Abstract
Oral mucositis is an important side effect of hematopoietic stem cell transplantation (HCST), mainly due to toxicity of conditioning regimens. It produces significant pain and morbidity. The present study reports a prospective, randomized, non-blinded study testing the efficacy of a new mouthwash, called Baxidil Onco® (Sanitas Farmaceutici Srl, Tortona, Italy) in 60 hematologic patients undergoing HCST (28 autologous, 32 allogeneic). Baxidil Onco®, used three times a day from Day -1 to Day +30, in addition to standard prophylactic schedules, was administered to 14 patients undergoing autologous and 14 patients undergoing allogeneic HCST. The remaining 32 patients (14 autologous and 18 HCST) were treated only with standard prophylactic schedules and served as control. In our study, the overall incidence of oral mucositis, measured according to the World Health Organization 0-4 scale, was 50% in the Baxidl Onco® group versus 82% in the control group (P=0.022). In addition, a significant reduction in scale 2-4 oral mucositis was observed in the Baxidil Onco® group (25% vs 56.2%; P=0.0029). The results obtained indicate that incidence, severity and duration of oral mucositis induced by conditioning regimens for HCST can be significantly reduced by oral rinsing with Baxidil Onco®, in addition to the standard prophylaxis scheme. Since Camelia Sinensin extract, which is used to produce green tea, is the main agent in this mouthwash, we hypothesize that the anti-oxidative properties of polyphenolic compounds of tea might exert protective effects on oral mucosa.
Key words: Camelia Sinensis, mucositis, transplantation
Introduction
Oral mucositis (OM) is one of the most frequent and important side effects of many anticancer therapies and has a significant physical and psychological impact for a large number of patients treated for neoplasias.1-4 The most important consequences of OM are pain, dysphagia, requirement of parenteral nutrition, increased risk of mucosal and systemic infections, poor quality of life, delayed discharge from hospital.
Polychemotherapy is associated with OM in approximately 5-15% of hematologic patients treated with standard doses.5 A higher incidence can be expected when agents such as thymidylate synthase inhibitors (methotrexate), topoisomerase II inhibitors (etoposide, irinotecan), pyrimidine analogs (cytarabine), purine analogs (6-mercaptopurine and 6-thioguanine), alkylating agents (busulfan, melphalan and cyclophosphamide), and intercalating drugs (idarubicin, doxorubicin, daunorubicin) are used at high doses.5
The frequency of OM further increases in the setting of stem cell transplantation.5,6 Conditioning regimens used in autologous peripheral blood hematopoietic stem cell transplantation (HSCT) cause OM in approximately 35-75% of patients,7,8 while in the allogeneic HCST setting the incidence of OM reaches 75-100%, depending on the type of disease, transplant procedure and conditioning regimen.9-11 OM induced by chemo- and radiotherapy seems to be caused by a combination of several pathways, including direct DNA and mucosal damage, increased production of reactive oxygen species, increased production of transcription factors, production of proinflammatory cytokines. As a consequence, loss of epithelial integrity, and increased apoptosis and necrosis of mucosal cells occur, followed by various degrees of bleeding, ulceration, bacterial colonization, and increased risk of systemic infection.9,12 For all of these reasons, the clinical approach to OM should include both prevention and treatment of manifestations. Prevention may include various measures, such as topical antimicrobials, barrier protectants, ice, and adequate oral hygiene.12 For treatment of OM, various agents have been proposed and used, such as cryotherapy,13 low-level laser therapy,14 chlorhexidine,15,16 a naturally occurring antimicrobial agent,17 and measures aimed at increasing epithelial cell regeneration and attenuating pro-inflammatory cytokine production. 5,18 In the present paper, we present the results of a randomized, non-blinded clinical study carried out with the use of a new mouthwash, called Baxidil Onco® (Sanitas Farmaceutici Srl, Tortona, Italy), in hematologic patients undergoing peripheral blood stem cell (PBSC) transplantation. All patients were treated with standard schedules for OM prevention, while Baxidil Onco® was used as an additive agent in a subgroup of patients after randomization.
Materials and Methods
Patients
Sixty patients (males 39, females 21, age 21-73 years) undergoing either autologous or allogeneic HCST were evaluated. They were managed in a purpose built leukemia and bone marrow transplantation unit. The patients were suffering from: multiple myeloma (MM, n=23), B-cell non-Hodgkin’s lymphomas (NHL, n=14), acute myeloblastic leukemia (AML, n=10), acute lymphoblastic leukemia (ALL, n=7), chronic lymphocytic leukemia (CLL, n=2), primary myelofibrosis (PMF, n=1), myelodysplastic syndrome (MDS, n=1), paroxysmal nocturnal hemoglobinuria (PNH, n=1), Hodgkin’s lymphoma (HL, n=1). Twenty-eight patients were treated with transplantation of autologous PBSC and the remaining 32 patients underwent transplantation of allogeneic PBSC (Table 1). The conditioning regimens used to prepare patients for HCST, which are routinely used in the HCST setting,19-27 are shown in Table 2.
Table 1.
General characteristics of patients.
| Disease | Autologous HCST | Allogeneic HCST |
|---|---|---|
| NHL | 7 | 7 |
| AML | 0 | 10 |
| ALL | 0 | 7 |
| MDS | 0 | 1 |
| HL | 0 | 1 |
| MM | 21 | 2 |
| B-CLL | 0 | 2 |
| PMF | 0 | 1 |
| PNH | 0 | 1 |
NHL, B-cell non-Hodgkin’s lymphomas; AML, acute myeloblastic leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; HL, Hodgkin’s lymphoma; MM, multiple myeloma; B-CLL, chronic lymphocytic leukemia; PMF, primary myelofibrosis; PNH, paroxysmal nocturnal hemoglobinuria.
Table 2.
Conditioning regimens used in autologous and allogeneic hematopoietic stem cell transplantation.
| Conditioning regimen | Autologous n. | Allogeneic n. |
|---|---|---|
| Melphalan-100 | 12 | - |
| Melphalan-140 | 2 | - |
| Melphalan-200 | 7 | - |
| BEAM | 5 | 3 |
| FEAM | 1 | - |
| Melphalan/mitoxantrone | 1 | 1 |
| Busulfan/cyclophosphamide | - | 9 |
| Fludarabine/TBl | - | 5 |
| Cyclophosphamide/TBl/ATG | - | 3 |
| Cyclophosphamide/thiotepa/TBI | - | 1 |
| Thiotepa/fludarabine/melphalan | - | 2 |
| Busulfan/cyclophosphamide/melphalan | - | 8 |
BEAM, association of carmustine, etoposide, cytosine arabinoside, melphalan; FEAM, association of fotemustine, etoposide, cytosine arabinoside, melphalan; TBI, total body irradiation; ATG, anti-thymocyte globulin.
All patients gave their informed consent to undergo HCST, to be treated with growth factors and other drugs including antibiotics, antifungals, antiviral agents, and to undergo OM prophylaxis with either standard schedules or Baxidil Onco® mouthwash.
Oral cavity examination
Three experienced dental hygienists carried out oral cavity examinations, with the support of a mirror, a tongue retractor, and gauzes, before starting conditioning (Day -1) and over the 30-day period of the study. The visit included examination and palpation of lips, frenum, buccal mucosa, gingivae, hard palate, soft palate, oropharynx, tonsils, tongue, floor of the mouth. Finally, the oral hygiene index (OHI-S) according to Greene and Vermilion was calculated for each patient.28 All data were registered on a dental chart.
Oral mucositis measurement
OM was measured according to the scoring system published by the WHO.29 This scale defines the state of the mucositis injuries according to their severity (scale 0-4). This not only values the presence of erythema and ulceration, but also the patient’s capacity to eat. In brief: grade 0, absence of symptoms; grade 1, soreness and erythema, no further symptoms; grade 2, ulcers present, but solid diet possible; grade 3, only liquids can be swallowed; grade 4, oral alimentation impossible.
Standard prophylaxis and treatment for oral mucositis
According to internal guidelines developed over time and approved by all physicians involved in patient management, all patients underwent the following prophylactic and therapeutic procedures aimed at reducing the incidence, duration and clinical impact of OM. Initial management of OM (grade 0-1) was with mouthwashes with 0.9% saline/sodium bicarbonate solution, 0.12% chlorhexidine and amphotericin B.
A soft toothbrush could be used in grade 0-1 OM, but in OM of grade 2 or over oral hygiene was maintained with a careful use of sponges or soft gauzes. Patients were invited to wash their mouth with 0.9% saline before topical medication was applied to remove debris and saliva. All patients received prophylaxis with acyclovir 400 mg twice daily from Day -3 to 16 weeks, fluconazole 400 mg once daily, ciprofloxacin 500 mg once daily, and cotrimoxazole 960 mg twice daily until day of transplant.
Mouthwash administration
Baxidil Onco® mouthwash was given to 28 patients (14 undergoing autologous and 14 allogeneic PBSC transplantation) after randomization. Baxidil Onco® was used at 20 mL four times daily, from Day -1 to Day +30. Patients were asked to rinse their mouth for at least one minute without swallowing. The remaining 32 patients served as control.
General patient management
All patients received granulocyte colony-stimulating factor (rhG-CSF) 5 mg/kg/day until white blood cell (WBC) count reached 0.5 x 109/L starting on Day 5 after PBSC infusion; irradiated blood products were infused to maintain hemoglobin and platelet levels above 8 g/dL and 10×109/L, respectively. All patients with an absolute neutrophil count of less than 0.5×109/L (grade IV neutropenia) received total parenteral nutrition (TPN); this was performed only during the period of profound reduction of neutrophils. Analgesic therapy with morphine 20 mg daily in continuous intravenous (i.v.) infusion was given to patients with grade 3-4 OM.
Statistical analysis
The χ2 test was used to compare results obtained in the group of patients treated with Baxidil Onco® versus the control group.
Results
In the control group, 26 of 32 patients (81.2%) experienced OM according to one of the four grades of the WHO score system. There was a statistically significant difference in the overall incidence of OM in the group treated with Baxidil Onco® (50%, 14 of 28 patients) (P=0.022) (Table 3, Figure 1). When intermediate (grade 2) plus severe (grade 3-4) OM were taken into account, a significant difference (P=0.029) between controls and patients treated with Baxidil Onco® was still registered, with 18 of 32 patients (56.2%) versus 7 of 28 patients (25%), respectively (Table 4). A lower incidence of grade 3-4 OM was registered in the Baxidil Onco® group (controls: 12 of 32, 37.5%; patients treated with Baxidil Onco®: 5 of 28, 17.8%), but a significant difference was not reached (P=0.16) despite the clear trend towards a different incidence of ulcerative OM.
Table 3.
Cumulative results obtained in patients treated with Baxidil Onco® and comparison with controls.
| Grade oral mucositis | Controls | Baxidil Onco® | ||
|---|---|---|---|---|
| n. | % | n. | % | |
| 0 | 6 | 18.75 | 14 | 50 |
| 1 | 8 | 25 | 7 | 25 |
| 2 | 6 | 18.75 | 2 | 7 |
| 3 | 5 | 15.6 | 3 | 10.7 |
| 4 | 7 | 21.8 | 2 | 7 |
Figure 1.
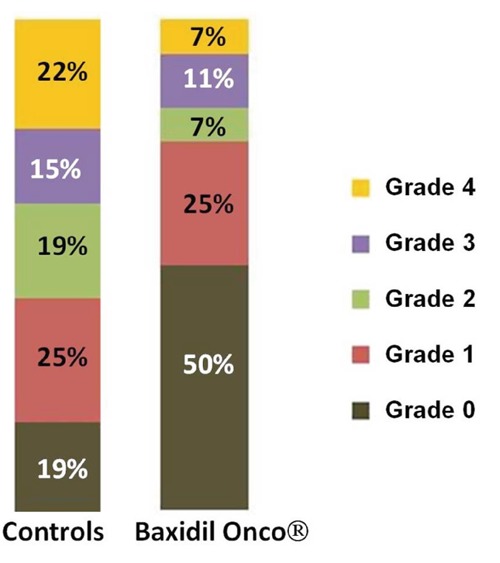
Percentage of distribution of oral mucositis according to World Health Organization grades in controls and in patients treated with Baxidil Onco®.
Table 4.
Detailed results in autologous and allogeneic hematopoietic stem cell transplantation.
| Grade oral mucositis | Controls | Baxidil Onco® | ||||
|---|---|---|---|---|---|---|
| Total | Autologous | Allogeneic | Total | Autologous | Allogeneic | |
| 0 | 6 | 4 | 2 | 14 | 9 | 5 |
| 1 | 8 | 4 | 4 | 7 | 5 | 2 |
| 2 | 6 | 5 | 1 | 2 | 0 | 2 |
| 3 | b | 0 | 5 | 3 | 0 | 3 |
| 4 | 7 | 1 | 6 | 2 | 0 | 2 |
The higher incidence of OM was observed in patients undergoing allogeneic HCST, both in controls and in the group treated with Baxidil Onco®. Mean duration of OM, when present, was seven days for autologous HSCT and 13 days for allogeneic HCST, without any difference between controls and patients treated with Baxidil Onco®. All patients experienced grade IV neutropenia; this lasted in patients undergoing allogeneic HCST. No difference was found between the control group and the group of patients treated with Baxidil Onco® (Table 5). There was no difference between controls and treated patients in terms of OHI-S values as measured before PBSC infusion. In addition, there was no correlation between OHI-S values and severity of OM (Table 6).
Table 5.
Duration of grade IV neutropenia.
| Patients | Duration of grade IV neutropenia (days) | P | |
|---|---|---|---|
| Controls | Baxidil Onco® | ||
| Allogeneic HCST | 18.06±2.18 (n=18) | 17.50±2.47 (n=14) | NS |
| Autologous HCST | 9.21±0.97 (n=14) | 9.00±1.18 (n=14) | NS |
Table 6.
OHI-S values before conditioning and relationship with oral mucositis grade in controls and in patients treated with Baxidil Onco® mouthwash
| Grade oral mucositis | Controls | Baxidil Onco® | ||||||
|---|---|---|---|---|---|---|---|---|
| n. | OHI-S 0 | OHI-S 1-2 | OHI-S 3 | n. | OHI-S 0 | OHI-S 1-2 | OHI-S 3 | |
| 0 | 6 | 4 | 1 | 1 | 14 | 12 | 1 | 1 |
| 1 | 8 | 7 | 0 | 1 | 7 | 6 | 1 | 0 |
| 2 | 6 | 6 | 0 | 0 | 2 | 1 | 0 | 1 |
| 3 | 5 | 5 | 0 | 0 | 3 | 3 | 0 | 0 |
| 4 | 7 | 7 | 0 | 0 | 2 | 2 | 0 | 0 |
According to our internal guidelines, all patients were subjected to TPN during the period of grade IV neutropenia, independently of OM grading. The days of TPN were, therefore, different in the two groups of patients, being longer in patients undergoing allogeneic HCST. TPN was suspended once engraftment was achieved, i.e. when absolute neutrophil count reached 0.5×109/L or over. A reduced requirement of morphine was observed in patients treated with Baxidil Onco® because of the reduced number of patients who developed grade 3-4 OM. The total requirement of morphine was 1300 mg for patients treated with Baxidil Onco® and 2880 mg in controls. No adverse events were reported after use of Baxidil Onco® mouthwash and all patients considered it to have a good taste.
Discussion
This study provides evidence that Baxidil Onco® mouthwash reduces both incidence and severity of OM in hematologic patients undergoing either autologous or allogeneic HCST. Although obtained in only a small series of patients, our observation shows that Baxidil Onco® mouthwash, used together with the standard prophylactic measures adopted in our transplant unit, was able to exert a protective action, especially in patients treated with autologous HCST who were conditioned with melphalan alone or with the BEAM regimen. The overall results showed that 50% of patients did not experience OM. Interestingly, Baxidil Onco® exerts its action locally as a topical agent. It is composed of Camelia Sinensis leaf extract (which is used to produce green tea and has anti-oxidative properties) and palmitoyil hydrolyzed wheat protein (a condensation product of palmitic acid chloride and hydrolyzed wheat protein, which might have healing properties on skin and mucosa). Thus, this mouthwash seems to be able to counteract both increased production of reactive oxygen species and the ulcerative damage that induces and worsens OM.
The most relevant activity of Baxidil Onco® can be attributed to Camelia Sinensis extract. The anti-oxidative properties of green tea are well known. Most of the beneficial effects of tea have been attributed to its polyphenolic compounds, with particular evidence of three catechins, such as epigallocatechin-3-gallate, epigallocatechin and epicatchin-3-gallate.30 Catechins show direct antioxidative properties, leading to hydrogen atom transfer or single electron transfer reactions, inhibition of lipid peroxidation, and free radical scavenging. 31,32 A particular protective effect is exerted by epigallocatechin-3-gallate because of its ability to decrease lipid peroxidation, oxidative stress and the production of nitric oxide radicals. Epigallocatechin-3-gallate also ameliorates the overproduction of pro-inflammatory cytokines and mediators, and reduces the activity of NF-KB and AP-1 and the subsequent formation of peroxynitrite with nitric oxide and reactive oxygen species.33 This antioxidant effect of tea is supported by several clinical studies and it is noteworthy that green tea extracts retain the beneficial effects of green and black tea.34,35 In addition, a beneficial effect of Camelia Sinensis extracts on the integrity of the oral mucosa after exposure to cytotoxic drugs is supported by studies which demonstrate that catechins extracted from green tea are chemopreventive, natural healing, and anti-aging agents for human skin.36
Other medical mouthwashes, topical agents (such as chlorhexidine, PVP-iodide or benzy-damine) or cryotherapy have been evaluated in medical studies carried out on small series of patients. For example, Adamietz et al.37 evaluated 20 patients undergoing radio-chemotherapy and concluded that rinsing with povidoneiodine was an easy, cheap and safe prophylactic method and that it could be recommended as a supportive treatment during antineoplastic treatment of the head and neck region. Pfeiffer et al.38 evaluated sucralfate (a wellknown gastric mucosal protective agent) in OM prophylaxis in patients undergoing therapy with cisplatin and continuous infusion with 5-fluorouracil, and among 23 evaluable patients found a significant reduction in an objective score of edema, erythema, erosion and ulcerations. However, 10 patients did not complete the study since swishing the mouthwash around the mouth aggravated chemotherapy-induced nausea. Salvador et al.18 studied 23 patients undergoing transplantation with autologous HCST and treated with a 60-minute regimen of oral cryotherapy. They found that the overall mean of oral mucositis severity for the experimental arm was significantly lower than that for the control group, and that similar results could be observed in terms of overall mean mucositis-related pain score. They concluded that oral cryotherapy plus an oral care protocol appeared to be beneficial in preventing severe OM.
Some systematic reviews analyzed the results of randomized studies carried out with single agents that were considered potentially useful in reducing incidence and/or severity of OM in cancer patients. In one of these review articles,39 four agents, each in single trials, were found to exert a weak protective action allopurinol, granulocyte macrophage-colony stimulating factor (GM-CSF), immunoglobulin, human placental extracts. The following agents were not found to be effective: benzy-damine, HCl, sucralfate, tetrachlorodecaoxide, chlorhexidine.
Niscola et al.5 reviewed the results of clinical trials involving various growth factors and cytokines and reported no beneficial effects for GM-CSF, transforming growth factor-ß3, whey-derived growth factor extract, rhIL-11, epidermal growth factor.
There is consistent evidence for a protective action of two agents that are given intravenously, such as repifermin (keratinocyte growth factor-2) and palifermin (recombinant human keratinocyte growth factor-1).
Only one study evaluated the usefulness of repifermin in patients undergoing autologous hematopoietic stem cell transplantation.40
More information is available about palifermin. Following the first clinical study, reported by Spielberger et al.41 carried out in patients treated with chemotherapy, radiotherapy or autologous HCST, a significant protective action of palifermin has been confirmed, as discussed by Blijlevens and Sonis.42 As far as the allogeneic HCST setting is concerned, two multicenter studies reported a reduced incidence of OM (range 30-40%) in patients treated with palifermin, with a more significant efficacy in the lower WHO OM grades.43,44
Amifostine is another systemic agent capable of reducing incidence and/or severity of OM in hematologic patients undergoing HCST. Both Capelli et al.45 and Thieblemont et al.46 reported a significant protective action of amifostine in patients with multiple myeloma receiving high-dose melphalan as a conditioning agent for autologous HCST, with a 50% decrease in the occurrence of severe OM.
Conclusions
If we compare the results of our study with these reports, which are very similar in terms of both patients’ characteristics and number of patients evaluated, we find that Baxidl Onco® mouthwash exerted a protective action that was comparable to that shown both by palifermin and by amifostine, without their possible adverse effects. The results obtained in our study indicate that oral rinsing with Baxidil Onco® mouthwash in addition to the standard prophylaxis can significantly reduce incidence, severity and duration of OM. Compared to other prophylactic agents, such as palifermin and amifostine, we think that Baxidil Onco® is cheaper, easier to use and capable of exerting similar effects when used in hematologic patients undergoing HCST. Further studies involving a larger number of patients are needed to validate the results obtained so far.
References
- 1.Blijlevens NM, Donnelly JP, De Pauw BE. Mucosal barrier injury: biology, pathology, clinical counterparts and consequences of intensive treatment for haematological malignancy: an overview. Bone Marrow Transplant. 2000; 25:1269-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elting LS, Cooksley C, Chambers M, et al. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003; 98:1531-9 [DOI] [PubMed] [Google Scholar]
- 3.Duncan M, Grant G. Review article: oral and intestinal mucositis - causes and possible treatments. Aliment Pharmacol Ther 200853-74 [DOI] [PubMed] [Google Scholar]
- 4.Walsh LJ. Clinical assessment and management of the oral environment in the oncology patient. Aust Dent J 2010;55 Suppl 1:66-77 [DOI] [PubMed] [Google Scholar]
- 5.Niscola P, Romani C, Cupelli L, et al. Mucositis in patients with hematologic malignancies: an overview. Haematologica. 2007; 92:222-31 [DOI] [PubMed] [Google Scholar]
- 6.Wardley AM, Jayson GC, Swindell R, et al. Prospective evaluation of oral mucositis in patients receiving myeloablative conditioning regimens and haemopoietic progenitor rescue. Br J Haematol 2000;110:292-9 [DOI] [PubMed] [Google Scholar]
- 7.Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002; 99:731-5 [DOI] [PubMed] [Google Scholar]
- 8.Wang EH, Chen YA, Corringham S, et al. High-dose CEB vs BEAM with autologous stem cell transplant in lymphoma. Bone Marrow Transplant. 2004; 34:581-7 [DOI] [PubMed] [Google Scholar]
- 9.Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100 Suppl 9:1995-2025 [DOI] [PubMed] [Google Scholar]
- 10.Vera-Llonch M, Oster G, Ford CM, et al. Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support Care Cancer. 2007; 15:491-4 [DOI] [PubMed] [Google Scholar]
- 11.Ozturk M, Komurcu S, Kilic S, et al. Self-reported experience of mucositis in cancer patients who underwent conditioning regimen and stem cell transplantation. Support Care Cancer. 2009; 17:1295-9 [DOI] [PubMed] [Google Scholar]
- 12.Redding SW. Cancer Therapy-Related Oral Mucositis. J Dent Educ. 2005; 69:919-29 [PubMed] [Google Scholar]
- 13.Salvador P, Azusano C, Wang L, Howell D. A pilot randomized controlled trial of an oral care intervention to reduce mucositis severity in stem cell transplant patients. J Pain Symptom Manage. 2012; 4:64-73 [DOI] [PubMed] [Google Scholar]
- 14.Silva GB, Mendonça EF, Bariani C, et al. The prevention of induced oral mucositis with low-level laser therapy in bone marrow transplantation patients: a randomized clinical trial. Photomed Laser Surg. 2011; 29:27-31 [DOI] [PubMed] [Google Scholar]
- 15.Papas A, Johansen E. Prevention of mucositis in oncology patients undergoing radiation therapy. J Dent Res IADR 1984;63:311 [Google Scholar]
- 16.Ferretti GA, Ash RC, Brown AT, et al. Control of oral mucositis and candidiasis in marrow transplant patients: a prospective double-blind trial of chlorhexidine gluconate oral rinse. Bone Marrow Transplant. 1988; 3:483-93 [PubMed] [Google Scholar]
- 17.Vesole DH, Fuchs HJ. IB-367 Phase II Investigators. IB-367 reduces the number of days of severe oral mucositis complicating myeloablative chemotherapy. Blood 1999;94:154a [Google Scholar]
- 18.Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients with cancer. Dent Clin North Am 2008;52:61-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, van Besien K, Khouri I, et al. Carmustine, etoposide, cytarabine and melphalan as a preparative regimen for allogeneic transplantation for high-risk malignant lymphoma. Ann Oncol 1999;10:527-32 [DOI] [PubMed] [Google Scholar]
- 20.Musso M, Scalone R, Marcacci G, et al. Fotemustine plus etoposide, cytarabine and melphalan (FEAM) as a new conditioning regimen for lymphoma patients undergoing auto-SCT: a multicenter feasibility study. Bone Marrow Transplant. 2010; 45:1147-53 [DOI] [PubMed] [Google Scholar]
- 21.Beaven AW, Moore DT, Sharf A, et al. Infusional mitoxantrone plus bolus melphalan as a stem cell transplant conditioning regimen for multiple myeloma. Cancer Invest. 2011; 29:214-9 [DOI] [PubMed] [Google Scholar]
- 22.Santos GW. Busulfan (Bu) and cyclophosphamide (Cy) for marrow transplantation. Bone Marrow Transplant 1989;4 Suppl 1:236-9 [PubMed] [Google Scholar]
- 23.Nakamae H, Storer BE, Storb R, et al. Low-dose total body irradiation and fludarabine conditioning for HLA class I-mismatched donor stem cell transplantation and immunologic recovery in patients with hematologic malignancies: a multicenter trial. Biol Blood Marrow Transplant. 2010; 16:384-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta T, Kannan S, Dantkale V, Laskar S. Cyclophosphamide plus total body irradiation compared with busulfan plus cyclophosphamide as a conditioning regimen prior to hematopoietic stem cell transplantation in patients with leukemia: a systematic review and meta-analysis. Hematol Oncol Stem Cell Ther. 2011; 4:17-29 [DOI] [PubMed] [Google Scholar]
- 25.Fujimaki K, Tanaka M, Takasaki H, et al. Thiotepa/cyclophosphamide/TBI as a conditioning regimen for allogeneic hematopoietic stem cell transplantation in patients aged 50 years and over. Intern Med. 2008; 47:379-83 [DOI] [PubMed] [Google Scholar]
- 26.Ciurea SO, Saliba R, Rondon G, et al. Reduced-intensity conditioning using fkudarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant. 2010; 45:429-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venigalla ML, Azar D, Becker P, et al. A novel conditioning regimen with excellent DFS using thiotepa, melphalan and cyclophosphamide in bone marrow transplantation of multiple myeloma. Proc Am Soc Clin Oncol 2002:21 [Google Scholar]
- 28.Greene JC, Vermillion JK. The simplified oral hygiene index. J Am Dent Assoc. 1964; 68:7-13 [DOI] [PubMed] [Google Scholar]
- 29.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results in cancer patients. Cancer. 1981; 47:207-14 [DOI] [PubMed] [Google Scholar]
- 30.Yang CS, Lambert JD, Sang S. Antioxidative and anti-carcinogenetic activities of tea polyphenols. Arch Toxicol. 2009; 83:11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003; 43:89-143 [DOI] [PubMed] [Google Scholar]
- 32.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010; 501:65-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets. 2007; 7:135-44 [DOI] [PubMed] [Google Scholar]
- 34.Rietveld A, Wiseman S. Antioxidant effects of tea: evidence from human clinical trials. J Nutr 2003;133:3285S-92S [DOI] [PubMed] [Google Scholar]
- 35.Henning SM, Niu Y, Lee NH, et al. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004; 80:1558-64 [DOI] [PubMed] [Google Scholar]
- 36.Hsu S. Green tea and the skin. J Am Acad Dermatol. 2005; 52:1049-59 [DOI] [PubMed] [Google Scholar]
- 37.Adamietz IA, Rahn R, Böttcher HD, et al. Prophylaxis with povidone-iodine against induction of oral mucositis by radioche - motherapy. Support Care Cancer 1998;6:373-7 [DOI] [PubMed] [Google Scholar]
- 38.Pfeiffer P, Hansen O, Madsen EL, May O. Effect of prophylactic sucralfate suspension on stomatitis induced by cancer chemotherapy. A randomized, double-blind cross-over study. Acta Oncol. 1990; 29:471-3 [DOI] [PubMed] [Google Scholar]
- 39.Clarkson JE, Worthington HV, Eden OB. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2007;18:CD001973. [DOI] [PubMed] [Google Scholar]
- 40.Freytes CO, Ratanatharathorn V, Taylor C, et al. Phase I/II randomized trial evaluating the safety and clinical effects of Repifermin administered to reduce mucositis in patients undergoing autologous hematopoietic stem cell transplantation. Clin Cancer Res. 2004; 10:8318-24 [DOI] [PubMed] [Google Scholar]
- 41.Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004; 351:2590-8 [DOI] [PubMed] [Google Scholar]
- 42.Blijleven N, Sonis S. Palifermin (recombinant keratinocyte growth factor-1): a pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann Oncol. 2007; 18:817-26 [DOI] [PubMed] [Google Scholar]
- 43.Nasilowska-Adamska B, Rzepecki P, Manko J, et al. The influence of palifermin (Kepivance) on oral mucositis and acute graft versus host disease in patients with hematological diseases undergoing hematopoietic stem cell transplant. Bone Marrow Transplant. 2007; 40:983-8 [DOI] [PubMed] [Google Scholar]
- 44.Langner S, Staber PB, Schub N, et al. Palifermin reduces incidence and severity of oral mucositis in allogeneic stem-cell transplant recipients. Bone Marrow Transplant. 2008; 42:275-9 [DOI] [PubMed] [Google Scholar]
- 45.Capelli D, Santini G, De Souza C, et al. Amifostine can reduce mucosal damage after high-dose melphalan conditioning for peripheral blood progenitor cell autotransplant: a retrospective study. Br J Haematol. 2000; 110:300-7 [DOI] [PubMed] [Google Scholar]
- 46.Thieblemont C, Dumontet C, Saad H, et al. Amifostine reduces mucosal damage after high-dose melphalan conditioning and autologous peripheral blood progenitor cell transplantation for patients with multiple myeloma. Bone Marrow Transplant. 2002; 30:769-75 [DOI] [PubMed] [Google Scholar]


