46. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 3.Levy O, Zaremba KA, Roy R, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-α induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–34. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 4.Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M, Willems F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–81. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Maródi L. Down-regulation of Th1 responses in human neonates. Clin Exp Immunol. 2002;128:1–2. doi: 10.1046/j.1365-2249.2002.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burchett SK, Corey L, Mohan KM, Westall J, Ashley R, Wilson CB. Diminished interferon-γ and lymphocyte proliferation in neonatal and postpartum primary herpes simplex virus infection. J Infect Dis. 1992;165:813–8. doi: 10.1093/infdis/165.5.813. [DOI] [PubMed] [Google Scholar]
- 8.Vekemans J, Ota MO, Wang EC, et al. T cell responses to vaccines in infants: defective IFN γ production after oral polio vaccination. Clin Exp Immunol. 2002;127:495–8. doi: 10.1046/j.1365-2249.2002.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ota MO, Vekemans J, Schlegel-Haueter SE, et al. Hepatitis B immunization induces higher antibody and memory Th2 responses in newborns than in adults. Vaccine. 2004;22:511–9. doi: 10.1016/j.vaccine.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Taylor S, Bryson YJ. Impaired production of γ-interferon by newborn cells in vitro is due to a functionally immature macrophage. J Immunol. 1985;134:1493–7. [PubMed] [Google Scholar]
- 11.Hunt DWC, Huppertz HI, Jiang HJ, Petty RE. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood. 1994;84:4333–43. [PubMed] [Google Scholar]
- 12.Goriely S, Vincart B, Stordeur P, Vekemans J, Willems F, Goldman M, De Wit D. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166:2141–6. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 13.Liu E, Tu W, Law HKW, Lau YL. Decreased yield, phenotypic expression and function of immature monocyte-derived dendritic cells in cord blood. Br J Haematol. 2001;113:240–6. doi: 10.1046/j.1365-2141.2001.02720.x. [DOI] [PubMed] [Google Scholar]
- 14.Devergne O, Hummel M, Koeppen H, LeBeau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein–Barr virus infection in B lymphocytes. J Virol. 1996;70:1143. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprecher CA, Grant FJ, Baumgartner JW, et al. Cloning and characterization of a novel class I cytokine receptor. Biochem Biophys Res Commun. 1998;246:82–90. doi: 10.1006/bbrc.1998.8576. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, deSauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–20. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 17.Pflanz S, Timans JC, Cheunget J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naïve CD4+ T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 18.Hamano S, Himeno K, Miyazaki Y, et al. WSX-1 is required for resistance to Trypanosome cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–67. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 19.Villarino A, Hibbert L, Lieberman L, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–50. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 20.Pearl J, Khader SA, Solache A, Gilmartin L, Ghilardi N, deSauvage F, Cooper AD. IL-27 signaling compromises control of bacterial growth in Mycobacteria-infected mice. J Immunol. 2004;173:7490–6. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 21.Hölscher C, Hölscher A, Rückerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3524–44. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 22.Artis D, Villarino A, Silverman M, et al. The IL-27 receptor is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–34. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 23.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 24.Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 25.Awasthi A, Carrier Y, Peron JPS, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory cells. Nat Immunol. 2007;8:1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 26.Robinson CM, Nau GJ. Interleukin-12 and Interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J Infect Dis. 2008;198:359–66. doi: 10.1086/589774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson CM, Nau GJ. Cytokines involved in interferon-γ production by human macrophages. J Innate Immun. 2010;2:56–65. doi: 10.1159/000247156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson CM, Jung J-Y, Nau GJ. Interferon-γ, tumor necrosis factor, and interleukin-18 cooperate to control growth of Mycobacterium tuberculosis in human macrophages. Cytokine. 2012;60:233–41. doi: 10.1016/j.cyto.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rückerl D, Heßmann M, Yoshimoto T, Ehlers S, Holscher C. Alternatively activated macrophages express the IL-27 receptor α chain WSX-1. Immunobiology. 2006;211:427–36. doi: 10.1016/j.imbio.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Bogaert D, Weinberger D, Thompson C, Lipsitch M, Malley R. Impaired innate and adaptive immunity to Streptococcus pneumoniae and its effect on colonization in an infant mouse model. Infect Immun. 2009;77:1613–22. doi: 10.1128/IAI.00871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krumbiegel D, Anthogalidis-Voss C, Markus H, Zepp F, Meyer CU. Enhanced expression of IL-27 mRNA in human newborns. Pediatr Allergy Immunol. 2008;19:513–6. doi: 10.1111/j.1399-3038.2007.00685.x. [DOI] [PubMed] [Google Scholar]
- 32.Piccinni MP, Giudizi MG, Biagiotti R, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clone. J Immunol. 1995;155:128–33. [PubMed] [Google Scholar]
- 33.Heikkinen J, Möttönen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol. 2003;131:498–505. doi: 10.1046/j.1365-2249.2003.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beagley KW, Gockel CM. Regulation of innate adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 35.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–30. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO. Weekly Epidemiological Record. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 37.Fine PEM, Carneiro IAM, Milstien JB, Clements CJ. Issues Relating to Use of BCG in Immunization Programmes. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 38.Hanekom WA. The immune response to BCG vaccination of newborns. Ann N Y Acad Sci. 2005;1062:69–78. doi: 10.1196/annals.1358.010. [DOI] [PubMed] [Google Scholar]
- 39.Gordon S, Hamann J, Lin H, Stacey M. F4/80 and the related adhesion G-PCRs. Eur J Immunol. 2011;41:2470–525. doi: 10.1002/eji.201141715. [DOI] [PubMed] [Google Scholar]
- 40.Idoyaga J, Suda N, Suda K, Park CG, Steinman RM. Antibody to Langerin/CD207 localizes large numbers of CD8 α+ dendritic cells to the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2009;106:1524–9. doi: 10.1073/pnas.0812247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGarry MP, Stewart CC. Murine eosinophil granulocytes bind the murine macrophage-monocyte specific monoclonal antibody F4/80. J Leukoc Biol. 1991;50:471–8. doi: 10.1002/jlb.50.5.471. [DOI] [PubMed] [Google Scholar]
- 42.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–82. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 43.Mas AE, Petitbarat M, Dubanchet S, Fay S, Ledée N, Chaouat G. Immune regulation at the interface during early steps of murine implantation: involvement of two new cytokines of the IL-12 family (IL-23 and IL-27) and of TWEAK. Am J Reprod Immunol. 2008;59:323–38. doi: 10.1111/j.1600-0897.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 44.Chaouat G, Petitbarat M, Bulla R, et al. Early regulators in abortion and implications for a preeclampsia model. J Reproductive Immunol. 2009;82:132–41. doi: 10.1016/j.jri.2009.08.004. [DOI] [PubMed] [Google Scholar]


