Abstract
Inflammatory bowel disease is characterized by dysregulated immune responses in inflamed intestine, with dominance of interleukin-17 (IL-17) -producing cells and deficiency of regulatory T (Treg) cells. The aim of this study was to investigate the effect and mechanisms of sirolimus, an inhibitor of the mammalian target of rapamycin, on immune responses in a murine model of Crohn's disease. Murine colitis was induced by intrarectal administration of 2,4,6-trinitrobenzene sulphonic acid at day 0. Mice were then treated intraperitoneally with sirolimus daily for 3 days. The gross and histological appearances of the colon and the numbers, phenotype and cytokine production of lymphocytes were compared with these characteristics in a control group. Sirolimus treatment significantly decreased all macroscopic, microscopic and histopathological parameters of colitis that were analysed. The therapeutic effects of sirolimus were associated with a down-regulation of pro-inflammatory cytokines tumour necrosis factor-α, IL-6 and IL-17A. Intriguingly, sirolimus administration resulted in a prominent up-regulation of the regulatory cytokine transforming growth factor-β. Supporting the hypothesis that sirolimus directly affects the functional activity of CD4+ CD25+ Treg cells, we observed a remarkable enhancement of FoxP3 expression in colon tissues and isolated CD4+ T cells of sirolimus-treated mice. Simultaneously, sirolimus treatment led to a significant reduction in the number of CD4+ IL-17A+ T cells in the mesenteric lymph node cells as well as IL-17A production in mesenteric lymph node cells. Therefore, sirolimus may offer a promising new therapeutic strategy for the treatment of inflammatory bowel disease.
Keywords: colitis, inflammation, regulatory T cells, sirolimus, T helper type 17 cells
Introduction
Inflammatory bowel diseases (IBDs), such as Crohn's disease and ulcerative colitis, are characterized by chronic relapsing intestinal diseases that affect the human digestive tract.1,2 Although evidence implies that genetic susceptibility and environmental triggers accelerate the immunopathogenic process,3 the aetiology of IBD is still unknown. The current studies showed that intrinsic factors, such as inappropriate immune responses, exert an essential role in the development of IBD.4 Excessive or dysregulated intestinal mucosal immunity leads to an over-production of pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6) and IL-1β released primarily from macrophages and lymphocytes. These pro-inflammatory cytokines play a major role in the perpetuation of intestinal inflammation and result in an imbalance of pro-inflammatory and anti-inflammatory responses in IBD.5 Down-regulating the production of these pro-inflammatory cytokines in inflamed intestine can suppress the established inflammatory reaction and attenuate IBD effectively, as suggested by clinical and experimental studies.6,7
Recently, a body of evidence suggested that imbalance of the development and function of T helper type 17 (Th17) cells and regulatory T (Treg) cells plays a critical role in autoimmune diseases, including IBD.8,9 The Th17-cell-derived cytokines IL-17, IL-17F, IL-21 and IL-22 are supposed to participate in the protection of the host against various bacterial and fungal infections, particularly at mucosal surfaces.10 Meantime, there are also findings that uncontrolled and persistent effector Th17 cell responses can contribute to autoimmune disease, such as rheumatoid arthritis,11 multiple sclerosis,12 systemic lupus erythematosus13 and type 1 diabetes.14 On the other hand, Treg cells, also known as CD4+ CD25+ FoxP3+ T cells, are involved in the maintenance of peripheral tolerance and the control of immune responses by initiating suppressive effects on activated immune cells.15 The development of IBD has been associated with an imbalance between pro-inflammatory, effector Th17 cells and anti-inflammatory, tolerating Treg cell subsets in inflamed mucosa. Pro-inflammatory cytokines released by Th17 cells are expressed extensively in the mucosa and serum of patients with IBD,16 and Treg cells show only moderate expansion in mucosal lymphoid tissues,17 which is far from counterbalancing the mucosal inflammation in IBD. Several studies demonstrated that polarization of Th17 cells, in addition to Th1 cells, can profoundly accelerate the perpetuation of IBD.18 On the contrary, switching of a Th1/Th17 profile to the enhancement of Treg cells or inhibition of Th17 polarization is beneficial for restraining immune response and ameliorating intestinal inflammation.19–21
The immunophilin ligand sirolimus, a macrolide antibiotic produced by Streptomyces hygroscopicus, exhibits potent immunosuppressive properties and is used therapeutically in countering autoimmunity and preventing allograft rejection.22,23 Specific inhibition by sirolimus of the serine/threonine protein kinase mammalian target of rapamycin (mTOR) in T cells blocks co-stimulation and cytokine-induced signalling but allows T-cell receptor-mediated signal transduction.24 Consequently, sirolimus promotes T-cell anergy and deletion.25,26 Unlike other commonly used immunosuppressants, such as cyclosporine A and FK506, sirolimus does not appear to interfere with tolerance induction27,28 and permits the in vitro proliferation and suppressive function of Treg cells.29,30 Whether sirolimus influences the imbalance between Th17 and Treg cells in the development of IBD, however, has not been fully elucidated. In this study, we investigated the immunomodulatory effect of sirolimus in a 2,4,6-trinitrobenzene sulphonic acid (TNBS) -induced murine colitis model. We also explored the potential mechanisms involved, especially in the balance of Treg and Th17 cells.
Materials and methods
Animals
Male BALB/c mice (8–10 weeks old) were purchased from the Center of Experimental Animals of Guangdong Province, and maintained at an animal facility under pathogen-free conditions. All studies involving mice were approved by the Guangdong Pharmaceutical University Animal Care and Use Committee.
Induction of colitis and sirolimus treatment
Colitis was induced by administration of TNBS in mice at day 0 as described previously.31 In brief, mice were anaesthetized lightly, and a 3·5-F catheter was inserted intrarectally to 4 cm proximal to the anus. To induce colitis, 120 μl 2·5 mg TNBS (Sigma-Aldrich, St Louis, MO) in 50% ethanol was injected slowly into the lumen via the catheter. Control mice received the same volume of 50% ethanol alone. To study the therapeutic effect of sirolimus, 1·25 mg/kg sirolimus (LC Laboratories, Woburn, MA) was administered intraperitoneally for three consecutive days starting at day 0 after TNBS administration. Animals were monitored daily for appearance of diarrhoea, loss of body weight and survival. The disease activity index was used to assess the grade of colitis based on a previously published scoring system by Reinecke et al.31 All of the mice were killed at the indicated time after administration of TNBS.
Macroscopic and microscopic (histological) assessment
Colonic morphology was evaluated as a gross indicator of colitis. Macroscopic assessment of the colitis severity was graded according to a previously established scoring system as follows: 0, no ulcer and no inflammation; 1, local hyperaemia without ulceration; 2, ulceration without hyperaemia; 3, ulceration and inflammation at one site only; 4, two or more sites of ulceration and inflammation; and 5, ulceration extending more than 2 cm.32 For histological analysis, colons were fixed, sectioned and stained with haematoxylin & eosin. Histological changes were graded from 0 to 4 in a blind fashion according to previously described criteria as follows: 0, no signs of inflammation; 1, very low level of leucocyte infiltration; 2, low level of leucocyte infiltration; 3, high level of leucocyte infiltration, high vascular density, and thickening of the colon wall; 4, transmural leucocyte infiltration, loss of goblet cells, high vascular density and thickening of the colon wall.32
Myeloperoxidase assay
Myeloperoxidase (MPO) activity of the colon was measured according to the method described previously.33 Briefly, tissues were homogenized and centrifuged (30 000 g, 30 min at 4°). Pellets were resuspended in hexadecyltrimethylammonium bromide in 50 mm potassium phosphate buffer and then freeze–thawed three times. The supernatants were diluted in potassium phosphate buffer (pH 6·0) containing 0·167 mg O-dianisidine dihydrochloride (Sigma-Aldrich) and 0·0006% (vol/vol) H2O2. Changes in absorbance at 460 nm were recorded with kinetic readings over 3 min. Sample protein concentrations were determined (bicinchoninic acid assay), and the results are presented as MPO units per milligram of protein.
Isolation and culture of mesenteric lymph node cells
Mesenteric lymph node (MLN) cells were isolated and incubated in complete RPMI-1640 with 10% fetal calf serum at a concentration of 1 × 106 cells/ml for 48 hr in the presence or absence of PMA (10 ng/ml) and concanavalin A (Con A; 2 μg/ml) (Sigma-Aldrich). Cytokine production in culture supernatants was determined by ELISA.
Cytokine measurement
The levels of IL-6, IL-17A and transforming growth factor-β (TGF-β) in MLN cell culture supernatants were determined by sandwich ELISA using the kits supplied by eBioscience (San Diego, CA). ELISA was performed according to the manufacturer's instructions.
Intracellular cytokine staining
Mesenteric lymph node cells were isolated and suspended in complete RPMI-1640 with 10% fetal calf serum at a density of 1 × 106/ml. The cell suspensions were re-stimulated with PMA (20 ng/ml), ionomycin (1 μg/ml) and 2 μm of monensin (Sigma-Aldrich) for 4 hr. Cells were harvested, blocked with rat anti-mouse CD16/32 antibodies, and stained with phycoerythrin-cy5-conjugated anti-mouse CD4 antibody (BD Pharmingen, San Jose, CA). Cells were then fixed and permeabilized with Cytofix/Cytoperm (BD Pharmingen) and stained with phycoerythrin-conjugated anti-mouse IL-17A antibody. Intracellular FoxP3 was determined according to the manufacturer's instructions. Data were acquired on a FACScalibur (BD Biosciences, San Jose, CA) and analysed with the CellQuest v3.3 software as instructed.
Real-time quantitative PCR
Two micrograms of total RNA, extracted from the colon tissue and MLN using TRIzol reagent (Invitrogen, Carlsbad, CA) and treated with DNase I, was used to synthesize cDNA using the first-strand cDNA synthesis kit (Invitrogen). PCR mixture was prepared using SYBR Green qPCR kit (Invitrogen) and using the primers as follows: tumour necrosis factor-α (TNF-α) (forward 5′-CATCTTCTCAAAATTCGAGTGACAA-3′, reverse 5′-TG-GGAGTAGACAAGGTACAACCC-3′), IL-6 (forward 5′-GAGACTTCCATCCAGTTGCC-3′, reverse 5′-AAGTGCATCATCGTTGTTCATACA-3′), IL-4 (forward 5′-ACAGGAGAAGGGACGCCAT-3′, reverse 5′-GAAGCCCTAC-AGACGAGCTCA-3′), IL-17A (forward 5′-TCTCTGATG-CTGTTGCTGCT-3′, reverse 5′-AGGAAGTCCTTGGCCTCAGT-3′), TGF-β (forward, 5′-TTGCTTCAGCTCCACAGAGA-3′, reverse 5′-TGGTTGTAGAGGGCAAGGAC-3′), Foxp3 (forward, 5′-CCCAGGAAAGACAGCAACCTT-3′, reverse 5′-CCTTGCCTTTCTCATCCAGGA-3′), Retinoid-related orphan receptor γ t (RORγt) (forward, 5′-CAGCCAACATGTGGAAAAGCT-3′, reverse 5′-GGGAAGGCGGCTTGGA-3′), and GAPDH (forward 5′-TTCACCACCATGGAGAAGGC-3′, reverse 5′-GGCAT-GGACTGTGGTCATGA-3′). All real-time quantitative PCR (RT-qPCR) were performed with an ABI PRISM® 7000 Sequence Detector Systems (Applied Biosystems, Foster City, CA), and expression values were normalized to the housekeeping gene GAPDH using the comparative threshold cycle (CT) method.
Proliferation assays
Mesenteric lymph node cells from sirolimus- or PBS-treated mice were separated into CD4+ CD25+ T cells and CD4+ CD25− T cells using a CD4+ CD25+ regulatory T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). CD4+ CD25− T cells (2·5 × 105) were cultured with Mitomycin-treated BALB/c CD4− cells (2 × 105) as antigen-presenting cells for 48 hr in round-bottom 96-well plates in complete PRMI-1640 medium. Cells were also stimulated with 1 mg/ml anti-CD3 monoclonal antibody (BD Pharmingen). In co-culture experiments, titrated CD4+ CD25+ T cells and 2·5 × 105 responder cells (CD4+ CD25− T cells) were simultaneously added into the wells. Following an 18-hr pulse with [3H]thymidine at 1 μCi/well, proliferation was analysed in a scintillation counter.
Statistical analysis
The values are expressed as mean ± SEM. Data were analysed by using Student's t-test or one-way analysis of variance. Differences were considered statistically significant when P < 0·05.
Results
Sirolimus protects mice from development of TNBS-induced colitis
The pathological changes in mice after the induction of TNBS colitis have been described in detail.9 To investigate the effect of sirolimus in intestinal inflammation in vivo, colitis was induced in BALB/c mice. As expected, mice given TNBS showed severe colitis characterized by bloody diarrhoea, rectal prolapse and a profound and sustained weight loss, which resulted in a high mortality rate (65%), whereas control mice rapidly recovered weight after the starvation period and did not die (Fig. 1). In contrast, sirolimus-treated mice rapidly recovered the lost body weight, regained a healthy appearance similar to control mice, and had a survival rate of 85% (Fig. 1).
Figure 1.
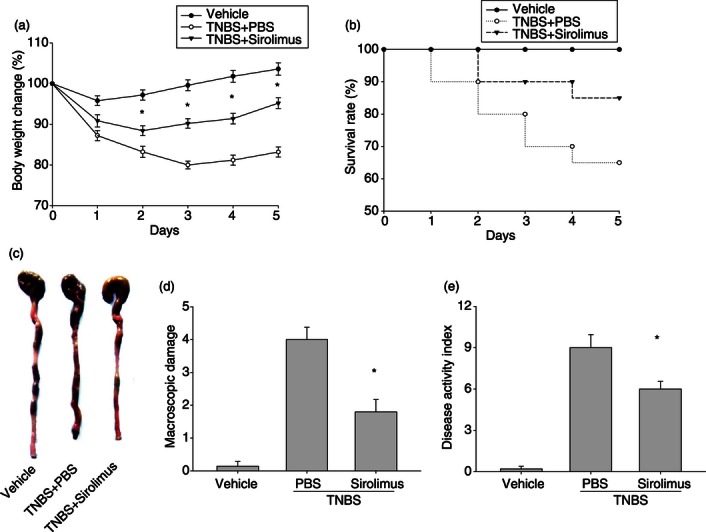
Treatment with sirolimus ameliorates the severity of 2,4,6-trinitrobenzene sulphonic acid (TNBS) -induced murine colitis. Throughout the experiment, mice were monitored for body weight loss and overall mortality, and colitis severity was assessed at 72 hr after TNBS administration. (a) Body weight changes of each group after colitis induction. (b) Animal survival during the first 5 days after TNBS administration. (c) Typical colon morphology of each group. (d) Macroscopic score of each group. (e) Disease activity index (DAI) of each group. Data are presented as the mean ± SEM (n = 6 to n = 10 in each group). *P < 0·05 versus the PBS-treated group.
Sirolimus attenuates colonic damage in BALB/c mice with TNBS colitis
Histological analysis of the colons of mice with TNBS-induced colitis treated with PBS exhibited transmural inflammation affecting the entire colonic mucosa characterized by ulceration, loss of goblet cells, degradation of crypt integrity, and massive inflammatory cell infiltration supported by elevated colonic MPO activity (Fig. 2). Microscopically the colons of mice given sirolimus displayed a marked reduction in the tissue disruption, mucosal ulcerations and mononuclear cell infiltration, which was accompanied by reduced MPO activity (Fig. 2). The mean histological score was significantly lower in sirolimus-treated mice when compared with PBS-treated mice (Fig. 2b).
Figure 2.
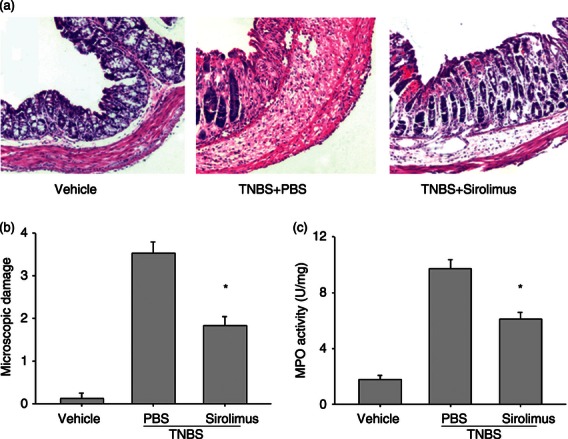
Treatment with sirolimus suppresses colonic damage in mice with induced colitis. Seventy-two hours following colitis induction, the histopathological tissue damage and the myeloperoxidase (MPO) activity were determined. (a) Colon sections from each experimental group were stained with haematoxylin & eosin (100 ×). (b) Microscopic score of each group. (c) MPO content was assessed in colonic tissue homogenates as described in Materials and methods. Data are presented as the mean ± SEM (n = 5 to n = 7 in each group). *P < 0·05 versus the PBS-treated group.
Sirolimus reduces the production of inflammatory cytokines in TNBS-induced colitis
Subsequently, we evaluated the effect of sirolimus on the production of inflammatory cytokines that are involved in the pathology of TNBS-induced colitis. We isolated colons and assessed the cytokine mRNA expression in tissue homogenates on day 3 by RT-qPCR. As shown in Figure 3, TNBS induced a marked increase in mRNA levels of pro-inflammatory cytokines, such as IL-6, TNF-α and IL-17A in the colon homogenates, whereas sirolimus treatment suppressed the mRNA expressions of these cytokines. On the contrary, significantly enhanced amounts of anti-inflammatory cytokines IL-4 and TGF-β were observed in the colon homogenates of sirolimus-treated mice compared with that of PBS-treated mice.
Figure 3.
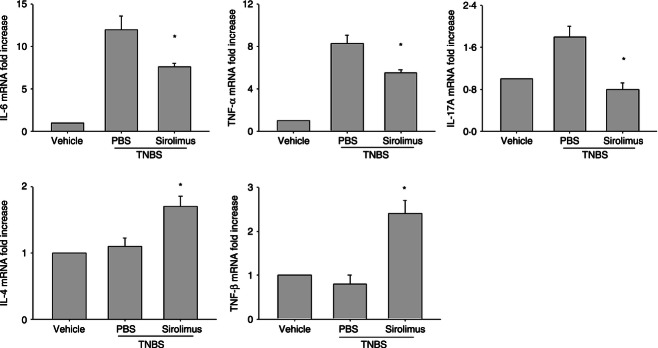
Treatment with sirolimus inhibits inflammatory cytokine production in colon tissue. Colon tissue, located precisely 3 cm above the anus, was isolated from the mice of each group at 72 hr after 2,4,6-trinitrobenzene sulphonic acid (TNBS) administration. Messenger RNA levels for cytokines (IL, interleukin; TNF, tumour necrosis factor) in colon tissue were determined by real-time quantitative PCR and expressed as relative increase compared with control animals. Data are presented as the mean ± SEM (n = 5 to n = 7 in each group). *P < 0·05 versus the PBS-treated group.
Sirolimus suppresses Th17-mediated inflammatory responses in colon tissue
We next determined the immunoregulatory effect of sirolimus on Th17 cells in TNBS-induced colitis. The MLN cells were isolated and cultured with PMA and Con A for 48 hr. As shown in Figure 4(a), MLN cells from sirolimus-treated mice exhibited a marked reduction of IL-6 and IL-17A secretion compared with those of PBS-treated mice. Notably, there was a significant increase in the levels of TGF-β with sirolimus treatment (Fig. 4a). Meanwhile, MLN cells from sirolimus-treated mice showed obviously lower percentages of Th17 cells and expression of RORγt mRNA by flow cytometry and RT-qPCR, respectively, compared with PBS-treated mice (Fig. 4b,c).
Figure 4.
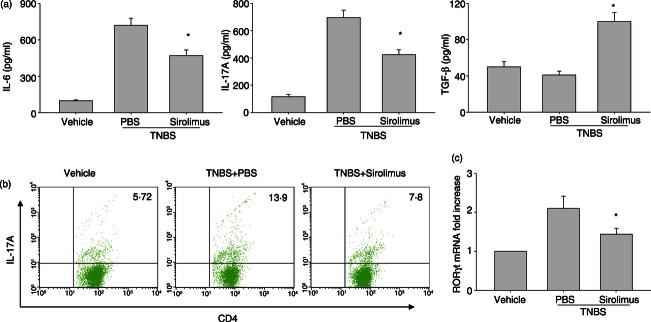
Sirolimus impedes T helper type 17 (Th17) -mediated responses of mesenteric lymph node (MLN) cells. Colitis was induced in male BALB/c mice using 2,4,6-trinitrobenzene sulphonic acid (TNBS) as described in the Materials and methods. (a) MLN cells from each group were isolated and cultured with PMA (10 ng/ml) and concanavalin A (Con A; 2 μg/ml) for 48 hr. Culture supernatants were harvested and assayed for cytokine production by ELISA (IL, interleukin; TGF, transforming growth factor). Data are presented as the mean ± SEM (n = 5 to n = 7 in each group). *P < 0·05 versus the PBS-treated group. (b) MLN cells were isolated from each group and subjected to intracellular interleukin-17A (IL-17A) staining. The percentages of CD4+ IL-17A+ T cells were determined by flow cytometry. Numbers display the percentages of IL-17A-expressing CD4+ T cells. The data are representative of three separate experiments and show similar results. (c) Messenger RNA levels of RORγt in MLN were determined by real-time qPCR and expressed as relative increase compared with control animals. Data are presented as the mean ± SEM (n = 5 to n = 7 in each group). *P < 0·05 versus the PBS-treated group.
Sirolimus expands CD4+ CD25+ Treg cells with suppressive ability in TNBS-mediated colitis
Regulatory immune cells such as CD4+ CD25+ T cells play a crucial role for the pathogenesis of both human IBD and the animal models.19,21 Hence, we further investigated whether the beneficial effect of sirolimus is associated with modulation of Treg cell function in TNBS-induced colitis. First, the cell populations of CD4+ CD25+ T cells in MLNs were analysed by flow cytometry. CD4+ CD25+ T cells are known to express high levels of Foxp3, a transcription factor that in a normal mouse is selectively expressed in CD25+ Treg cells.21 The number of CD25+ Foxp3+ Treg cells in MLNs from sirolimus-treated mice was significantly higher than that from PBS-treated mice (Fig. 5a). We next assessed the mRNA expression of Foxp3 in MLNs by RT-qPCR. Consistent with the results of flow cytometric analysis, mRNA expression of Foxp3 in MLNs from sirolimus-treated mice was markedly enhanced relative to that from PBS-treated mice (Fig. 5b). Furthermore, to clarify the function of Treg cells in MLN, a T-cell suppression assay was performed. Namely, CD4+ CD25− T cells from control mice were stimulated with plate-coated anti-CD3 monoclonal antibody and co-cultured with Treg cells from sirolimus-treated or untreated mice. As shown in Figure 6, the suppressive activity of Treg cells in MLN from sirolimus-treated mice was obviously stronger in comparison with that of PBS-treated mice.
Figure 5.
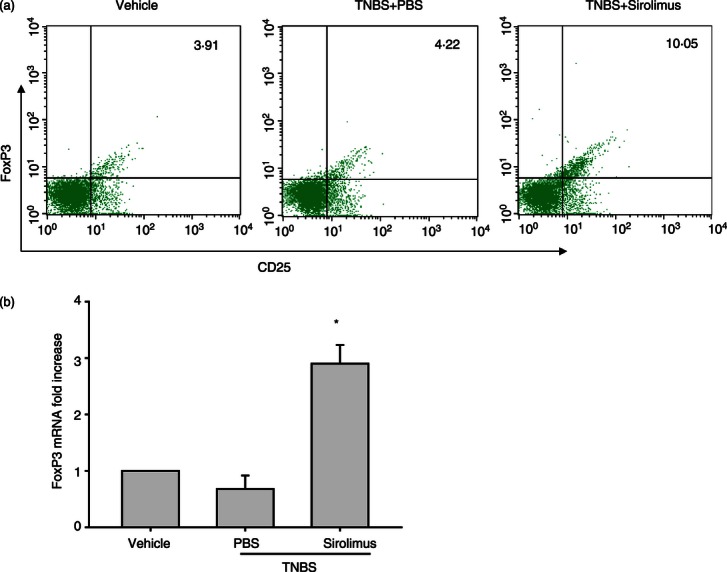
Sirolimus promotes the proliferation of CD4+ CD25+ regulatory T (Treg) cells in vivo. Colitis was induced in male BALB/c mice using 2,4,6-trinitrobenzene sulphonic acid (TNBS) as described in Materials and methods. (a) Mesenteric lymph node (MLN) cells were isolated from each group and subjected to intracellular FoxP3 staining. The percentages of CD4+ CD25+ T cells that are also positive for FoxP3 were determined by flow cytometry. Numbers display the percentages of FoxP3-expressing CD4+ CD25+ T cells. The data are representative of three separate experiments and show similar results. (b) Messenger RNA levels of FoxP3 in MLN were determined by real-time qPCR and expressed as relative increase compared with control animals. Data are presented as the mean ± SEM (n = 5–7 in each group). *P < 0·05 versus the PBS-treated group.
Figure 6.
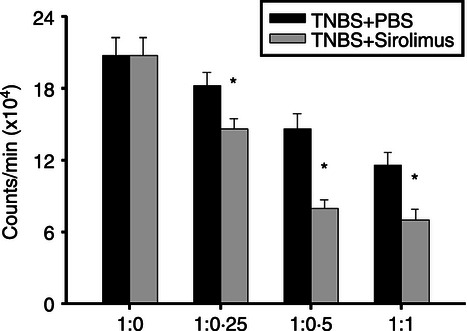
Sirolimus up-regulates the function of CD4+ CD25+ Treg cells derived from mesenteric lymph nodes (MLN). CD4+ CD25+ T cells were purified from MLN in sirolimus-treated or PBS-treated mice. The titrated CD4+ CD25+T cells were co-cultured with 2·5 × 105 CD4+ CD25− T cells as responder cells, which were obtained from PBS-treated mice in the presence of antigen-presenting cells (APC) and anti-CD3 antibody. The co-cultured cells were maintained for 48 hr and 1 μCi [3H]thymidine was added to the culture 18 hr before harvesting. The ratio of CD4+ CD25− T cells: CD4+ CD25+ T cells is indicated. Data are presented as the mean ± SEM (n = 4 in each group). *P < 0·05 versus the responder cell proliferation.
Discussion
The data in this study clearly indicate that the significant immunosuppressive capacities of sirolimus, an inhibitor of mTOR, in TNBS-induced colitis resulted from a prominent increase of the functional activity of CD4+ CD25+ Treg cells. The observed enhancement of the potency of Treg cells might additionally be the result of a differential down-regulation of pro-inflammatory signals of DC subsequently favouring the education of Treg cells. Furthermore, the beneficial effect of sirolimus was also involved in down-regulation of IL-17-producing T lymphocyte (Th17) response in the perpetuation of intestinal inflammation.
Recent compelling evidence demonstrated that Th17 cells play a crucial role in the induction of autoimmune diseases.11,34 On the contrary, Treg cells actively restrain the inflammatory response, suppress development of autoimmune diseases and dampen a wide spectrum of immune responses.8,9 The differentiation of naive Th cells into Th17 or Treg cells is mainly driven by cytokine milieu. For example, TGF-β is a critical differentiation factor for the generation of Treg cells35 and also directs FoxP3 expression, which is a specific marker in Treg cells and is responsible for the function of these cells.36 On the other hand, TGF-β, acting together with IL-6, induces the differentiation of pathogenic Th17 cells from naive T cells.37 In addition, Th cell differentiation is manipulated by distinct transcription factors. For example, STAT5 and FOXP3 direct Treg cell differentiation and induce the production of regulatory cytokines such as TGF-β and IL-10,38 and signal transducer and activator of transcription 3 (STAT3) and RORγt dominate Th17 cell formation and IL-17 production.39 Furthermore, the critical role of Th cell-intrinsic mTOR signalling, which regulates the differentiation between effector and Treg cells, has been well characterized.40 Inhibition of mTOR can modulate the expression of FoxP3, IL-17 and RORγt genes directly, which contribute to induction of FoxP3 and suppression of Th17 polarization.41 By inhibiting the mTOR signalling, sirolimus has been reported to promote Treg cell differentiation, proliferation and distribution42 and suppress the formation of Th17 cells.43 However, in inflammatory responses such as IBD, the role of mTOR inhibition in regulating Th17 and Treg cell differentiation has not been explored thoroughly. Here, we found that in the progression of TNBS-induced colitis, treatment with sirolimus, the inhibitor of mTOR, led to a significant increase in the percentage of CD4+ CD25+ Foxp3+ T cells in MLN and spleen (data not shown). Simultaneously, the number of CD4+ IL17A+ T cells and the expression of the RORγt gene in MLN were markedly decreased when compared with the untreated colitis group. This implies that the rehabilitating effect of inhibition of the activity of mTOR in IBD works through restoring the balance between Th17 and Treg cell differentiation.
Homeostasis of distinct Th cell subset-derived cytokines is important in intestinal mucosal immunity. An imbalance between pro-inflammatory and regulatory cytokines has been implicated in the pathogenesis of IBD, particularly Crohn's disease.4,9 Moreover, an increase in Th17 pro-inflammatory cytokines is also observed in patients with Crohn's disease, suggesting that Crohn's disease is closely related to a Th17-mediated disease.16 To expound the influence of mTOR inhibition on the production of Th17 and Treg cell-related cytokines, we cultured T-cell-enriched MLNs from different groups of mice with TNBS-induced colitis and determined the concentration of pro-inflammatory cytokines such as IL-6 and IL-17A and regulatory cytokine TGF-β. The MLNs from mice treated with sirolimus secreted lower concentrations of pro-inflammatory cytokines and produced higher levels of regulatory cytokines compared with cells from the untreated colitis group. As IL-17A is produced mainly by Th17 cells and TGF-β, acting as a major regulatory cytokine, is derived from Treg cells, these findings indicate that mTOR inhibition directs the production of regulatory cytokines and abrogates the production of IL-17A in the perpetuation of experimental colitis, in accordance with the expressions of FoxP3 and IL-17A in mesenteric lymph and colonic tissues. These results demonstrate that in intestinal inflammation, inhibition of the activity of mTOR by sirolimus manipulates the homeostasis of Th cell subgroups, which favours Treg cell function and inhibits the formation and activity of Th17 cells.
Pro-inflammatory cytokines, such as TNF-α and IL-6, contribute positively to the development of IBD and experimental colitis in animal models of IBD,4,44 and blockade of TNF-α and IL-6 bioactivity by specific antibodies such as infliximab and tocilizumab, respectively, can down-regulate the inflammatory response and limit the tissue damage of IBD and experimental colitis.7,45 As the production of TNF-α and IL-6 in inflamed tissues is driven by IL-17 but inhibited by the regulatory cytokines,19,46 we evaluated the effects of mTOR inhibition on the production of pro-inflammatory cytokines and other inflammatory parameters in TNBS-induced colitis. Our results showed that treatment with sirolimus markedly suppressed the expression of pro-inflammatory cytokines TNF-α and IL-6 in mesenteric lymph and colonic tissues. Intriguingly, sirolimus significantly inhibited TNBS-induced weight loss and reversed TNBS-induced shortening of the colon. Sirolimus also diminished the rectal bleeding index and attenuated the TNBS-induced reduction in haemoglobin levels. Moreover, microscopic histological analyses corroborated the macroscopic observations of sirolimus efficacy in protecting mice against TNBS-induced colitis. In particular, sirolimus dramatically suppressed oedema, reduced leucocyte infiltration and maintained mucosal integrity in TNBS-treated mice. These results apparently provide evidence of the therapeutic effect of sirolimus on experimental colitis and indicate that inhibition of the activity of mTOR is able to decrease the production of pro-inflammatory cytokines and disease parameters, thereby turning off the immune response of TNBS-induced experimental colitis.
In conclusion, the present study shows that pre-treatment with sirolimus, the inhibitor of mTOR, alleviated the perpetuation of TNBS-induced colitis. This amelioration was paralleled by promoting differentiation of Treg cells and inhibiting the generation of Th17 cells. Sirolimus treatment resulted in a significant histological improvement, protecting against mucosal ulcerations. This study suggests that sirolimus-based pharmaceutical strategies may offer a promising alternative to our current approaches of managing IBD.
Acknowledgments
The project was supported by Guangdong Natural Science Foundation (Grant S2012010009409) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry [No (2011)1139].
Disclosures
The authors declare no conflict of interest.
References
- 1.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 3.Ishihara S, Aziz MM, Yuki T, Kazumori H, Kinoshita Y. Inflammatory bowel disease: review from the aspect of genetics. J Gastroenterol. 2009;44:1097–108. doi: 10.1007/s00535-009-0141-8. [DOI] [PubMed] [Google Scholar]
- 4.Brown SJ, Mayer L. The immune response in inflammatory bowel disease. Am J Gastroenterol. 2007;102:2058–69. doi: 10.1111/j.1572-0241.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 5.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 6.Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182–97. doi: 10.1053/j.gastro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Waldner MJ, Neurath MF. Novel cytokine-targeted therapies and intestinal inflammation. Curr Opin Pharmacol. 2009;9:702–7. doi: 10.1016/j.coph.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–91. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–9. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 10.Mucida D, Salek-Ardakani S. Regulation of TH17 cells in the mucosal surfaces. J Allergy Clin Immunol. 2009;123:997–1003. doi: 10.1016/j.jaci.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–9. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- 12.Graber JJ, Allie SR, Mullen KM, et al. Interleukin-17 in transverse myelitis and multiple sclerosis. J Neuroimmunol. 2008;196:124–32. doi: 10.1016/j.jneuroim.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–93. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 14.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–11. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roncarolo MG, Battaglia M. Regulatory T cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–98. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 16.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham AH, Papadakis KA. Expression and functional characterization of FOXP3+ CD4+ Treg in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191–9. doi: 10.1002/ibd.20053. [DOI] [PubMed] [Google Scholar]
- 18.Kanai T, Mikami Y, Sujino T, Hisamatsu T, Hibi T. RORγt-dependent IL-17A-producing cells in the pathogenesis of intestinal inflammation. Mucosal Immunol. 2012;5:240–7. doi: 10.1038/mi.2012.6. [DOI] [PubMed] [Google Scholar]
- 19.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–71. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 20.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–70. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 21.Himmel ME, Yao Y, Orban PC, Steiner TS, Levings MK. Regulatory T-cell therapy for inflammatory bowel disease: more questions than answers. Immunology. 2012;136:115–22. doi: 10.1111/j.1365-2567.2012.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Chen L, Niu X, Liu J, Ping M, Li R, Xie X, Guo L. Immunomodulatory synergy by combining atorvastatin and rapamycin in the treatment of experimental autoimmune encephalomyelitis (EAE) J Neuroimmunol. 2012;250:9–17. doi: 10.1016/j.jneuroim.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Shin HJ, Baker J, Leveson-Gower DB, Smith AT, Sega EI, Negrin RS. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood. 2011;118:2342–50. doi: 10.1182/blood-2010-10-313684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–11. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS ONE. 2009;4:e5994. doi: 10.1371/journal.pone.0005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–9. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 27.Levitsky J, Gallon L, Miller J, et al. Allospecific regulatory effects of sirolimus and tacrolimus in the human mixed lymphocyte reaction. Transplantation. 2011;91:199–206. doi: 10.1097/TP.0b013e318200e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bocian K, Borysowski J, Wierzbicki P, et al. Rapamycin, unlike cyclosporine A, enhances suppressive functions of in vitro-induced CD4+CD25+ Tregs. Nephrol Dial Transplant. 2010;25:710–7. doi: 10.1093/ndt/gfp586. [DOI] [PubMed] [Google Scholar]
- 29.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–8. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 30.Singh K, Kozyr N, Stempora L, Kirk AD, Larsen CP, Blazar BR, Kean LS. Regulatory T cells exhibit decreased proliferation but enhanced suppression after pulsing with sirolimus. Am J Transplant. 2012;12:1441–57. doi: 10.1111/j.1600-6143.2011.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinecke K, Eminel S, Dierck F, et al. The JNK inhibitor XG-102 protects against TNBS-induced colitis. PLoS ONE. 2012;7:e30985. doi: 10.1371/journal.pone.0030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai A, Lu N, Guo Y, Liu Z, Chen J, Peng Z. All-trans retinoic acid down-regulates inflammatory responses by shifting the Treg/Th17 profile in human ulcerative and murine colitis. J Leukoc Biol. 2009;86:959–69. doi: 10.1189/jlb.0109006. [DOI] [PubMed] [Google Scholar]
- 33.Yin H, Li X, Gong Q, et al. Heme oxygenase-1 upregulation improves lipopolysaccharide-induced acute lung injury involving suppression of macrophage migration inhibitory factor. Mol Immunol. 2010;47:2443–9. doi: 10.1016/j.molimm.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–73. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 37.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 38.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 39.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;26:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 40.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–38. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7:1819–24. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–37. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delgoffe GM, Pollizzi KN, Waickman AT, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878–82. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 45.Baert F, Noman M, Vermeire S, Van Assche G, D' Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601–8. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 46.Siakavellas SI, Bamias G. Role of the IL-23/IL-17 axis in Crohn's disease. Discov Med. 2012;14:253–62. [PubMed] [Google Scholar]


