Abstract
Although reports of typical acute promyelocytic leukemia (APL) cases rarely mention dysplastic changes, this report concerns a rare case of APL with tri-lineage dysplastic changes resembling the characteristic features of myelodysplastic syndrome (MDS). The patient, a 77-year-old Japanese male, was diagnosed as having pancytopenia with hematologic morphological abnormalities comprising micro - megakaryocytes, neutrophils with hypo-granulation and negative peroxidase activity, and erythroblasts containing nuclei with abnormalities such as karyorrhexis. Although there is one report of a case of transformation of de novo MDS into APL and several reports of cases of therapy-related MDS transformed into APL, our patient had no history of cytopenia or of either chemo or radiation therapy. Our case can thus be considered to constitute a rare case of APL with dysplastic morphology.
Key words: acute promyelocytic leukemia, dysplasia, all-trans retinoic acid
Introduction
Myelodysplastic syndrome (MDS) frequently transforms into acute myelogenous leukemia, but rarely into acute promylocytic leukemia (APL).1 We experienced a rare case of elderly APL featuring various morphological abnormalities resembling characteristics of MDS, although the patient did not go through a stage perceptibly complicated with any form of cytopenia before the diagnosis of APL.
Case Report
A 77-year-old male was suffering from lowgrade fever and general fatigability in April 2011. After the possibility of pancytopenia was pointed out by a general physician, he was referred to Kakogawa West City Hospital, Japan. A chest X-ray indicated the presence of pneumonia and yielded the following laboratory findings: leukocyte count, 0.84×109/L; red blood cell count, 2.75×1012/L; hemoglobin value, 8.5 g/dL; hematocrit value, 26.5%; platelet count, 55×109/L; prothrombin time, 13.8 sec; fibrinogen level, 372 mg/dL and fibrin degradation product (FDP) level, 88.7 micro g/mL. These findings pointed to the presence of pancytopenia and a hyperfibrinolytic state.
Differential leukocyte counts were 10% for neutrophils, 83% for lymphocytes and 1% for basophils. Morphological examination detected frequent hypo-granular and peroxidase (POX)-negative neutrophils (Figure 1A and B).
Figure 1.
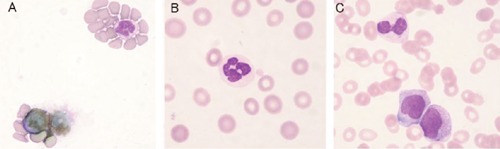
Peripheral blood leukocytes. A) peroxidase (POX) negative neutrophil; B) hypogranular neutrophil (May-Giemsa staining); C) neutrophil containing Auer body after initiation of ATRA treatment (May-Giemsa staining).
Blood chemistry test findings were: CRP, 20.45 mg/dL; AST, 20 IU/L; ALT, 8 IU/L; LD, 175 IU/L; ALP, 238 IU/L; BUN, 55 mg/dL; creatinine, 2.74 mg/dL and uric acid, 8.7 mg/dL, indicating severe inflammation due to pneumonia and renal dysfunction.
Iliac bone marrow aspiration produced a total nucleated cell count of 59×109/L and a megakaryocyte count of 25×106/L, while the differential count showed the bone marrow consisted of 28.2% myeloblasts and 55.2% abnormal promyelocytes, frequently containing Auer rods or faggot cells (Figure 2A and B). These cells were strongly positive for POX staining (Figure 2C). Micromegakaryocytes and megakryocytes with non-lobulated nuclei or isolated multi-nuclei (Figure 3A-D) as well as erythroblasts showing karyorrhexis or nuclei with abnormal configuration (Figure 3E and F) were also detected. Surface marker analysis produced positive results for CD13 (88.9%), CD33 (98.7%), CD64 (70.8%), CD65 (42.9%), CD71 (51.8%), CD117 (84.8%) and negative results for CD2, CD7, CD19, CD14, CD15, CD11b, CD34, HLA-DR, and gycophorin-A. RT-PCR detected the presence of a PMLRARA chimeric mRNA of 2.0×104 copies/micro g RNA, and chromosome analysis showed 46XY, t(15;17)(q22;q12) in 25% of the cells analyzed. These results indicated the patient had APL with MDS-like morphological abnormalities complicated with disseminated intravascular coagulation (DIC), pneumonia and renal insufficiency.
Figure 2.
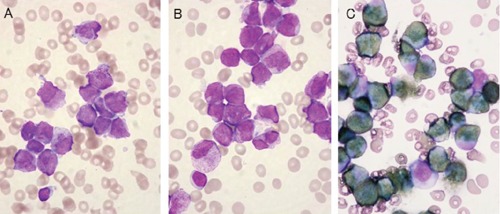
Typical acute promyelocytic leukemia (APL) cells in bone marrow. A) and B) APL cells with hypergranular cytoplasm and Auer body or faggot cells (May-Giemsa staining); C) strongly positive staining for peroxidase.
Figure 3.
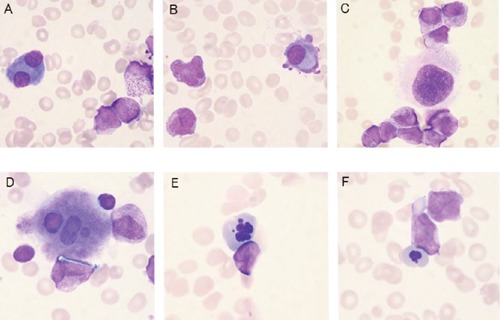
Atypical cells in bone marrow smear (May-Giemsa staining). A) and B) micro megakaryocytes; C) megakryocyte with non-lobulated nucleus; D) megakaryocyte with isolated multi-nuclei; E) erythroblast showing karyorrhexis; F) erythroblast with a nucleus showing abnormal configuration.
Although treatment with all-trans-retinoic acid (ATRA) combined with antibiotics therapy was started immediately, renal insufficiency deteriorated and the patient died on the 14th day after admission. The renal insufficiency was considered to be result of pre-existing dysfunction and adverse effects of antibiotics or anti-inflammatory drugs, although the possibility of differentiation syndrome could not be completely excluded. After the initiation of ATRA administration, the leukocyte counts increased from 1×109/L to 5.7×109/L and the neutrophil percentage from 21% to 62%. Because mature neutrophils with Auer rods were observed in peripheral blood (Figure 1C), the patient’s leukemia was considered to be responsive to ATRA treatment.
Discussion and Conclusions
Initially, our patient was thought to have MDS complicated with pneumonia and DIC, but the laboratory findings disclosed he was suffering from APL. There are three possible explanations for the morphological abnormalities resembling the characteristic features of MDS. First, this was possibly a rare case of MDS transformed into APL. Ogawa et al. reported a case of RAEB in transformation with 15% t(15;17),2 which changed to APL after several months and is the only case report of de novo MDS transformed into APL. On the other hand, there are no reports of even APL cases with chromosome 7 abnormalities that possess morphological abnormalities.3 Although Varma et al. reported that variant forms of APL cases frequently express myelodysplastic abnormalities, our patient was a typical APL.4 However, the diagnosis of MDS could not be confirmed in our case because there was no past history of cytopenia.
There are several reports of cases of therapy-related MDS transformed into APL. Anderson et al. reported three such cases among 41 secondary APL cases.5 Furthermore, Wolach et al. reported a case responsive to treatment with ATRA and arsenic trioxide that developed APL from therapy-related MDS over the course of several years.6 However, our patient had no history of chemotherapy or irradiation.
The third possibility is that, according to the WHO classification of tumors of hematopoietic and lymphoid tissues, our patient could have been classified as acute myelogenous leukemia (AML) with myelodysplasia-related changes.7 Although this specific category excludes cases characterized by genetic abnormalities of AML accompanied by recurrent abnormalities such as t(15;17), our case might be classified in this category.
According to Haferlach et al., morphologic dysplasia in de novo AML reportedly does not correlate with prognosis.8 Although our patient also seemed to respond to ATRA, he died from complicating renal insufficiency and infection.
To summarize, we encountered an elderly case with APL associated with dysplastic changes resembling myelodysplastic syndrome.
References
- 1.de Souza Fernandez T, Ornellas MH, Otero de Carvalho LD, et al. Chromosomal alterations associated with evolution from myelodysplastic syndrome to acute myeloid leukemia. Leuk Res. 2000; 24:839-48 [DOI] [PubMed] [Google Scholar]
- 2.Ogawa K, Shineha H, Abe R, et al. Acute promyelocytic leukemia with a history of RAEB in transformation and the 15/17 translocation. Rinsho Ketsueki 1989;30:67-71 [Article in Japanese] [PubMed] [Google Scholar]
- 3.Vial JP, Mahon FX, Pigneux A, et al. Derivative (7)t(7;8)(q34;q21): a new additional cytogenetic abnormality in acute promyelocytic leukemia. Cancer Genet Cytogenet. 2003; 140:78-81 [DOI] [PubMed] [Google Scholar]
- 4.Varma N, Dash S, Sarode R. Correlation between morphological and cytochemical heterogeneity of acute promyelocytic leukemia (APL) and its association with myelodysplasia. Indian J Cancer. 1990; 27:166-71 [PubMed] [Google Scholar]
- 5.Anderson MK, Larson RA, Mauritzson N, et al. Balanced chromosome abnormalities inv(16) and t(15;17) in therapy-related myelodysplastic syndromes and acute leukemia: Report from an international workshop. Genes Chromosomes Cancer. 2002; 33:395-400 [DOI] [PubMed] [Google Scholar]
- 6.Wolach O, Yeshurun M, Amariglio N, et al. Acute promyelocytic leukemia with a smoldering course associated with therapy-related myelodysplastic syndrome. Acta Haematol 2011;126;152-6 [DOI] [PubMed] [Google Scholar]
- 7.Arber DA, Brunning RD, Orazi, Bain BJ. Acute myeloid leukemia with myelodysplastia-related changes. Swerdlow SH, Campo E, Harris NL, et al.WHO classification of tumors of haematopoietic and lymphoid tissues, 4th ed Lyon, France: International agency for research on cancer (IARC); 2008. pp.124-126 [Google Scholar]
- 8.Haferlach T, Schoch C, Loffler H, et al. Morphologic dysplasia in de novo acute myeloid leukemia (AML) is related to unfavorable cytogenetics but has no independent prognostic relevance under the conditions of intensive induction therapy: results of a multicenter analysis from the German AML cooperative group studies. J Clin Oncol. 2003; 21:256-65 [DOI] [PubMed] [Google Scholar]


