Abstract
Abstract
Early stage testicular germ cell tumors are highly curable malignancies, but the need for close radiologic and biomarker surveillance is pivotal. Even in the setting of recurrence, rescue therapy has been successfully implemented. The present report describes a patient that had rapid and aggressive recurrence after radical orchiectomy for a testicular germ cell tumor and presented with bulky disease necessitating reconstruction of the inferior vena cava at the time of salvage retroperitoneal debulking.
Key words: testicular germ cell tumor, retroperitoneal tumor, teratoma
Introduction
Testicular germ cell tumors (TGCT) affect young men between the second and forth decades of life. Overall TGCTs are relatively rare (1-1.5%) male malignancies, however, TGCTs carry an excellent prognosis. Of an estimated 8600 cases diagnosed in 2012, only 360 men died.1 Broadly, TGCTs are classified into seminomas and non-seminomatous GCTs (NSGCT) (Table 1).2 In general, seminomatous tumors are more likely to present with localized disease, and are highly sensitive to radiation therapy. On the other hand, chemotherapy and surgical resection is the mainstay of treatment in advanced NSGCT.
Table 1.
WHO classification of testicular tumors.
| Germ cell tumors | Sex cord-stromal tumors |
|---|---|
| Seminoma | Sertoli cell tumor |
| Seminoma with syncytiotrophoblastic cells | Leydig cell tumor |
| Spermatocytic seminoma | Granulosa cell tumor |
| Spermatocytic seminoma with sarcoma | Mixed types (e.g. Sertoli-Leydig cell tumor) |
| Non-seminomatous germ cell tumors | Unclassified |
| Embryonal carcinoma | |
| Teratoma | |
| a. Dermoid cyst | |
| b. Monodermal teratoma | |
| c. Teratoma with somatic type malignancy | |
| Trophoblastic tumors (choriocarcinoma) | |
| Yolk sac tumor (endodermal sinus tumor) | |
| Mixed germ cell tumors |
The successful outcome of TGCTs stems from an aggressive multidisciplinary approach in therapeutics and surveillance modalities. This approach has changed the five-year survival from 66 percent forty years ago to 95 percent today.3 The successful improvement in therapeutics is the result of better understanding of natural history of the disease, improved surgical techniques, and use of platinumbased combination chemotherapy. Surveillance has drastically improved owing to the use of tumor markers and modern cross-sectional imaging as a guide in patient management.4
Early stage TGCTs are treated with radical inguinal orchiectomy. The role of retroperitoneal lymph node dissection (RLND) in early disease is limited such that in the absence of pathologic enlarged retroperitoneal lymph nodes, RLND is not indicated. However many centers perform staging RPLND for selected high risk patients with stage I disease. Patients are defined as having clinical stage I NSGCT if radiographic studies are negative and serum tumor markers post-orchiectomy are normal. The majority of stage I patients are cured with orchiectomy alone. However, it is difficult to identify which subset of these patients are at highest risk for recurrence and may benefit from adjuvant treatment.
Numerous attempts have been made to identify men with clinical stage I NSGCT at high risk for relapse. The risk factors for recurrence include: lymphovascular invasion (LVI), pathologic tumor stage above T2 (LVI or tumor extending through the tunica albuginea with involvement of the tunica vaginalis), and predominance of an embryonal component in the primary tumor.
In the present report, we discuss a patient without risk factors who had rapid enlargement of retroperitoneal disease, consisting of primarily teratoma elements with marginal response to chemotherapeutic intervention. Treatment options and outcomes are outlined.
Case Report
The patient is a 29 year-old Caucasian male who initially presented to an outside hospital emergency department with a right testicular mass. His past medical history was significant for substance abuse. He had no known history of cryptorchidism. His family history was positive for prostate cancer in his grandfather. He had no family history of testicular cancer. Physical exam was only remarkable for a right solid testicular mass.
A right scrotal sonographic examination revealed a heterogeneous testicular mass suspicious for testicular cancer. This was accompanied with an alpha fetoprotein (AFP) serum level of 670 ng/mL and beta-Human Chorionic Gonadotropin (HCG) level of 10.3 IU/mL. A computed tomographic (CT) examination revealed no retroperitoneal lymphadenopathy.
He underwent right radical orchiectomy during the same hospital stay. An intra-operative frozen section showed a teratoma with germ cell elements. Pathological analysis demonstrated a 5.3 cm tumor with no lymphovascular invasion, negative margins and a stage 1A (T1N0M0S1) lesion. He was then discharged home with plans to continue with close surveillance, but did not maintain his follow up appointment.
Seven months later, he presented to our institution with three month history of increasing back and abdominal pain. He also complained of generalized weakness accompanied with 15 pound weight loss as well as nausea and vomiting for three days. Physical exam showed palpable abdominal mass with no peritoneal signs. Tumor markers revealed an AFP level of 14,000 ng/mL. CT scan showed a large retroperitoneal mass encasing the great vessels (Figure 1). CT of the chest and the brain showed no further lesions.
Figure 1.
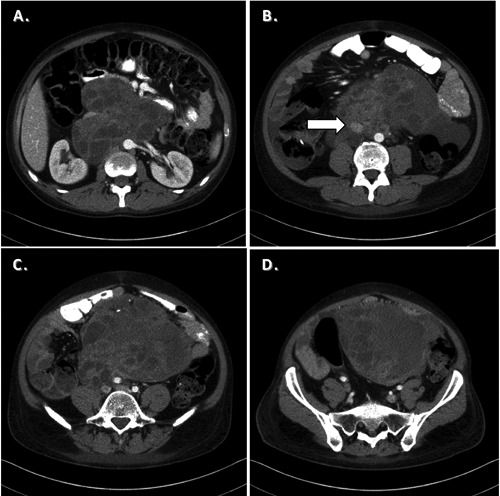
Computed tomography of the abdomen. A) shows a heterogeneous mass consistent with retroperitoneal teratoma. The mass extends caudally encasing the IVC (B, arrow). C, D) demonstrate further caudal extension of the tumor.
He was immediately initiated on chemotherapy consisting of bleomycin, etoposide, and cisplatin (BEP). Following four cycles of treatment, he demonstrated a decrease in tumor markers with AFP level of 27 ng/mL. However, these levels stayed consistently elevated and a second chemotherapeutic regimen was started consisting of vinblastine, isophosphamide, and cisplatin (VeIP). Unfortunately, after three cycles of treatment, the tumor demonstrated minimal decrease in size determined by computed tomography measurements.
With marginal response to chemotherapy and persistent retroperitoneal disease, retroperitoneal debulking was undertaken. He was taken to the operating room and underwent exploratory laparotomy, bilateral full template retroperitoneal lymph node dissection and bilateral pelvic lymph node dissection. The inferior vena cava had to be transected and primarily reconstructed to allow access to retrocaval tumor and complete debulking (Figure 2). He also underwent resection of sigmoid colon en block with the tumor for complete debulking as it was not feasible to preserve mesenteric blood supply. He has an end colostomy is in place. Gross pathological examination demonstrated a 27x20x18 cm mass. Representative Hematoxylin and Eosin stains demonstrated metastatic germ cell tumor composed exclusively of mature teratoma involving multiple lymph nodes (Figure 3).
Figure 2.
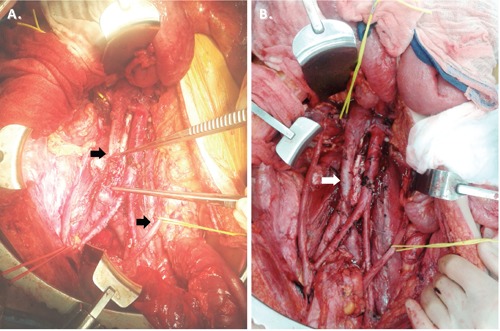
Intraoperative photographs of the retroperitoneum. The IVC was divided for successful debulking on the tumor (A, arrows), which was later reconstructed with primary end to end vascular anastemosis (B).
Figure 3.
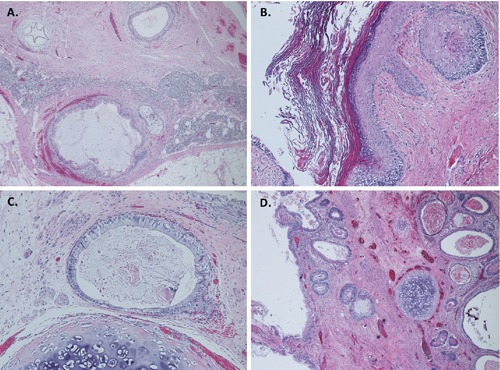
Histological photographs of the tumor. Hematoxylin and Eosin (H&E) stains at low power (40x, A, D) show lymphoid tissue with metastatic germ cell tumor composed of mixture of respiratory, and gastrointestinal type epithelium, smooth muscle, and cartilage. Higher magnification (H&E, 100x) of the tumor showing squamous, and gastrointestinal type epithelium, and cartilage (B, C).
Discussion and Conclusions
This case report represents a unique situation necessitating debulking surgery because of rapid progression of tumor and marginal response to chemotherapy inherent to teratomas. Most TGCTs respond well to chemotherapy following orchiectomy. While failure to follow up and the inability to provide early chemotherapy led to the rapid progression of this tumor, massive retroperitoneal disease was encountered leaving debulking surgery as the only optional therapeutic treatment.
Regional metastases first appear in the retroperitoneal lymph nodes. RPLND provides accurate pathologic staging, as well as identifying patients who may benefit from postoperative chemotherapy. This is indicated in patients who have radiographic evidence of enlarged retroperitoneal lymph nodes, which initially were not present in this case. Some centers performed this routinely even in early stage disease.5
In these patients, micro-metastatic disease cannot be captured by CT scan. Furthermore, false negative rates occur with a rate as high as 44 percent.6 Thus, 20 to 25 percent of patients with clinical stage I disease develop retroperitoneal metastatic disease.7 Histological features such as lymphovascular invasion, T stage greater than II, and embryonal carcinoma features predicted recurrence in 58 percent of patients.8 These patients are considered high risk and would benefit from RPLND.
TGCTs typically relapse within the first five years. Late recurrences are rare. In one study, 0.8 percent of men with seminoma and 1.8 percent of men with non-seminomatous (NS) TGCTs recurred more than five years after the original diagnosis.9 For patients with NSTGCT, over 95 percent of relapses occur within the first two years following orchiectomy.10 There are no prospective, randomized trials that have defined the optimal follow-up strategy after orchiectomy. Multiple urologic and oncologic groups have published guidelines that rely upon interval history and physical examination, assessment of serum tumor markers, and radiological evaluation of chest, abdomen and pelvis. Guidelines for follow up are also provided by the National Comprehensive Cancer Network (NCCN).11
The International Germ Cell Cancer Collaborative Group (IGCCCG) has developed a prognostic model for advanced testicular cancer. In this model, metastatic TGCTs are divided into good-, intermediate-, and poor-risk groups, based upon the primary site of the tumor, metastatic sites of involvement, and levels of serum tumor markers. These criteria also segregate tumors into seminomas and NSTGCTs (Table 2).12 This scheme also predicts the 5-year survival for patients based on these factors. In a study inclusive of 5202 patients with NSTGCTs and 660 with seminomas, the 5-year survival was 90%, 86%, and 72% for patients with good, intermediate, and poor risk; respectively. NSTGCTs had a 92%, 80%, and 48% 5-year survival for patients with good, intermediate and poor risk; respectively.
Table 2.
Risk stratification system for advanced testicular germ cell tumors.
| Risk | Seminomas | Non-seminomatous germ cell tumors |
|---|---|---|
| Good | Any primary site | Testicular or retroperitoneal primary tumors |
| No non-pulmonary visceral metastases | No non-pulmonary visceral metastases | |
| Normal serum AFP, any hCG or LDH | Post orchiectomy markers AFP<1000 ng/mL beta-hCG<5000 LDH<1.5 times upper limit of normal | |
| Intermediate | Any primary site | Testicular or retroperitoneal primary tumors |
| Non-pulmonary visceral metastases | No non-pulmonary visceral metastases | |
| Normal serum AFP, any hCG or LDH | Post orchiectomy markers AFP 1000 to 10,000 ng/mL beta-hCG 5000 to 50,000 mIU/mL LDH 1.5 to 10 times upper limit of normal | |
| Poor | No patients classified as poor prognosis | Mediastinal primary Non-pulmonary visceral metastases Post orchiectomy markers AFP>10,000 ng/mL beta-hCG>50,000 LDH>1.5 times upper limit of normal |
The patient in the present report did not have known recurrence risk factors including Lymphovascular invasion (LVI), Predominance of an embryonal component in the primary tumor and Pathologic tumor stage above T2. Thus, this patient had low risk factors for recurrence. However, he developed rapid growth of retroperitoneal disease that was not captured early as this patient was lost to follow up. On emergent presentation, he had substantially elevated serum tumor markers suggesting the presence of active germ cell elements. He had persistently elevated serum tumor markers after 4 cycles of BEP, indicating presence of non-teratomatous elements. In this setting, we proceeded with second line salvage chemotherapy prior to salvage RPLND.
Following two courses of platinum based chemotherapy he had decrease in his serum tumor markers but no radiographic change in tumor size. We then proceeded with surgical debulking of the tumor. He had an uneventful intraoperative course, but a long postoperative course that was complicated by a chyle leak and sepsis. He recovered well from these complications and recently presented to clinic for a colostomy take down. Follow up radiographic and serum markers demonstrated that he was free of disease.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10. [DOI] [PubMed] [Google Scholar]
- 2.Walsh TJ, Grady RW, Porter MP, et al. Incidence of testicular germ cell cancers in U.S. children: SEER program experience 1973 to 2000. Urology 2006;68:402. [DOI] [PubMed] [Google Scholar]
- 3.Einhorn LH. Treatment of testicular cancer: a new and improved model. J Clin Oncol 1990;8:1777. [DOI] [PubMed] [Google Scholar]
- 4.Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med 1997;337:242. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich A, Pfister D. Retroperitoneal lymphadenectomy and resection for testicular cancer: an update on best practice. Ther Adv Urol 2012;4:187-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richie JP, Garnick MB, Finberg H. Computerized tomography: how accurate for abdominal staging of testis tumors? J Urol 1982;127:715. [DOI] [PubMed] [Google Scholar]
- 7.Gels ME, Hoekstra HJ, Sleijfer DT, et al. Detection of recurrence in patients with clinical stage I nonseminomatous testicular germ cell tumors and consequences for further follow-up: a single-center 10-year experience. J Clin Oncol 1995;13:1188. [DOI] [PubMed] [Google Scholar]
- 8.Freedman LS, Parkinson MC, Jones WG, et al. Histopathology in the prediction of relapse of patients with stage I testicular teratoma treated by orchidectomy alone. Lancet 1987;2:294. [DOI] [PubMed] [Google Scholar]
- 9.Oldenburg J, Alfsen GC, Waehre H, Fosså SD. Late recurrences of germ cell malignancies: a population-based experience over three decades. Br J Cancer 2006;94:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugaard G, Roerth M. Observation and expectant management for low-stage seminoma and nonseminoma. Comprehensive Textbook of Genitourinary Oncology, 2nd, Vogelzang NJ, Scardino PT, Shipley WU, Coffey DS.(Eds), Lippincott, Williams and Wilkins, Philadelphia; 2000. p.976 [Google Scholar]
- 11.Motzer RJ, Agarwal N, Beard C, et al. Testicular cancer. J Natl Compr Canc Netw 2012;10:502. [DOI] [PubMed] [Google Scholar]
- 12.International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 1997;15:594. [DOI] [PubMed] [Google Scholar]


