Abstract
Abstract
Mitochondrion-rich adenocarcinomas represent a rare variant of gastric adenocarcinomas composed predominantly of columnar adenocarcinoma cells with eosinophilic cytoplasm, a strong supranuclear immunoreactivity for antimitochondrial antibody, and a marked neutrophil infiltration associated to tumor cell death. The purpose of this work is to investigate, using correlated light and electron microscopy, mitochondrion-rich gastric adenocarcinomas focusing on the nature of the death in neoplastic cells and in infiltrating neutrophils. Adenocarcinoma cells, single or in small clusters, showed convoluted nuclei, irregularly condensed chromatin, loss of microvilli, and nuclear envelope dilatation. No nuclear fragmentation was observed in these dying cells and the plasma membrane did not show signs of disruption. These ultrastructural findings represent intermediate aspects between apoptosis and necrosis and are compatible with apoptosis-like programmed cell death. By contrast, some infiltrating neutrophils showed ultrastructural signs of classic apoptosis such as chromatin condensation into compact geometric (globular, crescentshaped) figures, tightly packed cytoplasmic granules and intact cell membrane. Our study provides ultrastructural evidence of apoptosislike tumour cell death in mitochondrion-rich gastric carcinomas and confirms that stereotyped outcome either as apoptosis or necrosis of tumor cells cannot always be expected in human neoplasms.
Key words: mitochondrion-rich gastric carcinoma, neutrophils, apoptosis-like programmed cell death, electron microscopy
Introduction
The term cell necrobiology was introduced to comprise the life processes associated with morphological, biochemical, and molecular changes which predispose, precede, and accompany cell death, as well as the consequences and tissue response to cell death.1 Cell necrobiology will focus on the physiology of dying cells and will aim to identify molecular targets for therapeutic intervention, either with the presently available or newly obtained drugs.1 Two alternative modes of cell death can be distinguished, apoptosis and necrosis. The expression apoptosis has been coined by Kerr et al.2 to describe the phenomenon as a mode of cell death morphologically separate from coagulative necrosis. Apoptosis is characterized by the rounding of the cell, chromatin condensation (pyknosis), nuclear fragmentation (karyorhexis), and engulfment by neighboring cells.3 Despite subsequent introduction of numerous molecular assays, the morphological changes, detected by light and electron microscopy, still remain the gold standard to differentiate these two distinct modes of cell death.3 Noteworthy, recent reports led to the characterization of alternative cell demise modes such as caspase-independent apoptosis-like programmed cell death (PCD), autophagy, necrosis-like PCD, and mitotic catastrophe.4,5 Although still a matter of debate, these noncanonical pathways appear to have wide reaching connotations in pathogenesis and treatment of human diseases.6,7
In our previous paper, we described clinicopathologic characteristics of mitochondrionrich variant gastric carcinomas and we showed focal neutrophil infiltration associated with tumor cell death.8 This second paper of mitochondrion-rich gastric carcinomas was undertaken with two specific aims: i) to characterize from an ultrastructural viewpoint the type of cell death in adenocarcinoma cells, and ii) to study the relationship between neutrophils and cancer cells. In addition, some of the experimental and clinical data which are pertinent to the interpretation of ultrastructural observations will also be discussed.
Materials and Methods
The fragments of tumor tissue were obtained from gastric carcinomas resected from 9 patients. Only tissue available for study within 30 minutes of surgical removal was included to ensure optimal fixation. The fragments of fresh tumor tissue were divided into two portions with a sharp razor blade. The first member of the pair was processed for routine paraffin embedding together with additional tissue samples taken from the tumor as well as from surgical borders of the specimen. These sections were stained with hematoxylin-eosin. The second piece of the paired samples was minced into smaller pieces and destined for electron microscopy. This material was fixed in 3% phosphate-buffered glutaraldeyde pH 7.2 and post-fixed in 1% osmium tetroxide. Semithin sections of these araldite-embedded blocks were stained with Giemsa’s reagent. Thin sections were double-stained with uranyl acetate and lead citrate; they were then examined and photographed under a JEOL 1200 EX electron microscope at 70 kv.
Electron Microscopy Evaluation of Cell Death Phenotype
Cell death phenotype was classified into four subclasses, according to their nuclear ultrastructure.5 Apoptosis is defined by chromatin condensation into compact and apparently simple geometric (globular, crescentshaped) figures. Apoptosis-like PCD is characterized by chromatin condensation that is less compact than in apoptosis (geometrically more complex and lumpier shape). In contrast, in necrosis-like PCD no chromatin condensation is observed, but at best, chromatin clustering to loose speckles, whereas necrosis is characterized by cytoplasmic swelling and cell membrane rupture.5
The Nomenclature Committee on Cell Death (NCCD) has also proposed that a cell should be regarded as dead when i) the cell has lost the integrity of its plasma membrane and/or ii) the cell, including its nucleus, has undergone complete disintegration, and/or iii) its corpse (or its fragments) has been engulfed by a neighboring cell in vivo.3
Results
The main clinicopathologic characteristics of mitochondrion-rich gastric adenocarcinomas are summarized in Table 1.
Table 1.
Clinicopathologic findings of mitochondrion-rich gastric adenocarcinomas.
| Case | Age | Gender | Site | Size | pTNM stage | Histology |
|---|---|---|---|---|---|---|
| 1 | 68 | M | Antrum | 3.5 | T2N0M0 | Cribriform |
| 2 | 55 | F | Antrum | 3 | T1N1M0 | Tubulopapillary |
| 3 | 80 | M | Antrum | 4 | T2N0M0 | Cribriform |
| 4 | 67 | M | Antrum | 6 | T3N0M0 | Tubulopapillary |
| 5 | 65 | F | Body | 3 | T2N0M0 | Tubulopapillary |
| 6 | 69 | M | Antrum | 4.5 | T2N0M0 | Cribriform |
| 7 | 70 | F | Body | 2.5 | T2N0M0 | Tubulopapillary |
| 8 | 75 | M | Antrum | 4 | T3N0M0 | Tubulopapillary |
| 9 | 65 | M | Antrum | 2 | T2N0M0 | Tubulopapillary |
On microscopic examination, tumors exhibited a tubulopapillary growth pattern with a focal intratumoural neutrophil infiltrate (Figure 1A). Cribriform pattern was also seen. Distinct focal gland disruptions and neutrophil infiltration were detected in tumor tissue (Figure 1B). The size of disruption varied substantially among glands, ranging from a few cells to over 80% of the neoplastic gland. Adenocarcinoma cells in contact with neutrophils showed signs of injury such as shrinkage, condensed hyperchromatic chromatin, cytoplasmic vacuolization (Figure 1B). Cell shrinkage, chromatin condensation and cytoplasmic vacuolization were also seen in single adenocarcinoma cells not contacted by neutrophils (Figure 2A).
Figure 1.
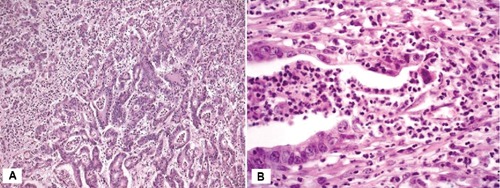
A) Mitochondrion-rich gastric adenocarcinoma (case 1). Adenocarcinoma glands lined by columnar cells with eosinophilic cytoplasm. Neutrophils are seen both in the tumor stroma and within neoplastic glands. Haematoxylin & Eosin x100. B) Mitochondrion-rich gastric adenocarcinoma (case 2). Focal adenocarcinoma gland disruption associated with neutrophil transepithelial migration. Dying adenocarcinoma cells show cytoplasmic vacuolization and dark nuclei. Haematoxylin & Eosin x200.
Figure 2.
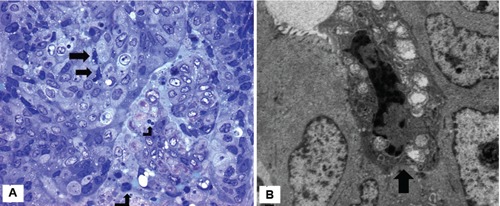
A) Single adenocarcinoma cells show convoluted nuclei and cytoplasmic vacuolization (arrows). A few neutrophils are seen in the tumour stroma (case 3). Semithin section; Giemsa x400; B) single adenocarcinoma cell characterized by marked chromatin condensation, convoluted nucleus, loss of microvilli and mitochondrial swelling (arrow). The adjacent tumor cells appear morphologically well preserved (case 4), x6000.
Under electron microscope tumour cells, considered as healthy, exhibited intact membranes, numerous mitochondria and nuclei which were relatively homogeneous and displayed low electron density (Figure 2B). Other adenocarcinoma cells, single or in small clusters, showed progressive increase in the electron density of the cytoplasmic matrix, intact cell membrane, loss of microvilli, mitochondrial swelling and nuclear envelope dilalatation. Chromatin was condensed, but irregularly, and the nuclei were heavily convoluted (Figure 2B). No nuclear fragmentation was observed in these dying cells and the plasma membrane did not show signs of disruption. The ultrastructural observations of dying cells were coincident with those described in apoptosis-like PCD, according to Leist and Jäättelä classification.5 A remarkable finding was the presence of scattered single adenocarcinoma cells with this type of PCD in contact with neutrophils (Figures 3A,B and 4A). Other neutrophils showed classic ultrastructural signs of apoptosis such as chromatin condensation into compact geometric (globular, crescent-shaped) figures, tightly packed cytoplasmic granules and intact cell membrane (Figure 4B).
Figure 3.
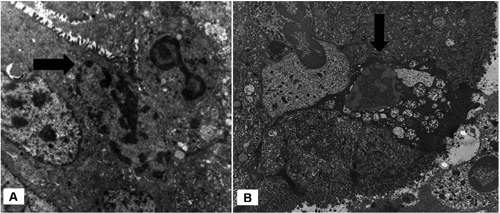
A) Intraluminal migration of neutrophil in adenocarcinoma gland. The nucleus of tumor cell in contact with the neutrophil shows patches of localized partially condensed chromatin along the inner part of nuclear membrane (arrow) (case 6), x8000. B) neutrophils in contact with adenocarcinoma cell showing chromatin condensation and dilatation of the nuclear envelope (arrow) (case 7), x6000.
Figure 4.
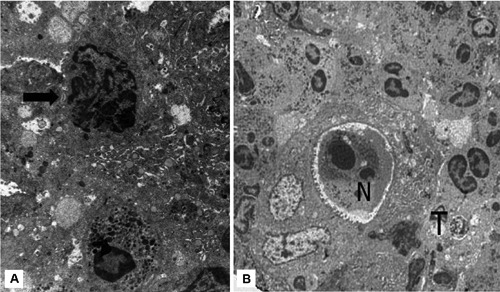
A) Adenocarcinoma cell showing loss of microvilli, heavily convoluted nucleus and patches of localized condensed chromatin abutting along the inner part of the nuclear membrane. Note the presence of intraepithelial neutrophil (arrow) (case 8), x6000. B) Intraglandular apoptotic neutrophil (N) showing characteristic geometric condensation of nuclear chromatin. By contrast, adenocarcinoma cell (T) displays apoptotis-like PCD features including convoluted nuclei, irregular chromatin condensation, cytoplasmic vacuolization and loss of microvilli (arrow) (case 9), x4000.
Discussion
We have studied cell death in mitochondrion-rich gastric carcinomas by histological and ultrastructural methods. At the light microscopic level, dying tumor cells may be recognized by their condensed chromatin and reduced cell volume, and cytoplasmic vacuolization. A clear identification of morphologic characteristics, achievable exclusively by electron microscopy analysis, is considered crucial for assessment of occurrence of cell death as well as to identify dying cells that have not yet concluded their demise.3,9
Our ultrastructural data show tumor cells characterized by convoluted nuclei, chromatin condensation at the nuclear periphery, loss of microvilli, variably swollen mitochondria and dilatation of the nuclear envelope. Therefore, dying tumor cells had structures that are neither classically apoptotic nor necrotic, being similar to that described in apoptosis-like PCD.5 It is generally believed that necrosis triggers substantial inflammatory response while apoptosis does not. However, in recent years, it was recognized that apoptotic cell death can trigger neutrophil recruitment.10-12 Our ultrastructural study extends these observations showing association between viable infiltrating neutrophils and apoptotic-like tumor cells.
Much of what is known about neutrophil transepithelial migration comes from in vitro studies using intestinal epithelial monolayers. Le Negrate et al.13 provide the first evidence that neutrophil transmigration across cultured intestinal cell monolayers triggers apoptosis of epithelial cells. After neutrophil transmigration for 12 hr, intestinal cell monolayers exhibited the morphological features of apoptotic cell death, such as brush border alteration, chromatin condensation, and cytoplasmic shrinkage. On the other hand, experimental data suggest that neutrophils do not attack healthy cells, but respond to distressed or dying cells.14 Taken together, experimental models suggest two alternative possibilities: i) neutrophils may be responsible of apoptoticlike PCD observed in adenocarcinoma cells; ii) neutrophil recruitment is due to dying tumor cells. Thus, if neutrophils sense the stressed tumor cell through chemoattractant mediators or direct cell contact, they will attack and kill these tumor cells. We prefer the second hypothesis because single dying tumor cells could be found not associated with migrating neutrophils. As this is a purely morphologic study, however, no direct evidence of cytotoxicity can be provided. It remains to be investigated whether focal adenocarcinoma gland disruption is triggered off by infiltrating neutrophils. This question could be resolved through experimental studies of developing disruptions, as opposed to existing disruptions described above. Apoptosis is an ubiquitous and highly regulated mechanism by which cell undergo programmed cell death. However, cancer cells frequently harbor acquired mutations that allow them to escape spontaneous and therapy-induced apoptosis.15 For example, the upregulation of antiapoptotic Bcl-2 family members and mutations in the p53 tumor suppressor protein are common in human tumors. Apoptosis-resistant cancer cells are, however, not completely resistant to cell death, but can die via alternative cell death pathways.16 Accordingly, new strategies to kill cancer cells by nonapoptotic mechanisms have flourished during the past decade, and many inducers of alternative caspase-independent cell death pathways have been identified. Examples of this include: the topoisomerase inhibitor camptothecin induces cathepsin-mediated apoptosis-like PCD in hepatocellular carcinoma cells;17 antibodies to CD99 trigger a rapid apoptosis-like PCD in transformed T cells;18 breast cancer cells can be triggered to undergo caspase-independent PCD by treatment with vitamin D analogues;19 apoptosis-like cell death of human breast cancer cell line MCF-7 is induced by buprenorphine hydrochloride;20 human alpha-lactalbumin made lethal to tumor cells (HAMLET) kills human glioblastoma cells in brain xenografts by an apoptosislike mechanism;21 nano-bubble hydrogen water with platinum colloid is potent as an anti-tumor agent and induces apoptosis-like cell death.22 Nonapoptotic mechanisms of cell death have been largely overlooked in studies of cancer causation, progression and therapy. It is important for cancer researchers to consider the presence and impact of apoptotic-like cell death and similar less studied processes when interpreting clinical trials and developing drugs for modulation of aberrant cellular pathways.
Conclusions
In conclusion, our ultrastructural study provides morphological evidence of apoptosis-like PCD in a subset of gastric adenocarcinoma, where dying tumor cells showed intermediate aspects between apoptosis and necrosis. This intermediate form of tumor cell death was associated with infiltration of neutrophils, some of which showed classic apoptotic changes. Elucidation of the molecular mechanisms of apoptosis-like cell death is expected to contribute to the development of novel therapeutic strategies against cancer, which often acquires resistance against apoptotic cell death.
References
- 1.Darzynkiewicz Z, Juan G, Li X, et al. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis). Cytometry 1997;27:1-20 [PubMed] [Google Scholar]
- 2.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wideranging implications in tissue kinetics. Br J Cancer 1972;26:239-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009;16:3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci 2007;32:37-43 [DOI] [PubMed] [Google Scholar]
- 5.Leist M, Jäättelä M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2001;2:589-98 [DOI] [PubMed] [Google Scholar]
- 6.Lockshin RA, Zakeri Z. Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol Cell Biol 2001;2:545-50 [DOI] [PubMed] [Google Scholar]
- 7.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer 2004;4:592-603 [DOI] [PubMed] [Google Scholar]
- 8.Caruso RA, Napoli P, Nania A, et al. Mitochondrion-rich differentiated adenocarcinomas of the stomach: clinicopathological, immunohistochemical and electron microscopy study of nine case. Virchows Arch 2010;456:499-505 [DOI] [PubMed] [Google Scholar]
- 9.Galluzzi L, Aaronson SA, Abrams J, et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ 2009;16:1093-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol 2009;9:353-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Restifo NP. Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptive immunity. Curr Opin Immunol 2000;12:597-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawson JA, Fisher MA, Simmons CA, et al. Parenchymal cell apoptosis as a signal for sinusoidal sequestration and transendothelial migration of neutrophils in murine models of endotoxin and Fasantibody-induced liver injury. Hepatology 1998;28:761-7 [DOI] [PubMed] [Google Scholar]
- 13.Le’Negrate G, Selva E, Auberger P, et al. Sustained polymorphonuclear leukocyte transmigration induces apoptosis in T84 intestinal epithelial cells. J Cell Biol 2000;150:1479-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol 2006;290:G1083-8 [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70 [DOI] [PubMed] [Google Scholar]
- 16.Kroemer G, Jäättelä M. Lysosomes and autophagy in cell death control. Nat Rev Cancer 2005;5:886-97 [DOI] [PubMed] [Google Scholar]
- 17.Roberts LR, Adjei PN, Gores GJ. Cathepsins as effector proteases in hepatocyte apoptosis. Cell Biochem Biophys 1999;30:71-88 [DOI] [PubMed] [Google Scholar]
- 18.Pettersen RD, Bernard G, Olafsen MK, et al. CD99 signals caspase-independent T cell death. J Immunol 2001;166:4931-42 [DOI] [PubMed] [Google Scholar]
- 19.Mathiasen IS, Lademann U, Jäättelä M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res 1999;59:4848-56 [PubMed] [Google Scholar]
- 20.Kugawa F, Matsumoto K, Aoki M. Apoptosis-like cell death of human breast cancer cell line MCF-7 induced by buprenorphine hydrochloride. Life Sci 2004;75:287-99 [DOI] [PubMed] [Google Scholar]
- 21.Fischer W, Gustafsson L, Mossberg AK, Gronli J, Mork S, Bjerkvig R, Svanborg C. Human alpha-lactalbumin made lethal to tumor cells (HAMLET) kills human glioblastoma cells in brain xenografts by an apoptosis-like mechanism and prolongs survival. Cancer Res 2004;64:2105-12 [DOI] [PubMed] [Google Scholar]
- 22.Asada R, Kageyama K, Tanaka H, et al. Antitumor effects of nano-bubble hydrogen-dissolved water are enhanced by coexistent platinum colloid and the combined hyperthermia with apoptosis-like cell death. Oncol Rep 2010;24:1463-70 [DOI] [PubMed] [Google Scholar]


