Abstract
Three families of membrane-active peptides are commonly found in nature and are classified according to their initial apparent activity. Antimicrobial peptides are ancient components of the innate immune system and typically act by disruption of microbial membranes leading to cell death. Amyloid peptides contribute to the pathology of diverse diseases from Alzheimer's to type II diabetes. Preamyloid states of these peptides can act as toxins by binding to and permeabilizing cellular membranes. Cell-penetrating peptides are natural or engineered short sequences that can spontaneously translocate across a membrane. Despite these differences in classification, many similarities in sequence, structure, and activity suggest that peptides from all three classes act through a small, common set of physical principles. Namely, these peptides alter the Brownian properties of phospholipid bilayers, enhancing the sampling of intrinsic fluctuations that include membrane defects. A complete energy landscape for such systems can be described by the innate membrane properties, differential partition, and the associated kinetics of peptides dividing between surface and defect regions of the bilayer. The goal of this review is to argue that the activities of these membrane-active families of peptides simply represent different facets of what is a shared energy landscape.
Keywords: cell-penetrating peptides, antimicrobial peptides, amyloid, lipid biophysics, membrane protein folding
Introduction
Membrane:protein interactions are routinely defined in terms that suggest a passive role for membrane structure. BAR domains bend membranes to defined curvatures.1 SNARES are responsible for inducing membrane fusion.2 Cell-penetrating peptides, as their names state, penetrate the membrane.3 Antimicrobial peptides insert into the membrane bilayer.4 Amyloid peptides porate membranes.5 These protein-centric phrases reveal a dogma that membrane proteins are the active species whose folding and self-assembly drive the gains of membrane function. In contrast, the bilayer is viewed as a passive substrate for formation of these proteinaceous structures.
Phospholipid membranes, however, are not passive.6 The effects of Brownian dynamics result in spontaneous thinning and the continuous formation and dissipation of membrane microdefects. Larger scale effects such as pore formation, fusion, and fission are also possible. In pure artificial membranes, these thermal fluctuations are sufficiently minor that they can be taken as irrelevant to biological processes. However, alterations of the energetic landscape of the lipid bilayer by the binding of protein can cause these transient defects to contribute significantly in changing membrane structure.
In this review, we describe an alternative universe in which membrane perturbation and protein interactions are cooperative phenomena.7 Specifically, we examine recent and not-so-recent reports regarding the interaction of short amphiphilic peptides with biological membranes. These interactions cover the physical basis of cytotoxicity caused by preamyloid toxins (PAT) in many amyloid diseases including Alzheimer's and type II diabetes.8 They are also addressed by the expansive field of antimicrobial peptides (AMP), a prominent subdiscipline of innate immunity.4 Understanding of their properties and mechanism is vital given the increasing emergence of antibiotic resistant pathogens. Lastly, these interactions are critical to the behavior of cell-penetrating peptides (CPP) whose capacity to transport cargo or gain access to intracellular compartments is central to their activity.9 This defining property of CPPs is finding increased importance as these proteins are being developed as delivery vehicles for cargos as diverse as small molecule drugs to oligonucleotides for gene therapy.3 We aim to show that these three seemingly disparate systems are simply subsets of a common physical chemistry.
General Properties
A well defined and large subset of amyloid proteins, antimicrobials, and cell-penetrating peptides are amphipathic, short (<50 amino acids), and readily soluble in both aqueous and membrane environments (Fig. 1).3,4,8 They are typically unstructured in solution,13–15 but undergo a disorder-to-order transition upon membrane association which promotes either α-helical or β-strand secondary structural states that are generally amphipathic in nature.5,15,16 This amphipathic character allows the structures to bind membranes such that the peptide initially intercalates between lipid headgroups.
Figure 1.
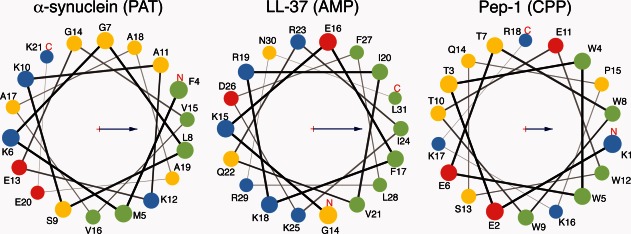
Helical wheel diagrams for three representative peptides discussed in this review. These are α-synuclein,8 which forms a PAT associated with disease progression in Parkinson's disease, LL-37,10 a human AMP, and Pep-1,11 a designed CPP. Members of these three classes of peptide generally possess an amphipathic structure, as seen here, with nonpolar and polar/charged residues segregated on opposite faces of an α-helix. This allows peptides to partition onto membranes, with the nonpolar regions residing in the acyl core and the polar regions contacting the lipid headgroups and solvent. Green circles represent nonpolar residues, yellow polar uncharged residues, red acidic residues, and blue basic residues. Arrows represent the hydrophobic moment of the shown helix. Helical wheel diagrams produced using MPEx.12
These initial adsorption events usually result in peptides orienting parallel to the plane of the bilayer with the polar side of the helix exposed to the lipid headgroups and solvent, while the nonpolar side is exposed to the membrane acyl core.17,18 Preformed amphipathic oligomeric structures may also bind via a similar mechanism.19 The orientations of these binding events have been measured in many systems using a variety of tools. For example, the orientation of AMP cecropin A was found to be parallel with the surface of a phospholipid bilayer by solid state NMR.20 Similarly, EPR measurements of membrane-bound amyloid peptides α-synuclein (aSyn) and islet amyloid polypeptide (IAPP) indicated an orientation parallel to the bilayer surface.17,21 In contrast, a combined SFG vibrational spectroscopy and ATR-FTIR spectroscopy study indicated that the orientation of CPP Pep-1 was perpendicular to the bilayer at low peptide concentration.22 The degree of membrane curvature has been shown to alter the orientation of bound peptides,23 leading to multiple binding modes or even a dynamic switching between alternate modes.24 For larger proteins, and for proteins such as CPPs carrying cargo, these initial binding events are suggested to be performed by a subdomain of the larger protein.13,25
A set of coupled phenomena is associated with the membrane interactions of these peptides, including: lateral adsorption/desorption (see above), oligomerization, leakage, and translocation. Oligomerization is typically identified indirectly. Membrane adsorption often leads to a change in secondary structure, which can be observed by circular dichroism or IR spectroscopy.26–28 This allows measurement of concentration-dependent membrane binding affinity, which can be taken as evidence of oligomerization.26 Alternatively, direct measures of oligomerization can be made. Several pore-like structures have been directly observed using atomic force microscopy.29 Direct visualization of oligomers30 or puncta31 on both cells and artificial membranes has also been observed using fluorescently labeled protein. Finally, neutron scattering,32 X-ray diffraction,33 or single-particle FRET34 of pores or oligomers in membranes has given direct evidence of protein–protein interactions on membranes.
Membrane leakage induced by these peptides has been observed for both artificial membranes and live cells and has given some insight into the mechanism of membrane disruption. Measurements of delayed membrane leakage show that the amyloid protein IAPP forms membrane pores that are indefinitely stable,35 eliminating transient mechanisms of permeabilization such as detergent or carpeting. Similarly, leakage of dyes or fluorescent quenchers from synthetic liposomes showed AMP cecropin A to induce leakage in an all-or-nothing fashion,36 while CPP transportan 10 causes gradual, graded leakage from all liposomes exposed to the peptide.37 Several amyloid proteins also give conductance recordings with discrete levels and clear transitions similar to bona fide ion channels.38,39 Such observations have also been made for the AMP protegrin-1.40,41 Finally, in vivo leakage has been observed for several peptides through the introduction of intracellular calcium sensitive fluorophores42 or through the measurement of efflux of intracellular or mitochondrial proteins such as lactate dehydrogenase43 or cytochrome c.44
Translocation, as a property of state, simply represents equilibration across a bilayer. As a phenomenon observed with this class of peptides, it captures attention as a result of the presence of charged moieties on the peptides which would not normally be expected to cross the hydrophobic layer. The capacity of cationic peptides and proteins to cross a bilayer in a cellular system was first observed in 1965 using polyarginine and histones.45 This work foreshadowed the discovery of CPPs (see below). Thus, it has long been recognized that while membranes may be relatively impermeable to inorganic cations, amphipathic structures have a strong capacity to be membrane-active and translocate.
This review is predicated on the idea that within the literature, differences within and between AMP, CPP, and PAT activities are simply the result of differences in each protein's intrinsic capacity to bind, desorb, oligomerize, permeabilize, and translocate across a given membrane. Similarly, within a given system, changes in behavior are noted and assigned to changes in properties resulting from altered membrane composition. In AMPs, for example, enhanced anionicity of bacterial cell membranes is often cited as a possible origin for selective toxicity toward a foreign organism relative to a host.46 Membrane compositions are also invoked to account for gains of toxic function in amyloid proteins. For example, IAPP has long been known to form channels in membranes with greater anionic lipid content and less likely in membranes with cholesterol.39 Likewise, many well-studied CPPs have been observed to have a higher propensity to assume secondary structural motifs in the presence of lipid vesicles of greater anionic composition.47,48 Membranes differ between organisms and can even change as an origin or result of disease.49 These differences allow for changes in protein–membrane interactions and can lead to dramatically different behaviors between proteins or even for the same protein in alternate environments. Thus, the constellation of behaviors described here may be related by a common set of physical principles.
Systems
Amyloid peptides
Amyloid peptides are a class of protein characterized by their ability to form stable β-sheet-rich structures known as amyloid fibers.50 Amyloid proteins play a role in many functional biological processes including biofilm formation51 and hormone storage.52 There are also a wide variety of diseases in which amyloid proteins play a central role, including Alzheimer's disease (AD), Parkinson's disease, and type II diabetes mellitus (TDM).5,8 Here, we focus on the subset of peptide amyloids that lead to cellular toxicity, such as amyloid beta (Aβ) in AD, α-Syn in Parkinson's, and IAPP in TDM. Historically, considerable research was focused on the fibrillar state as central to disease progression. More recently, however, it has been shown that the toxic species of amyloid proteins are nonfibrillar PATs, with the mature fibers being relatively benign.8
The discovery that amyloid proteins can be membrane permeabilizing in synthetic systems has led to the hypothesis that membrane disruption may be a significant contributor to pathology. Indirect evidence for a role for prefibrillar states had long been in evidence. For example, familial forms of AD show alterations in brain amyloid plaque burden that is not well correlated with dementia.53 In cell culture, it has been shown that addition of amyloid precursor to culture media is far more toxic than addition of preformed fibers.54 With such studies serving as backdrop, the discovery that PATs could induce membrane disruption in cells and in synthetic liposomes has drawn considerable interest.5,8
Amyloid precursor proteins have been observed to form a number of unique, nonamyloid structures on membranes. Aβ was observed to create a constellation of oligomeric structures, ranging from dimers to large oligomers.30 IAPP was similarly observed to oligomerize into puncta on cell membranes.54 Importantly, the development of a single antibody selective for the amyloid oligomers of a variety of different source proteins55 suggests there is a commonality of oligomer structure shared among different amyloid proteins.
A number of studies have now identified structures that are unique to preamyloid states on membranes. The prefibrillar membrane-bound states of many amyloid proteins, including Aβ,56,57 IAPP,13,21,26 and aSyn,17,58 have been found to be α-helical. This is surprising as these states often precede rapid formation of β-sheet filaments. These helices are generally slightly cationic, and membrane binding is usually driven by electrostatic effects.59–61 A number of tools have been used to implicate the role of these structures in disease. In AD, for example, small molecules designed and demonstrated to interact with the helical portions of Aβ are cytoprotective.62 A folded, helical tetramer of aSyn has also been observed to be capable of both binding membranes or existing freely in solution.19 In our own work with IAPP, we have shown that structural mutants affecting helix formation eliminate cytotoxicity in insulin secreting cell lines.63 Single-pair FRET measurements of IAPP have also shown the presence of a small family of membrane-bound antiparallel dimers.34
The connection between α-helical structures and the downstream formation of β-sheet rich amyloid is challenging to reconcile. One possible pathway between those species is offered by the structure determination of an oligomer formed by a fragment of the amyloid protein αβ crystallin (ABC).64 This peptide forms an oligomer from six antiparallel β-strands with a greasy, closed central cavity. This structure offers a link between small, membrane-bound oligomers and amyloid and is suggestive of a pore in a manner similar to those identified at low resolution using AFM.29 These do not yet, however, show that the pore-like shape is the basis for these molecules capacity to act as a toxic agent. Plainly, however, it illuminates one possibility of how PATs may interact with a membrane to induce a well-defined pore.
Bilayer permeation can additionally be driven by interactions which induce distortions and other disequilibria upon initial binding of protein to membranes.7 Amphiphiles, such as PATs, can act as detergents removing one leaf of a lipid bilayer. Relaxation of this condition requires flipping of phospholipids from the unaffected leaf to the damaged leaf in a process that can be coincident with leakage. A second possibility is the induction of membrane distortion as a result of a preference for specific membrane curvatures. Here, a portion of binding energy is available to distort membrane geometries that deviate from the protein's optimum. Curvature preferences that strongly affect lipid distributions are increasingly being observed in many proteins, such as cholera toxin subunit B.65 Several PATs, including IAPP,66 Aβ,67 and aSyn,68 have demonstrated preferences for membrane regions of negative or increased positive curvature. As with detergent, distortions require a relaxation of phopholipid from one leaf to another offering an opportunity for leakage events. Structure- and detergent-induced strain could thus be a general mechanism of poration by these peptides. Importantly, these mechanisms allow for leakage that is not dependent on specific proteinaceous structures (Fig. 3, see below).
Figure 3.
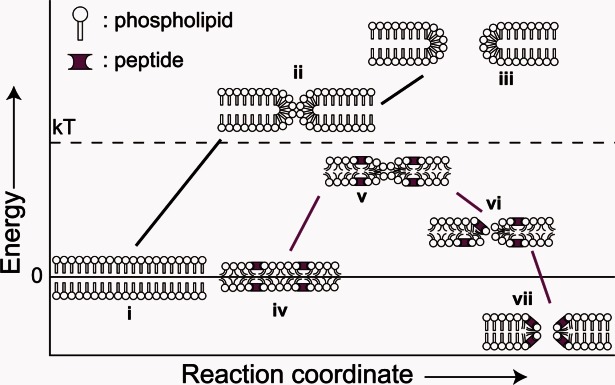
Schematic of the effect of protein binding on lipid bilayer integrity. (i) The reference state for energy change is an intact phospholipid bilayer. (ii) Spontaneous fluctuations result in the sampling of membrane defects. These are energetically unfavorable and therefore sampled infrequently. (iii) Widening of the defect to permit leakage results in a further energetic penalty. (iv) In the presence of surface-bound protein (magenta), membrane tension is induced. (v) Protein binding increases the frequency of defect formation. (vi) Surface tension is released by pore formation89 and stabilized by peptide binding resulting in equilibrium poration (vii). Note, many forms of defect, such as chaotic pores,36 can be accommodated by this model, and defect characteristics may differ between alternate peptides or the same peptide under alternate conditions.
It is clear from recent reports that PATs act on cells as a consequence of several gains of function. This includes CPP-like and/or antimicrobial gains of function such as in IAPP and Aβ.31,69–71 Given the much larger published set of insights and systems investigated for AMP and CPP properties, it is clear that the PAT community has much to gain by considering those disciplines' effort.
Antimicrobial peptides
Antimicrobial peptides are an ancient component of innate immunity acting to protect against invading pathogens. Insects, for example, ward off bacterial infection without lymphocytes or antibodies, in part, because they possess an arsenal of protective AMPs.72 These peptides are often toxic to a broad spectrum of bacteria, but are relatively inactive toward host eukaryotic cells at bactericidal concentrations. To date, more than 1200 peptides have been identified or predicted to be AMPs.73
A fundamental characteristic of nearly all AMPs is that they are shorter than 50 residues in length and rich in cationic and hydrophobic amino acids.72,74 Among these, the majority show a transition from disorder to order upon encountering a membrane.4,72,74 Many linear AMPs, such as cecropin, magainin, and melittin, form amphipathic helical structure in the presence of lipid bilayers.75 Increasing evidence, however, suggests it is the amphipathic nature of peptides, and not the specific structural motifs they adopt, that is responsible for biological and membrane activity.7
AMPs are thought to selectively target bacterial membranes because of an electrostatic attraction to negative charges originating from lipopolysaccharides and a higher abundance of phosphatidylglycerol and cardiolipin.76 However, this form of targeting may not completely explain selectivity as many nondiscriminant toxins also have high net positive charges.77,78 Alternatively, the overall hydrophobicity of AMPs and toxins may be a contributing factor to selectivity. Several studies have established that increased hydrophobicity results in enhanced cytotoxicity but does not lead to enhancement of antimicrobial activity.79–81 These observations indicate a delicate balance of amphipathic properties are essential for the design of potent and selective AMPs.
AMPs utilize a variety of mechanisms to kill bacteria. Poration of a membrane can result in cell death because of simple leakage of key components of the cell. In some cases, membrane disruption may serve instead as a precursor event. Translocation of the AMP through the membrane to access intracellular targets, for example, is a putative pathway for dermaseptin and histatins. These AMPs act by inhibiting protein and DNA synthesis or enzymatic activity.4,82 Additionally, pore formation can result in a disruption of the electrochemical balance across the lipid membrane (e.g., ion channel formation).4,82 Diverse models of membrane poration, including toroidal and barrel stave pores and carpeting, have been suggested for various AMPs,74 and it is possible that an individual AMP may utilize different mechanisms depending on experimental conditions (see below).
The mechanisms of a small group of AMPs have been identified based on measurements of the structure and orientation of peptides as well as the lipid membrane. Fluorescence and FRET-based experiments suggest helical peptides cecropin P1 and dermaseptin employ a carpeting mechanism, where bound amphipathic proteins act as detergents and solubilize membrane patches into proteolipid micelles.83,84 Magainin 2 was observed to form toroidal pores based on pore size and peptide orientation measured by oriented circular dichroism and neutron in-plane scattering.85 Comparatively, protegrin-1 forms toroidal pores based on the orientation of the peptide and lipids measured by solid-state NMR spectroscopy.86 Helical alamethicin is currently the only known antimicrobial peptide to embody the barrel-stave mechanism, where a peptide-lined pore is formed with lipid chains exposed to the hydrophobic faces of the transmembrane peptides. This was definitively investigated by X-ray diffraction, neutron in-plane scattering, and scanning tunneling microscopy.32,33,87 Finally, cecropin A and magainin 2 at low concentrations have been observed to induce leakage through a so-called chaotic pore, where proteins induce a dramatic but temporary leakage state that rapidly re-seals.36,88 These pores may resemble toroidal pores, though there is no requirement that these pores be well formed or stably lined with peptide.
Some AMPs have mechanisms that have been categorized in more than one way, indicating ambiguity when associating peptides with specific processes. Melittin, for example, a helical peptide toxin and AMP from honeybee venom, has been categorized as alternatively utilizing toroidal pore, barrel-stave, and carpeting mechanisms.89–91,92 Differences in experimental parameters such as peptide concentration, protein:lipid (P:L) ratio, and membrane composition may account for the variety of observations for a single peptide. In fact, the original segregation of AMPs, toxins, and other membrane-active peptides into separate categories may be attributable to variations in experimental parameters, which can greatly affect poration capacity and mechanism.7,93
Cell-penetrating peptides
Cell-penetrating peptides and proteins are systems with the intrinsic capacity to translocate across biological membranes. This capacity was first noted indirectly in 1965 as a property of polyarginine and histones applied exogenously to cell culture.45 A relationship of this property to protein folding was noted in 1984 where it was shown that apocytochrome C could spontaneously translocate across a bilayer in a synthetic liposome system.94 As a discipline, work on CPPs emerged as a result of the observation that systems retained these properties even when attached to other materials, termed cargo. The first peptide to show this capacity was the polycationic peptide from HIV, TAT. Numerous short CPPs have now been identified that are either naturally occurring or have been engineered.3,95
The range of potential cargo carried by CPPs is enormous, ranging from small molecules to polyanionic stretches of DNA. It is this capacity of CPPs that captures the imagination, as frequently cargo is dramatically larger than the CPP and/or highly membrane impermanent. As such, these proteins represent a potentially versatile mechanism for intracellular delivery appropriate for basic scientific research, translational research, and therapeutics. Indeed, over 20 clinical trials are currently underway in which a CPP forms an element of the delivery.3,95
The physicochemical properties of CPPs sound all too familiar to those working on AMPs and PATs. CPPs are typically polycationic. In one remarkable study, GFP was engineered so that the external surface carried a net +36 charges.96 This system has an ability to carry cargo far more efficiently than commonly used peptide systems such as penetratin. More typical, however, are smaller peptide-based CPPs that are intrinsically disordered in solution and adopt defined structures on biological membranes.
The mechanisms of CPP action are generally divided into two categories: those that are dependent on cellular processes, such as endocytosis and macropinocytosis, and those for which an active cellular process is not required for cargo delivery. As physical chemists, we view this distinction differently. Lysosomal escape represents a CPP that is affected by the acidification and/or crowding of its compartment. An illustrative example is the peptide pHlip, which includes a centrally located anionic residue. Cargo delivery with this peptide leverages its requirement for a drop in pH which neutralizes the buried charge.97
Structure formation by CPPs is a necessary component in the assessment of their properties. It is tempting to look to the high efficiency of cargo delivery by supercharged GFP as validation of the central role of cationic character. However, that system requires endocytosis as a prerequisite for CPP function. Standing in contrast is a combinatorial library of protein segments that was evaluated for CPP function in a cell free liposome system.98 One of the many remarkable features of this screen is that it allowed identification of sequences that could translocate cargo without an energy source and without induction of leakage. The 10,000 member library included sequences with as many as nine arginines and yet the most efficient hits carried as few as two and three positive charges. It is this observation that offers a route to connecting CPP mechanism with the CPP-like behavior observed for PATs. The sequences of PATs often contain only limited stretches of cationic residues. For example, IAPP has only three to four positive charges at neutral pH.
As membrane activity is related to the amphipathic nature of peptides, AMPs, CPPs, and PATs are likely mechanistically linked. Some AMPs, for example, are rich in one or two amino acids, a common characteristic of CPPs, and are capable of delivering cargo to cellular compartments.99,100 Likewise, amyloidogenic peptides are often short and amphipathic, with hydrophobic stretches that sample membrane bound structures8 indicating strong overlap in these, often regarded as disparate, fields.
A Membrane Deformation Model of Poration
The close physicochemical properties of AMPs, PATs, and CPPs makes it surprising that most biophysical work has been done in intellectual isolation. This has occurred in part because of the broad range of seemingly unrelated phenotypes that have been observed. The result is a bewildering array of proposed mechanisms. Carpeting, toroidal pores, barrel-stave pores, detergent effects, and additional models such as the sinking-raft model101 have been put forward, each with robust data to support it.
Direct observations of pore-like objects using EM and X-ray techniques have been made by several groups.29,64 Others, including ourselves, have used indirect measures of leakage.26,42,102 We observed, for example, that membrane leakage induced by IAPP has a high reaction order and nucleation-dependent formation of the leakage competent species.35 High reaction order is typically ascribed to protein oligomerization. We were therefore surprised to observe that mixtures of d- and l-enantiomeric IAPP behaved indistinguishably from single enantiomeric conditions (Fig. 2).69 Such an observation is difficult to rationalize with protein-specific pore formation. This and related observations have inspired us and other groups to look for a small set of governing principles which can instead manifest as the myriad of mechanisms listed above.
Figure 2.
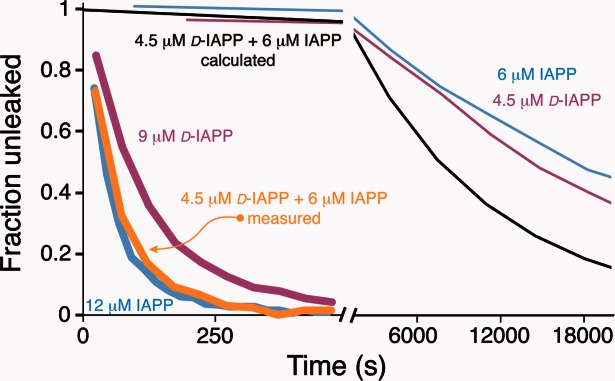
Cross-cooperative membrane leakage induced by l- and d-enantiomers of amyloid protein IAPP. Liposomes were prepared so as to encapsulate 70 kDa fluorescently labeled dextrans. These were incubated with the indicated concentration of protein for 48 h. Membrane integrity was then interrogated through the extraluminal introduction of the fluorescence quencher DPX, which transits into the liposome and quenches the entrapped fluors. Doubling the concentration of IAPP or d-IAPP individually (blue bold, purple bold) leads to an ∼100-fold increase in leakage rate constant over the initial concentrations (blue thin, purple thin). Instead, mixing equal activities of the two peptides brings about the same highly cooperative increase in leakage rate (orange). This observed leakage is more than an order of magnitude faster than the expected leakage rate if the peptides acted noncooperatively (black). Figure adapted from Ref.69.
One such set of principles focus on the membrane as an active participant in the formation of pores.6,7,89 Consider a phospholipid bilayer, above its melting transition and at equilibrium [Fig. 3(i)]. This is not a static state. Transient thermal fluctuations lead to localized membrane thinning and rare formation of pores [Fig. 3(ii,iii)]. The latter are highly unfavorable and rapidly vanish without inducing any leakage of membrane contents or, indeed, any significant contribution to solute permeation rates. While few would argue that this cannot be true, most would argue that the contribution of such phenomena to biological manifestations are rarely, if ever, significant.
Membrane-bound peptides can, however, alter the energetic landscape, allowing the stabilization of these membrane fluctuations. A framework has already been suggested for several AMPs [Fig. 3(iv–vii)]103–105 and we view such mechanisms as broadly capable of accounting for binding, pore formation, and translocation across PATs and CPPs as well.
The starting point for such a model is that peptide binding causes displacement of lipid headgroups without a concomitant expansion of the acyl chains.89,106 This leads to thinning of the bilayer107 and the induction of a surface tension due to the resultant nonideal packing of the lipid acyl chains.89 The presence of tension decreases the energetic penalty of pore formation, as formation of a pore will decrease the overall membrane surface area, allowing a fraction of the acyl chains to return to their preferred conformations. The combination of binding and surface tension increase the rate of stochastic poration, which can be nucleated by a combination of thermal fluctuations108 and local defects at the site of bound proteins.6
It is useful to first consider insights that are illustrated using membrane-only liposome systems. Deviation from equilibrium can be introduced into model systems using transient intense light illumination. The energy of a pore relative to a pore-free liposome in such a system varies with its radius [Eq. (1)]109,110:
| (1) |
E(R) is the energy of the system for a pore of radius R, σ is the membrane surface tension, and γ is the line tension. The term containing σ represents the loss of surface tension energy that results from forming a pore of radius R. The term containing γ represents the energy gained per unit circumference of an ideal circular pore due to nonideal packing around the pore rim. Line tension can be thought of as the energetic penalty caused by poration due to nonideal packing of lipids around the pore rim.111 Regardless of shape, line tension is the first derivative of the sum of energetic contributions to the stabilization of the edges of the pore.
At small radii (or more precisely R < γ/σ), the linear term is dominant. Line tension forces exceed forces due to surface tension release and the pore rapidly reseals and vanishes. Beyond a critical radius (R > γ/σ), pores move down their energy gradient, rapidly expanding until the liposome ruptures.
A critical change can occur in such a process if one simply asserts that amphipathic proteins bind differentially to the surface of a membrane compared to the rim of a pore. Many membrane-active peptides have been observed to both induce a curvature strain in membranes as well as preferentially bind to regions of higher curvature, as would be experienced in the interior of a toroidal pore. Observation of alterations in membrane phase transitions have shown a curvature preference for AMPs magainin 2112 and LL-37,113 IAPP,66 and the HIV TAT CPP.114 The binding of detergent-like proteins to a pore rim may also decrease the line tension, leading to more favorable lipid packing along the rim, and stabilizing the pore.111 In addition, cooperativity of protein binding to pore structures has been observed via oriented circular dichroism. When no pores are present, all bound proteins are observed to lie with their α-helices parallel to the plane of the membrane.107 Upon crossing a minimum concentration threshold, protein begins to reorient into the membrane's pore state. Once this threshold is passed, additional protein is now able to cooperatively orient into the pore. For example, at high concentrations, the AMP alamethicin orients completely perpendicular to the membrane, with all the protein that was initially surface bound in the pore state.115 Cooperative stabilization of pores by membrane-active peptides can thus allow otherwise transient membrane defects to be long lived.
This idea has been modeled to show that protein stabilization of a pore can relieve both line and surface tension.89 Protein binding results in negative feedback, where membrane tension drops as a pore expands due to loss of protein to the pore itself.106 In addition, protein binding to the rim of a nascent pore can relieve line tension and induce stabilization of a pore that would otherwise reseal. This provides an additional mechanism by which the apparent rate of pore formation is increased with protein concentration. Overall, the model creates a local energy minimum for a pore of radius greater than zero (Fig. 3), by allowing for the formation of a stable, tension-induced proteolipid pore.
A common model of membrane poration for PATs, AMPs, and CPPs can be put forward based on these physical chemical principles. Upon initial exposure, amphipathic peptides partition into the exposed face of a lipid bilayer, inducing a membrane tension [Fig. 3(iv)]. The membrane will initially be in a high energy but unporated state. While in a tense state, stochastic transient poration will occur [Fig. 3(v)]. Localized distortions caused by bound monomeric protein, or more dramatically by large oligomers, may catalyze this process. Sufficiently small pores will rapidly reseal. Pores larger than the previously described critical radius, or pores that are stabilized by stochastic binding of a line tension relieving peptide, may be stabilized and grow [Fig. 3(iv)]. This expansion will be met by further protein binding with concomitant curvature stabilization and tension release. Expansion will continue until the net membrane tension matches the line tension, resulting in a stable pore [Fig. 3(vii)].
Pores themselves are dynamic entities with sizes (in protein units) that vary in response to variation within and across the leaves of the bilayer. Above a critical P/L ratio, the porated state will be at a global energy minimum and therefore be stably present as has been often observed.89 Below this critical point, pores still form and can be transiently stabilized, but with lifetimes that are dictated by the interplay of line-binding and dissociation rates. A further evolution of pores has also been observed for several systems including IAPP,35 magainin 2,116 and a fragment of the Bax protein,117 an amphipathic pore forming peptide important in mammalian apoptosis. In these systems, pores have been observed to shrink from an initial highly leaky state to slower equilibrium leakage. These effects are likely a result, in part, of the equilibration of protein across the leaves of the bilayer. Indeed, we view CPP behavior to represent a coupling of pore formation with such translocation. In the case of unleaking CPPs, cargo would occupy the transiently formed hole thereby diminishing or eliminating leakage. Such a model would also account for the capacity of CPPs to transport cargo with a broad range of physicochemical properties.
A vital characteristic of the tension-based mechanism is that it is not dependent on any specific protein–protein interactions; the driving force for poration is the total tension that the membrane is under and the ability of individual peptides to stabilize the pore rim. As membrane tension is a distributed phenomenon across the entire liposome, tensions from different bound proteins sum on the membrane regardless of whether they share common primary structures. As a result, unrelated tension-inducing peptides would act cooperatively in the induction of a tension-based pore (Fig. 2).69 Protein–protein interactions may occur, and could contribute significantly to the nucleation or overall energy of some pore systems, but are not obligatory for the initiation and stabilization of pores.
Relationship to other mechanisms
Surface and line tension serve as useful underpinning for the breadth of proposed mechanisms and structures. The changes in energy that contribute to this breadth come from a small number of sources: (1) The energy associated with disorder <-> order transition upon membrane binding which may be favorable or unfavorable depending on whether the protein is surface or pore bound. (2) The energy associated with relocating protein from surface to pore. (3) The change in energies (1) and (2) that are dependent on the degree of self-assembly of the protein. Note that protein self-assembly may include strongly specific protein–protein interactions or may simply be described as colocalization resulting from a phase transition from a surface-bound to a pore-bound phase.
Lipid-lined toroidal pores, proteinaceous barrel-stave pores, and less well-defined chaotic pore dispersions of peptides and lipid headgroups can all provide a framework for membrane disruption. The specifics of the structures are less important than the capacity of the structures to stabilize curvature or decrease surface tension upon withdrawal of the constituent peptides into the pore state. For several systems, there is contradictory support for alternative structures. For example, the venom melittin has been observed to be pore forming in neutral vesicles118 but induce leakage via a detergent mechanism in anionic vesicles.91 Similarly, magainin 2 has been reported as a toroidal structure at high concentration85 and a chaotic pore at low P/L.88 The alternative possibilities can simply reflect a change in the balance of energies that satisfy the reduction in surface tension that occurs when the pore is bound. A compact toroidal structure formed from a discrete number of peptides might be favored at high concentration because tension and protein concentration is sufficiently high to bind and maintain a stable pore structure. In contrast, at low concentrations, a chaotic assembly may arise from transient stabilization of unstable pore states by the binding and release of peptides.
For both equilibrium and nonequilibrium leakage, a central practical distinction is the delineation between graded leakage and all-or-none. An ensemble of liposomes showing a net 50% leakage could reflect all liposomes with 50% of their contents leaked or 50% of liposomes fully leaked. The latter represents a change of state from an impermeable to a leakage competent state.35,36,116,118 Such observations can be associated with either transient or persistent pores, depending on specific experimental conditions such as protein concentration.35,116 Above a critical concentration and after equilibration, pore-like states are persistent. At lower concentrations, membranes can undergo transient cycles of poration, where membranes alternate between a porated and nonporated state.35,36 Such behavior might even account for the discrete, ion channel-like steps of current observed in electrophysiological measurements of IAPP39 and protegrin-3.40
Implications Going Forward
Over the course of this review, we have put forward a common framework for membrane disruption by PATs, AMPs, and CPPs, resulting from their shared physical properties. Many membrane-active proteins have been placed into one of those three categories based on a few defining traits, specifically their amyloidogenic, toxic, antimicrobial, or membrane penetrating potential. While central to many of the physiological activities of the peptides, these traits are not necessarily unique. In fact, there is significant sharing of AMP, CPP, or PAT characteristics between peptides of all three classes.
Numerous reports have now asserted significant crossover between PATs and AMPs, with examples from both classes of peptide displaying characteristics of the other. Amyloid protein Aβ was found to have antimicrobial activity against a wide spectrum of both bacterial and fungal microbes, including C. albicans, E. coli, and S. pneumoniae.70 In many cases, the minimum inhibitory concentration of Aβ required to halt microbial growth was less than that of LL-37, a canonical human antimicrobial peptide. IAPP has also been shown to have antimicrobial activity against E. coli, S. aureus, and P. denitrificans, a soil bacterium frequently used as a mimetic for eukaryotic mitochondria.69,71 As with AMPs, there appears to be a broad-spectrum character to the toxicity of amyloid proteins. This amyloid toxicity spans from bacteria through animals and further supports the nonspecific character of cellular attack.
AMPs have also been observed to have amyloid characteristics. Protegrin-1, a porcine β-sheet AMP, was observed to form amyloid fibrils upon deposition on hydrophilic mica surfaces with kinetics comparable to the formation of Aβ fibrils.119 Additionally, helical LL-37 forms fibrils visualized by bright field microscopy in the presence of anionic lipid vesicles.120 The LL-37 fibrillation is particularly interesting, as amyloid fiber formation by PATs are often reported to be catalyzed by anionic lipid vesicles.59,121,122 This similar behavior, both as amyloids and antimicrobials, demonstrates a possible conformational and functional link between two peptides previously allocated to different categories.
Crossover is similarly evident between PATs and CPPs. Consider, for example, the contentious issue of identifying the site of cellular toxicity mediated by PATs. Cellular experiments have focused on the plasma membrane (by exogenous introduction of PATs to culture media) and membranes of the secretory pathway (by transfection). However, reports have now shown that Aβ, aSyn, and IAPP all are found to localize to the mitochondrial membrane, an intracellular target.31,123,124 Indeed, mitochondrial damage is frequently evident in patients or animal models of these proteins' associated diseases.123,125,126 Furthermore, mitochondrial targeting and dysfunction by these peptides is found to occur before cell death is evident in these systems. In our own work, IAPP was found to have CPP characteristics, enabling it to cross plasma membranes and possibly lysosomal membranes to access the cytoplasm and mitochondrial membranes.31 Thus, the ability of amyloid proteins to act as CPPs may be as central to their mammalian cytotoxicity as their pore forming characteristics.
AMPs have also been shown to possess CPP-like behavior and characteristics. For example, apidaecins and indolicidin are rich in one or two amino acids, a common characteristic of CPPs. LL-37 has been observed to translocate the lipid bilayer and deliver cargo to cellular compartments.99,100,127 Additionally, analogs of magainin and buforin translocate the lipid bilayer, indicating AMPs can serve as a constructive template for engineering these properties once only attributed to CPPs.128–130 While membrane permeation is an obvious mechanism of cytotoxicity, many AMPs also induce cytotoxicity intracellularly through a variety of other mechanisms.4 For such peptides, their CPP-like characteristics are clearly central to their toxicity.
Cellular toxicity has been observed for PATs, and is a defining requirement of AMPs, but also has been observed for CPPs. The CPPs TP10 and pVEC were found to enter both bacterial and mammalian cells, but inhibited bacterial growth well below concentrations that damaged mammalian cells.131 Another study found penetratin, MAP, and pVEC to have strong antimicrobial activity against the Gram-positive bacteria B. megaterium.132 Beyond possible AMP activity, the amphipathic MAP and transporton family of CPPs have also demonstrated cytotoxicity toward mammalian cells.133–135 More typically, strongly cationic or arginine-rich CPPs are observed to be nontoxic.9 However, cytotoxicity or membrane destabilization and poration has been reported even for polyarginine.136 The membrane deformation required for protein internalization must thus be considered to ensure minimal possible toxicity for any CPP-based therapeutics.
In conclusion, it is useful, synergistic, and correct to cross compare the efforts of these three disciplines. CPP and AMP-like behavior is observed in PATs. PAT and CPP-like behavior is observed in AMPs. AMP and PAT-like behavior is observed in CPPs. The path going forward requires recognition of this cross-talk with particular attention given to elements of an under-recognized lexicon. Namely, that of a dynamic phospholipid bilayer whose interactions with proteins manipulate and are manipulated by alterations in surface tension and the induction and stabilization of bilayer defects.
Acknowledgments
DES is a post-doctoral fellow of the American Diabetes Association (ADA). This work supported, in part, by NIH GM094693 and the ADA.
References
- 1.Mim C, Cui H, Gawronski-Salerno JA, Frost A, Lyman E, Voth GA, Unger VM. Structural basis of membrane bending by the N-BAR protein endophilin. Cell. 2012;149:137–145. doi: 10.1016/j.cell.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17:850–860. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 5.Butterfield SM, Lashuel HA. Amyloidogenic protein-membrane interactions: mechanistic insight from model systems. Angew Chem Int Ed Engl. 2010;49:5628–5654. doi: 10.1002/anie.200906670. [DOI] [PubMed] [Google Scholar]
- 6.Fuertes G, Giménez D, Esteban-Martín S, Sánchez-Muñoz OL, Salgado J. A lipocentric view of peptide-induced pores. Eur Biophys J. 2011;40:399–415. doi: 10.1007/s00249-011-0693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wimley WC. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol. 2010;5:905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebda JA, Miranker AD. The interplay of catalysis and toxicity by amyloid intermediates on lipid bilayers: insights from type II diabetes. Annu Rev Biophys. 2009;38:125–152. doi: 10.1146/annurev.biophys.050708.133622. [DOI] [PubMed] [Google Scholar]
- 9.Vivès E, Schmidt J, Pèlegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta. 2008;1786:126–138. doi: 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Dürr UHN, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 12.Snider C, Jayasinghe S, Hristova K, White SH. MPEx: a tool for exploring membrane proteins. Protein Sci. 2009;18:2624–2628. doi: 10.1002/pro.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson JA, Loria JP, Miranker AD. Helix stabilization precedes aqueous and bilayer-catalyzed fiber formation in islet amyloid polypeptide. J Mol Biol. 2009;393:383–396. doi: 10.1016/j.jmb.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marion D, Zasloff M, Bax A. A two-dimensional NMR study of the antimicrobial peptide magainin 2. FEBS Lett. 1988;227:21–26. doi: 10.1016/0014-5793(88)81405-4. [DOI] [PubMed] [Google Scholar]
- 15.Mäler L. Solution NMR studies of cell-penetrating peptides in model membrane systems. Adv Drug Delivery Rev. 2012 doi: 10.1016/j.addr.2012.10.011. http://dx.doi.org/10.1016/j.addr.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta. 1999;1462:11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 17.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Structure of membrane-bound α-synuclein from site-directed spin labeling and computational refinement. Proc Natl Acad Sci USA. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang HW. Molecular mechanism of antimicrobial peptides: the origin of cooperativity. Biochim Biophys Acta. 2006;1758:1292–1302. doi: 10.1016/j.bbamem.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marassi FM, Opella SJ, Juvvadi P, Merrifield RB. Orientation of cecropin A helices in phospholipid bilayers determined by solid-state NMR spectroscopy. Biophys J. 1999;77:3152–3155. doi: 10.1016/S0006-3495(99)77145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apostolidou M, Jayasinghe SA, Langen R. Structure of α-helical membrane-bound human islet amyloid polypeptide and its implications for membrane-mediated misfolding. J Biol Chem. 2008;283:17205–17210. doi: 10.1074/jbc.M801383200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding B, Chen Z. Molecular interactions between cell penetrating peptide Pep-1 and model cell membranes. J Phys Chem B. 2012;116:2545–2552. doi: 10.1021/jp209604m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trexler AJ, Rhoades E. α-synuclein binds large unilamellar vesicles as an extended helix. Biochemistry. 2009;48:2304–2306. doi: 10.1021/bi900114z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreon ACM, Gambin Y, Lemke EA, Deniz AA. Interplay of α-synuclein binding and conformational switching probed by single-molecule fluorescence. Proc Natl Acad Sci USA. 2009;106:5645–5650. doi: 10.1073/pnas.0809232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleicken S, Classen M, Padmavathi PVL, Ishikawa T, Zeth K, Steinhoff H-J, Bordignon E. Molecular details of bax activation, oligomerization, and membrane insertion. J Biol Chem. 2010;285:6636–6647. doi: 10.1074/jbc.M109.081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight JD, Hebda JA, Miranker AD. Conserved and cooperative assembly of membrane-bound α-helical states of islet amyloid polypeptide. Biochemistry. 2006;45:9496–9508. doi: 10.1021/bi060579z. [DOI] [PubMed] [Google Scholar]
- 27.Wieprecht T, Dathe M, Schümann M, Krause E, Beyermann M, Bienert M. Conformational and functional study of magainin 2 in model membrane environments using the new approach of systematic double-D-amino acid replacement. Biochemistry. 1996;35:10844–10853. doi: 10.1021/bi960362c. [DOI] [PubMed] [Google Scholar]
- 28.Eiríksdóttir E, Konate K, Langel Ü, Divita G, Deshayes S. Secondary structure of cell-penetrating peptides controls membrane interaction and insertion. Biochim Biophys Acta. 2010;1798:1119–1128. doi: 10.1016/j.bbamem.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Quist A, Doudevski L, Lin H, Azimova R, Ng D, Frangione B, Kagan B, Ghiso J, Lal R. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc Natl Acad Sci USA. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schauerte JA, Wong PT, Wisser KC, Ding H, Steel DG, Gafni A. Simultaneous single-molecule fluorescence and conductivity studies reveal distinct classes of Aβ species on lipid bilayers. Biochemistry. 2010;49:3031–3039. doi: 10.1021/bi901444w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magzoub M, Miranker AD. Concentration-dependent transitions govern the subcellular localization of islet amyloid polypeptide. FASEB J. 2012;26:1228–1238. doi: 10.1096/fj.11-194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He K, Ludtke SJ, Worcester DL, Huang HW. Neutron scattering in the plane of membranes: structure of alamethicin pores. Biophys J. 1996;70:2659–2666. doi: 10.1016/S0006-3495(96)79835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian S, Wang W, Yang L, Huang HW. Structure of the alamethicin pore reconstructed by x-ray diffraction analysis. Biophys J. 2008;94:3512–3522. doi: 10.1529/biophysj.107.126474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nath A, Miranker AD, Rhoades E. A membrane-bound antiparallel dimer of rat islet amyloid polypeptide. Angew Chem Int Ed. 2011;50:10859–10862. doi: 10.1002/anie.201102887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Last NB, Rhoades E, Miranker AD. Islet amyloid polypeptide demonstrates a persistent capacity to disrupt membrane integrity. Proc Natl Acad Sci USA. 2011;108:9460–9465. doi: 10.1073/pnas.1102356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory SM, Cavenaugh A, Journigan V, Pokorny A, Almeida PFF. A quantitative model for the all-or-none permeabilization of phospholipid vesicles by the antimicrobial peptide cecropin A. Biophys J. 2008;94:1667–1680. doi: 10.1529/biophysj.107.118760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yandek LE, Pokorny A, Florén A, Knoelke K, Langel U, Almeida PFF. Mechanism of the cell-penetrating peptide transportan 10 permeation of lipid bilayers. Biophys J. 2007;92:2434–2444. doi: 10.1529/biophysj.106.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin H, Bhatia R, Lal R. Amyloid β protein forms ion channels: implications for Alzheimer's disease pathophysiology. FASEB J. 2001;15:2433–2444. doi: 10.1096/fj.01-0377com. [DOI] [PubMed] [Google Scholar]
- 39.Mirzabekov TA, Lin M-C, Kagan BL. Pore formation by the cytotoxic islet amyloid peptide amylin. J Biol Chem. 1996;271:1988–1992. doi: 10.1074/jbc.271.4.1988. [DOI] [PubMed] [Google Scholar]
- 40.Sokolov Y, Mirzabekov T, Martin DW, Lehrer RI, Kagan BL. Membrane channel formation by antimicrobial protegrins. Biochim Biophys Acta. 1999;1420:23–29. doi: 10.1016/s0005-2736(99)00086-3. [DOI] [PubMed] [Google Scholar]
- 41.Capone R, Mustata M, Jang H, Arce FT, Nussinov R, Lal R. Antimicrobial protegrin-1 forms ion channels: molecular dynamic simulation, atomic force microscopy, and electrical conductance studies. Biophys J. 2010;98:2644–2652. doi: 10.1016/j.bpj.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brender JR, Hartman K, Reid KR, Kennedy RT, Ramamoorthy A. A single mutation in the nonamyloidogenic region of islet amyloid polypeptide greatly reduces toxicity. Biochemistry. 2008;47:12680–12688. doi: 10.1021/bi801427c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garwood CJ, Pooler AM, Atherton J, Hanger DP, Noble W. Astrocytes are important mediators of Aβ-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis. 2011;2:e167. doi: 10.1038/cddis.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guillemin Y, Lopez J, Gimenez D, Fuertes G, Valero JG, Blum L, Gonzalo P, Salgado J, Girard-Egrot A, Aouacheria A. Active fragments from pro- and antiapoptotic BCL-2 proteins have distinct membrane behavior reflecting their functional divergence. PLoS One. 2010;5:e9066. doi: 10.1371/journal.pone.0009066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryser HJ-P, Hancock R. Histones and basic polyamino acids stimulate the uptake of albumin by tumor cells in culture. Science. 1965;150:501–503. doi: 10.1126/science.150.3695.501. [DOI] [PubMed] [Google Scholar]
- 46.Matsuzaki K. Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta. 2009;1788:1687–1692. doi: 10.1016/j.bbamem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Magzoub M, Eriksson LEG, Gräslund A. Conformational states of the cell-penetrating peptide penetratin when interacting with phospholipid vesicles: effects of surface charge and peptide concentration. Biochim Biophys Acta. 2002;1563:53–63. doi: 10.1016/s0005-2736(02)00373-5. [DOI] [PubMed] [Google Scholar]
- 48.Magzoub M, Gräslund A. Cell-penetrating peptides: small from inception to application. Q Rev Biophys. 2004;37:147–195. doi: 10.1017/s0033583505004014. [DOI] [PubMed] [Google Scholar]
- 49.Rustenbeck I, Matthies A, Lenzen S. Lipid composition of glucose-stimulated pancreatic islets and insulin-secreting tumor cells. Lipids. 1994;29:685–692. doi: 10.1007/BF02538912. [DOI] [PubMed] [Google Scholar]
- 50.Sunde M, Blake CC. From the globular to the fibrous state: protein structure and structural conversion in amyloid formation. Q Rev Biophys. 1998;31:1–39. doi: 10.1017/s0033583598003400. [DOI] [PubMed] [Google Scholar]
- 51.Barnhart MM, Chapman MR. Curli biogenesis and function. Annu Rev Microbiol. 2006;60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, Singru PS, Nilsson KPR, Simon R, Schubert D, Eisenberg D, Rivier J, Sawchenko P, Vale W, Riek R. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 54.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 55.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 56.Bokvist M, Lindström F, Watts A, Gröbner G. Two types of Alzheimer's β-amyloid (1–40) peptide membrane interactions: aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. J Mol Biol. 2004;335:1039–1049. doi: 10.1016/j.jmb.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 57.Ji S-R, Wu Y, Sui S-F. Cholesterol is an important factor affecting the membrane insertion of β-amyloid peptide (Aβ 1–40), which may potentially inhibit the fibril formation. J Biol Chem. 2002;277:6273–6279. doi: 10.1074/jbc.M104146200. [DOI] [PubMed] [Google Scholar]
- 58.Zakharov SD, Hulleman JD, Dutseva EA, Antonenko YN, Rochet J-C, Cramer WA. Helical α-synuclein forms highly conductive ion channels. Biochemistry. 2007;46:14369–14379. doi: 10.1021/bi701275p. [DOI] [PubMed] [Google Scholar]
- 59.Knight JD, Miranker AD. Phospholipid catalysis of diabetic amyloid assembly. J Mol Biol. 2004;341:1175–1187. doi: 10.1016/j.jmb.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 60.Shvadchak VV, Falomir-Lockhart LJ, Yushchenko DA, Jovin TM. Specificity and kinetics of α-synuclein binding to model membranes determined with fluorescent excited state intramolecular proton transfer (ESIPT) probe. J Biol Chem. 2011;286:13023–13032. doi: 10.1074/jbc.M110.204776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terzi E, Hölzemann G, Seelig J. Interaction of Alzheimer β-amyloid peptide(1–40) with lipid membranes. Biochemistry. 1997;36:14845–14852. doi: 10.1021/bi971843e. [DOI] [PubMed] [Google Scholar]
- 62.Nerelius C, Sandegren A, Sargsyan H, Raunak R, Leijonmarck H, Chatterjee U, Fisahn A, Imarisio S, Lomas DA, Crowther DC, Strömberg R, Johansson J. α-helix targeting reduces amyloid-β peptide toxicity. Proc Natl Acad Sci USA. 2009;106:9191–9196. doi: 10.1073/pnas.0810364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hebda JA, Saraogi I, Magzoub M, Hamilton AD, Miranker AD. A peptidomimetic approach to targeting pre-amyloidogenic states in type II diabetes. Chem Biol. 2009;16:943–950. doi: 10.1016/j.chembiol.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, Pensalfini A, Soriaga AB, Landau M, Teng PK, Cascio D, Glabe C, Eisenberg D. Atomic view of a toxic amyloid small oligomer. Science. 2012;335:1228–1231. doi: 10.1126/science.1213151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian A, Baumgart T. Sorting of lipids and proteins in membrane curvature gradients. Biophys J. 2009;96:2676–2688. doi: 10.1016/j.bpj.2008.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith PES, Brender JR, Ramamoorthy A. Induction of negative curvature as a mechanism of cell toxicity by amyloidogenic peptides: the case of islet amyloid polypeptide. J Am Chem Soc. 2009;131:4470–4478. doi: 10.1021/ja809002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuzaki K, Horikiri C. Interactions of amyloid β-peptide (1–40) with ganglioside-containing membranes. Biochemistry. 1999;38:4137–4142. doi: 10.1021/bi982345o. [DOI] [PubMed] [Google Scholar]
- 68.Middleton ER, Rhoades E. Effects of curvature and composition on α-synuclein binding to lipid vesicles. Biophys J. 2010;99:2279–2288. doi: 10.1016/j.bpj.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Last NB, Miranker AD. Common mechanism unites membrane poration by amyloid and antimicrobial peptides. Proc Natl Acad Sci USA. 2013;110:6382–6387. doi: 10.1073/pnas.1219059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD. The Alzheimer's disease-associated amyloid β-protein is an antimicrobial peptide. PLoS One. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, Liu Q, Chen J-C, Cui Y-X, Zhou B, Chen Y-X, Zhao Y-F, Li Y-M. Antimicrobial activity of human islet amyloid polypeptides: an insight into amyloid peptides' connection with antimicrobial peptides. Biol Chem. 2012;393:641–646. doi: 10.1515/hsz-2012-0107. [DOI] [PubMed] [Google Scholar]
- 72.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 73.Nakatsuji T, Gallo RL. Antimicrobial peptides: old molecules with new ideas. J Invest Dermatol. 2011;132:887–895. doi: 10.1038/jid.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011;29:464–472. doi: 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J Membr Biol. 1997;156:197–211. doi: 10.1007/s002329900201. [DOI] [PubMed] [Google Scholar]
- 76.Epand RM, Epand RF. Bacterial membrane lipids in the action of antimicrobial agents. J Peptide Sci. 2010;17:298–305. doi: 10.1002/psc.1319. [DOI] [PubMed] [Google Scholar]
- 77.Zelezetsky I, Tossi A. Alpha-helical antimicrobial peptides—using a sequence template to guide structure-activity relationship studies. Biochim Biophys Acta. 2006;1758:1436–1449. doi: 10.1016/j.bbamem.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 78.Dathe M, Nikolenko H, Meyer J, Beyermann M, Bienert M. Optimization of the antimicrobial activity of magainin peptides by modification of charge. FEBS Lett. 2001;501:146–150. doi: 10.1016/s0014-5793(01)02648-5. [DOI] [PubMed] [Google Scholar]
- 79.Chen Y, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS. Role of peptide hydrophobicity in the mechanism of action of α-helical antimicrobial peptides. Antimicrob Agents Chemother. 2007;51:1398–1406. doi: 10.1128/AAC.00925-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin LM, Edwards MA, Li J, Yip CM, Deber CM. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J Biol Chem. 2012;287:7738–7745. doi: 10.1074/jbc.M111.303602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Y, Mant CT, Farmer SW, Hancock REW, Vasil ML, Hodges RS. Rational design of α-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem. 2005;280:12316–12329. doi: 10.1074/jbc.M413406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nicolas P. Multifunctional host defense peptides: intracellular-targeting antimicrobial peptides. FEBS J. 2009;276:6483–6496. doi: 10.1111/j.1742-4658.2009.07359.x. [DOI] [PubMed] [Google Scholar]
- 83.Gazit E, Boman A, Boman HG, Shai Y. Interaction of the mammalian antibacterial peptide cecropin P1 with phospholipid vesicles. Biochemistry. 1995;34:11479–11488. doi: 10.1021/bi00036a021. [DOI] [PubMed] [Google Scholar]
- 84.Pouny Y, Rapaport D, Mor A, Nicolas P, Shai Y. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry. 1992;31:12416–12423. doi: 10.1021/bi00164a017. [DOI] [PubMed] [Google Scholar]
- 85.Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW. Membrane pores induced by magainin. Biochemistry. 1996;35:13723–13728. doi: 10.1021/bi9620621. [DOI] [PubMed] [Google Scholar]
- 86.Yamaguchi S, Hong T, Waring A, Lehrer RI, Hong M. Solid-state NMR investigations of peptide-lipid interaction and orientation of a beta-sheet antimicrobial peptide, protegrin. Biochemistry. 2002;41:9852–9862. doi: 10.1021/bi0257991. [DOI] [PubMed] [Google Scholar]
- 87.Pieta P, Mirza J, Lipkowski J. Direct visualization of the alamethicin pore formed in a planar phospholipid matrix. Proc Natl Acad Sci USA. 2012;109:21223–21227. doi: 10.1073/pnas.1201559110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gregory SM, Pokorny A, Almeida PFF. Magainin 2 revisited: a test of the quantitative model for the all-or-none permeabilization of phospholipid vesicles. Biophys J. 2009;96:116–131. doi: 10.1016/j.bpj.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee M-T, Chen F-Y, Huang HW. Energetics of pore formation induced by membrane active peptides. Biochemistry. 2004;43:3590–3599. doi: 10.1021/bi036153r. [DOI] [PubMed] [Google Scholar]
- 90.Naito A, Nagao T, Norisada K, Mizuno T, Tuzi S, Saitô H. Conformation and dynamics of melittin bound to magnetically oriented lipid bilayers by solid-state (31)P and (13)C NMR spectroscopy. Biophys J. 2000;78:2405–2417. doi: 10.1016/S0006-3495(00)76784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ladokhin AS, White SH. ‘Detergent-like’ permeabilization of anionic lipid vesicles by melittin. Biochem Biophys Acta. 2001;1514:253–260. doi: 10.1016/s0005-2736(01)00382-0. [DOI] [PubMed] [Google Scholar]
- 92.Vogel H, Jähnig F. The structure of melittin in membranes. Biophys J. 1986;50:573–582. doi: 10.1016/S0006-3495(86)83497-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krauson AJ, He J, Wimley WC. Determining the mechanism of membrane permeabilizing peptides: identification of potent, equilibrium pore-formers. Biochim Biophys Acta. 2012;1818:1625–1632. doi: 10.1016/j.bbamem.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dumont ME, Richards FM. Insertion of apocytochrome c into lipid vesicles. J Biol Chem. 1984;259:4147–4156. [PubMed] [Google Scholar]
- 95.van den Berg A, Dowdy SF. Protein transduction domain delivery of therapeutic macromolecules. Curr Opin Biotechnol. 2011;22:888–893. doi: 10.1016/j.copbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 96.McNaughton BR, Cronican JJ, Thompson DB, Liu DR. Mammalian cell penetration, siRNA transfection, and DNA transfection by supercharged proteins. Proc Natl Acad Sci USA. 2009;106:6111–6116. doi: 10.1073/pnas.0807883106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andreev OA, Karabadzhak AG, Weerakkody D, Andreev GO, Engelman DM, Reshetnyak YK. pH (low) insertion peptide (pHLIP) inserts across a lipid bilayer as a helix and exits by a different path. Proc Natl Acad Sci USA. 2010;107:4081–4086. doi: 10.1073/pnas.0914330107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marks JR, Placone J, Hristova K, Wimley WC. Spontaneous membrane-translocating peptides by orthogonal high-throughput screening. J Am Chem Soc. 2011;133:8995–9004. doi: 10.1021/ja2017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Splith K, Neundorf I. Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur Biophys J. 2011;40:387–397. doi: 10.1007/s00249-011-0682-7. [DOI] [PubMed] [Google Scholar]
- 100.Sandgren S, Wittrup A, Cheng F, Jönsson M, Eklund E, Busch S, Belting M. The human antimicrobial peptide LL-37 transfers extracellular DNA plasmid to the nuclear compartment of mammalian cells via lipid rafts and proteoglycan-dependent endocytosis. J Biol Chem. 2004;279:17951–17956. doi: 10.1074/jbc.M311440200. [DOI] [PubMed] [Google Scholar]
- 101.Pokorny A, Birkbeck TH, Almeida PFF. Mechanism and kinetics of δ-lysin interaction with phospholipid vesicles. Biochemistry. 2002;41:11044–11056. doi: 10.1021/bi020244r. [DOI] [PubMed] [Google Scholar]
- 102.Anguiano M, Nowak RJ, Lansbury PT., Jr Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–11343. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- 103.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta. 1999;1462:55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 104.Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta. 1999;1462:1–10. doi: 10.1016/s0005-2736(99)00197-2. [DOI] [PubMed] [Google Scholar]
- 105.Huang HW. Action of antimicrobial peptides: two-state model. Biochemistry. 2000;39:8347–8352. doi: 10.1021/bi000946l. [DOI] [PubMed] [Google Scholar]
- 106.Huang HW, Chen F-Y, Lee M-T. Molecular mechanism of peptide-induced pores in membranes. Phys Rev Lett. 2004;92:198304. doi: 10.1103/PhysRevLett.92.198304. [DOI] [PubMed] [Google Scholar]
- 107.Chen F-Y, Lee M-T, Huang HW. Evidence for membrane thinning effect as the mechanism for peptide-induced pore formation. Biophys J. 2003;84:3751–3758. doi: 10.1016/S0006-3495(03)75103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Polozov IV, Anantharamaiah GM, Segrest JP, Epand RM. Osmotically induced membrane tension modulates membrane permeabilization by class L amphipathic helical peptides: nucleation model of defect formation. Biophys J. 2001;81:949–959. doi: 10.1016/S0006-3495(01)75753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Litster JD. Stability of lipid bilayers and red blood cell membranes. Phys Lett A. 1975;53:193–194. [Google Scholar]
- 110.Taupin C, Dvolaitzky M, Sauterey C. Osmotic pressure induced pores in phospholipid vesicles. Biochemistry. 1975;14:4771–4775. doi: 10.1021/bi00692a032. [DOI] [PubMed] [Google Scholar]
- 111.Puech P-H, Borghi N, Karatekin E, Brochard-Wyart F. Line thermodynamics: adsorption at a membrane edge. Phys Rev Lett. 2003;90:128304. doi: 10.1103/PhysRevLett.90.128304. [DOI] [PubMed] [Google Scholar]
- 112.Matsuzaki K, Sugishita K, Ishibe N, Ueha M, Nakata S, Miyajima K, Epand RM. Relationship of membrane curvature to the formation of pores by magainin 2. Biochemistry. 1998;37:11856–11863. doi: 10.1021/bi980539y. [DOI] [PubMed] [Google Scholar]
- 113.Henzler Wildman KA, Lee D-K, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 114.Mishra A, Lai GH, Schmidt NW, Sun VZ, Rodriguez AR, Tong R, Tang L, Cheng J, Deming TJ, Kamei DT, Wong GCL. Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proc Natl Acad Sci USA. 2011;108:16883–16888. doi: 10.1073/pnas.1108795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen F-Y, Lee M-T, Huang HW. Sigmoidal concentration dependence of antimicrobial peptide activities: a case study on alamethicin. Biophys J. 2002;82:908–914. doi: 10.1016/S0006-3495(02)75452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tamba Y, Ariyama H, Levadny V, Yamazaki M. Kinetic pathway of antimicrobial peptide magainin 2-induced pore formation in lipid membranes. J Phys Chem B. 2010;114:12018–12026. doi: 10.1021/jp104527y. [DOI] [PubMed] [Google Scholar]
- 117.Fuertes G, García-Sáez AJ, Esteban-Martín S, Giménez D, Sánchez-Muñoz OL, Schwille P, Salgado J. Pores formed by Baxα5 relax to a smaller size and keep at equilibrium. Biophys J. 2010;99:2917–2925. doi: 10.1016/j.bpj.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee M-T, Hung W-C, Chen F-Y, Huang HW. Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc Natl Acad Sci USA. 2008;105:5087–5092. doi: 10.1073/pnas.0710625105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jang H, Arce FT, Mustata M, Ramachandran S, Capone R, Nussinov R, Lal R. Antimicrobial protegrin-1 forms amyloid-like fibrils with rapid kinetics suggesting a functional link. Biophys J. 2011;100:1775–1783. doi: 10.1016/j.bpj.2011.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sood R, Domanov Y, Pietiäinen M, Kontinen VP, Kinnunen PKJ. Binding of LL-37 to model biomembranes: insight into target vs host cell recognition. Biochim Biophys Acta. 2008;1778:983–996. doi: 10.1016/j.bbamem.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 121.Choo-Smith L-P, Garzon-Rodriguez W, Glabe CG, Surewicz WK. Acceleration of amyloid fibril formation by specific binding of Aβ-(1–40) peptide to ganglioside-containing membrane vesicles. J Biol Chem. 1997;272:22987–22990. doi: 10.1074/jbc.272.37.22987. [DOI] [PubMed] [Google Scholar]
- 122.Zhu M, Li J, Fink AL. The association of α-synuclein with membranes affects bilayer structure, stability, and fibril formation. J Biol Chem. 2003;278:40186–40197. doi: 10.1074/jbc.M305326200. [DOI] [PubMed] [Google Scholar]
- 123.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anandatheerthavarada HK, Biswas G, Robin M-A, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci USA. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gurlo T, Ryazantsev S, Huang C-J, Yeh MW, Reber HA, Hines OJ, O'Brien TD, Glabe CG, Butler PC. Evidence for proteotoxicity in β cells in type 2 diabetes. Am J Pathol. 2010;176:861–869. doi: 10.2353/ajpath.2010.090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang X, Oglęcka K, Sandgren S, Belting M, Esbjörner EK, Nordén B, Gräslund A. Dual functions of the human antimicrobial peptide LL-37—target membrane perturbation and host cell cargo delivery. Biochim Biophys Acta. 2010;1798:2201–2208. doi: 10.1016/j.bbamem.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 128.Kobayashi S, Chikushi A, Tougu S, Imura Y, Nishida M, Yano Y, Matsuzaki K. Membrane translocation mechanism of the antimicrobial peptide buforin 2. Biochemistry. 2004;43:15610–15616. doi: 10.1021/bi048206q. [DOI] [PubMed] [Google Scholar]
- 129.Takeshima K, Chikushi A, Lee K-K, Yonehara S, Matsuzaki K. Translocation of analogues of the antimicrobial peptides magainin and buforin across human cell membranes. J Biol Chem. 2003;278:1310–1315. doi: 10.1074/jbc.M208762200. [DOI] [PubMed] [Google Scholar]
- 130.Park CB, Yi K-S, Matsuzaki K, Kim MS, Kim SC. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc Natl Acad Sci USA. 2000;97:8245–8250. doi: 10.1073/pnas.150518097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nekhotiaeva N, Elmquist A, Rajarao GK, Hällbrink M, Langel U, Good L. Cell entry and antimicrobial properties of eukaryotic cell-penetrating peptides. FASEB J. 2004;18:394–396. doi: 10.1096/fj.03-0449fje. [DOI] [PubMed] [Google Scholar]
- 132.Palm C, Netzereab S, Hällbrink M. Quantitatively determined uptake of cell-penetrating peptides in non-mammalian cells with an evaluation of degradation and antimicrobial effects. Peptides. 2006;27:1710–1716. doi: 10.1016/j.peptides.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 133.Kilk K, Mahlapuu R, Soomets U, Langel U. Analysis of in vitro toxicity of five cell-penetrating peptides by metabolic profiling. Toxicology. 2009;265:87–95. doi: 10.1016/j.tox.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 134.Saar K, Lindgren M, Hansen M, Eiríksdóttir E, Jiang Y, Rosenthal-Aizman K, Sassian M, Langel U. Cell-penetrating peptides: a comparative membrane toxicity study. Anal Biochem. 2005;345:55–65. doi: 10.1016/j.ab.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 135.Jones SW, Christison R, Bundell K, Voyce CJ, Brockbank SMV, Newham P, Lindsay MA. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br J Pharmacol. 2005;145:1093–1102. doi: 10.1038/sj.bjp.0706279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Herce HD, Garcia AE, Litt J, Kane RS, Martin P, Enrique N, Rebolledo A, Milesi V. Arginine-rich peptides destabilize the plasma membrane, consistent with a pore formation translocation mechanism of cell-penetrating peptides. Biophys J. 2009;97:1917–1925. doi: 10.1016/j.bpj.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]


