Abstract
Using the isolated rat kidney perfused with an artificial medium containing glucose as the sole fuel, we studied the renal handling of immunoreactive arginine vasopressin (AVP) and determined the effect of various factors on the ability of the kidney to remove AVP.
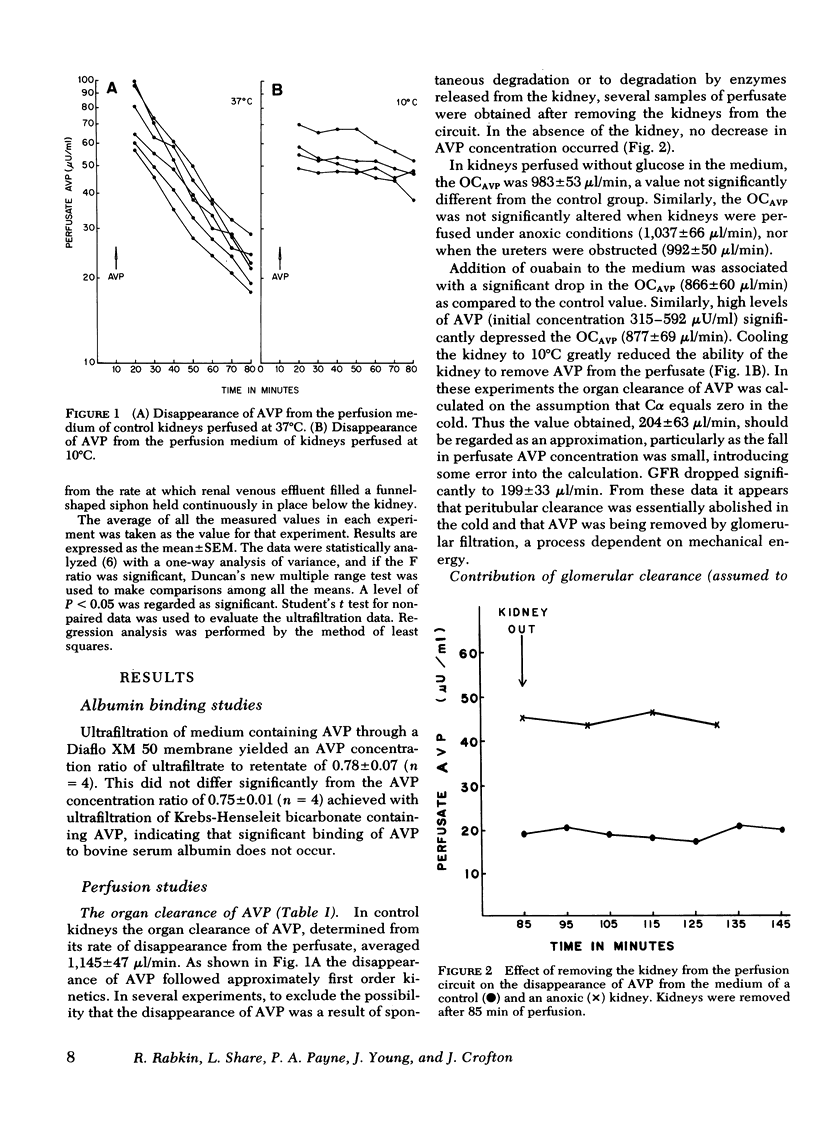
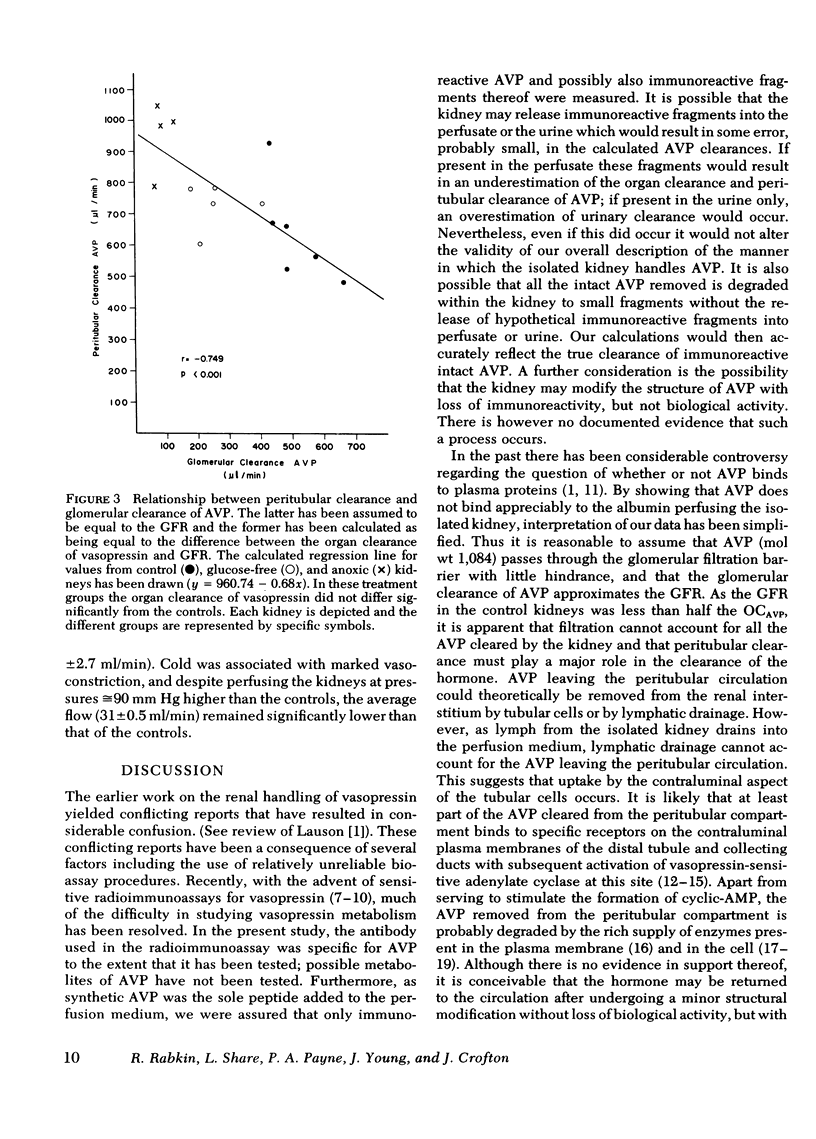
In control kidneys perfused with AVP at concentrations below 116 μU/ml, the organ clearance of AVP (OCAVP) was 1,145±47 (SE) μl/min, whereas glomerular filtration rate (GFR) averaged 515±37 μl/min. Filtration could thus account for up to 45% of the OCAVP, the balance presumably being cleared from the peritubular circulation. Of the AVP filtered, only 38% could be recovered in the urine (urinary clearance AVP averaged 205±12 μl/min) suggesting that the balance was taken up by the tubular epithelium and degraded. Fractional excretion of filtered AVP rose significantly in the presence of anoxia and cold (10°C) to 49 and 59%, respectively, but was not affected by ouabain or high levels of AVP (458±58 μU/ml).
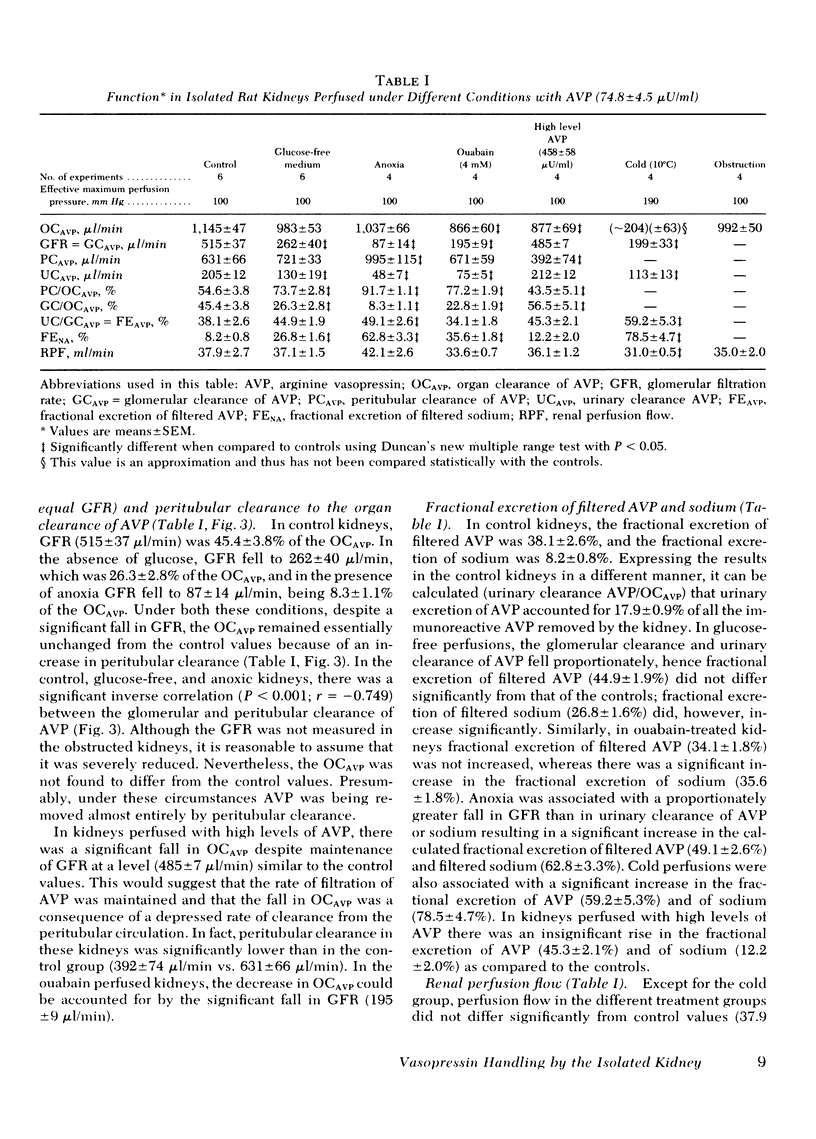
The OCAVP was not significantly altered by the absence of glucose in the perfusate, anoxia, or ureteral ligation, maneuvers that were associated with significant reductions in GFR. In these and the control experiments, there was a significant inverse correlation between GFR and peritubular clearance emphasizing the importance of the latter (r = −0.749; P < 0.001). Cold, ouabain, and high concentrations of AVP reduced the clearance of AVP by the kidneys.
On the basis of these studies we conclude that the kidney clears AVP from the circulation via two pathways, glomerular clearance and peritubular clearance. This exposes both the luminal and contraluminal surfaces of the tubular cells to the hormone, allowing these cells to remove AVP from the filtrate and the peritubular compartment. Noteworthy is the observation that under several conditions when GFR falls reducing the glomerular clearance of AVP, peritubular clearance increases and the total clearance of AVP by the kidney remains constant.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann G., Dingman J. F. Distribution, blood transport, and degradation of antidiuretic hormone in man. J Clin Invest. 1976 May;57(5):1109–1116. doi: 10.1172/JCI108377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardwell C. G. Radioimmunoassay of arginine vasopressin in human plasma. J Clin Endocrinol Metab. 1971 Aug;33(2):254–260. doi: 10.1210/jcem-33-2-254. [DOI] [PubMed] [Google Scholar]
- Bockaert J., Roy C., Rajerison R., Jard S. Specific binding of (3H) lysine-vasopressin to pig kidney plasma membranes. Relationship of receptor occupancy to adenylate cyclase activation. J Biol Chem. 1973 Sep 10;248(17):5922–5931. [PubMed] [Google Scholar]
- Campbell B. J., Woodward G., Borberg V. Calcium-mediated interactions between the antidiuretic hormone and renal plasma membranes. J Biol Chem. 1972 Oct 10;247(19):6167–6175. [PubMed] [Google Scholar]
- Carone F. A., Pullman T. N., Oparil S., Nakamura S. Micropuncture evidence of rapid hydrolysis of bradykinin by rat proximal tubule. Am J Physiol. 1976 May;230(5):1420–1424. doi: 10.1152/ajplegacy.1976.230.5.1420. [DOI] [PubMed] [Google Scholar]
- DICKER S. E., GREENBAUM A. L. Inactivation of the antidiuretic activity of vasopressin by tissue homogenates. J Physiol. 1956 Apr 27;132(1):199–212. doi: 10.1113/jphysiol.1956.sp005514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousa T., Hechter O., Schwartz I. L., Walter R. Neurohypophyseal hormone-responsive adenylate cyclase from mammalian kidney. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1693–1697. doi: 10.1073/pnas.68.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG M. The clearance of vasopressin from the splanchnic vascular area and the kidneys. J Endocrinol. 1957 Dec;16(2):217–226. doi: 10.1677/joe.0.0160217. [DOI] [PubMed] [Google Scholar]
- Green R., Giebisch G. Ionic requirements of proximal tubular sodium transport. I. Bicarbonate and chloride. Am J Physiol. 1975 Nov;229(5):1205–1215. doi: 10.1152/ajplegacy.1975.229.5.1205. [DOI] [PubMed] [Google Scholar]
- Harvey N., Jones J. J., Lee J. The renal clearance and plasma binding of vasopressin in the dog. J Endocrinol. 1967 Jun;38(2):163–171. doi: 10.1677/joe.0.0380163. [DOI] [PubMed] [Google Scholar]
- Husain M. K., Fernando N., Shapiro M., Kagan A., Glick S. M. Radioimmunoassay of arginine vasopressin in human plasma. J Clin Endocrinol Metab. 1973 Oct;37(4):616–625. doi: 10.1210/jcem-37-4-616. [DOI] [PubMed] [Google Scholar]
- Johnson M. D., Park C. S., Malvin R. L. Antidiuretic hormone and the distribution of renal cortical blood flow. Am J Physiol. 1977 Feb;232(2):F111–F116. doi: 10.1152/ajprenal.1977.232.2.F111. [DOI] [PubMed] [Google Scholar]
- LAUSON H. D., BOCANEGRA M., BEUZEVILLE C. F. HEPATIC AND RENAL CLEARANCE OF VASOPRESSIN FROM PLASMA OF DOGS. Am J Physiol. 1965 Jul;209:199–214. doi: 10.1152/ajplegacy.1965.209.1.199. [DOI] [PubMed] [Google Scholar]
- MORTIMORE G. E., TIETZE F., STETTEN D., Jr Metabolism of insulin-I 131; studies in isolated, perfused rat liver and hindlimb preparations. Diabetes. 1959 Jul-Aug;8(4):307–314. doi: 10.2337/diab.8.4.307. [DOI] [PubMed] [Google Scholar]
- Maack T. Renal handling of low molecular weight proteins. Am J Med. 1975 Jan;58(1):57–64. doi: 10.1016/0002-9343(75)90533-1. [DOI] [PubMed] [Google Scholar]
- Miller A. T., Jr, Hale D. M., Alexander K. D. Histochemical studies on the uptake of horseradish peroxidase by rat kidney slices. J Cell Biol. 1965 Nov;27(2):305–312. doi: 10.1083/jcb.27.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo J. M., Ross B. D., Krebs H. A. Metabolic activities of the isolated perfused rat kidney. Biochem J. 1967 Jun;103(3):852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullman T. N., Oparil S., Carone F. A. Fate of labeled angiotensin II microinfused into individual nephrons in the rat. Am J Physiol. 1975 Mar;228(3):747–751. doi: 10.1152/ajplegacy.1975.228.3.747. [DOI] [PubMed] [Google Scholar]
- Rabkin R., Kitabchi A. E. Factors influencing the handling of insulin by the isolated rat kidney. J Clin Invest. 1978 Jul;62(1):169–175. doi: 10.1172/JCI109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajerison R., Marchetti J., Roy C., Bockaert J., Jard S. The vasopressin-sensitive adenylate cyclase of the rat kidney. Effect of adrenalectomy and corticosteroids on hormonal receptor-enzyme coupling. J Biol Chem. 1974 Oct 25;249(20):6390–6400. [PubMed] [Google Scholar]
- Robertson G. L., Klein L. A., Roth J., Gorden P. Immunoassay of plasma vasopressin in man. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1298–1305. doi: 10.1073/pnas.66.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson G. L., Mahr E. A., Athar S., Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest. 1973 Sep;52(9):2340–2352. doi: 10.1172/JCI107423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. D., Epstein F. H., Leaf A. Sodium reabsorption in the perfused rat kidney. Am J Physiol. 1973 Nov;225(5):1165–1171. doi: 10.1152/ajplegacy.1973.225.5.1165. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Patlak C. S., Andreoli T. E. A component of fluid absorption linked to passive ion flows in the superficial pars recta. J Gen Physiol. 1975 Oct;66(4):445–471. doi: 10.1085/jgp.66.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L., Watkins M. L., Andreoli T. E. Volume absorption in the pars recta. I. "Simple" active Na+ transport. Am J Physiol. 1978 Apr;234(4):F332–F339. doi: 10.1152/ajprenal.1978.234.4.F332. [DOI] [PubMed] [Google Scholar]
- Shade R. E., Share L. Metabolic clearance of immunoreactive vasopressin and immunoreactive [131I]iodo vasopressin in the hypophysectomized dog. Endocrinology. 1976 Nov;99(5):1199–1206. doi: 10.1210/endo-99-5-1199. [DOI] [PubMed] [Google Scholar]
- Shade R. E., Share L. Renal vasopressin clearance with reductions in renal blood flow in the dog. Am J Physiol. 1977 Apr;232(4):F341–F347. doi: 10.1152/ajprenal.1977.232.4.F341. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Karnovsky M. J. Extravascular protein in the kidney. An ultrastructural study of its relation to renal peritubular capillary permeability using protein tracers. Lab Invest. 1972 Nov;27(5):435–444. [PubMed] [Google Scholar]
- Walter R., Bowman R. H. Mechanism of inactivation of vasopressin and oxytocin by the isolated perfused rat kidney. Endocrinology. 1973 Jan;92(1):189–193. doi: 10.1210/endo-92-1-189. [DOI] [PubMed] [Google Scholar]
- Walter R., Shlank H. Differences in the enzymatic inactivation of arginine vasopressin and oxytocin by rat kidney homogenate. Endocrinology. 1975 Mar;96(3):811–814. doi: 10.1210/endo-96-3-811. [DOI] [PubMed] [Google Scholar]


