Abstract
Reconstructive transplantation has emerged as clinical reality over the past decade. Long-term graft acceptance has been feasible in extremity and facial vascularized composite allotransplantation (VCA) under standard immunosuppression. Minimizing overall burden of lifelong immunosuppression is key to wider application of these non-life saving grafts. Allograft tolerance is the holy grail of many cell-based immunomodulatory strategies. Recent protocols using mesenchymal stem cells from bone marrow and adipose tissue offer promise and potential in VCA. This article provides an overview of the experimental basis, the scientific background and clinical applications of stem cell-based therapies in the field of reconstructive allotransplantation.
Keywords: adipose stem cells, cell-based therapy, immunomodulation, composite tissue allotransplantation, tolerance
Introduction
Complex reconstructions after major trauma are limited by available tissues, morbidity from extensive surgery, prolonged rehabilitation, suboptimal results, and costs of multiple surgeries. For such intricate injuries, vascularized composite allotransplantation (VCA) can achieve optimal restoration of tissue deficits with improved functional and esthetic results, enabling social and professional reintegration. During the past decade, more than 100 reconstructive transplant procedures have been performed around the world, including over 90 hand and 24 facial transplants with encouraging overall outcomes (1–3).
Like solid organ transplants, VCA requires long-term multidrug immunosuppression to prevent graft rejection mediated predominantly by the highly immunogenic skin component in these allografts. Medication toxicity could result in metabolic, infectious, or neoplastic complications. VCA is inherently different from solid organ transplantation in its non-life saving, yet life-enhancing impact on recipients. Further, unlike solid organs, clinical success is dictated not only by graft acceptance and survival, but also by nerve regeneration, which determines ultimate functional outcomes. These characteristics of VCA, drive the debate focused on the risks of lifelong immunosuppression mandated for graft survival balanced against the benefits of functional and quality of life outcomes. Thus, implementation of cellular therapies that integrate the concepts of immune regulation for graft acceptance with those of nerve regeneration could optimize the outcomes of these reconstructive modalities and minimize overall burden of immunosuppression. Such strategies could expand clinical feasibility and realize routine applicability of reconstructive transplantation.
Mesenchymal stem cells (MSCs) are pluripotent cells that are present in multiple tissues, including bone marrow (BM), adipose tissue, skin, muscle, blood, and placenta and can be isolated and expanded ex vivo. MSCs are capable of differentiation in vitro along multiple mesenchymal lineages such as osteocytes, chondrocytes, myocytes, adipocytes, and Schwann cells (SC) thereby emerging as a promising tool for tissue engineering and cell therapy.
Current literature on MSCs points to a wide range of immunological functions and interactions with other cell types (4, 5). Recent data support findings that MSCs mediate their actions through multiple mechanisms including paracrine effects. Various groups have shown some key differences between adipose-derived MSCs (AD-MSCs) and bone marrow derived MSCs (BM-MSCs) in vitro and in vivo (6–8). In particular, it has been demonstrated that MSCs have the capacity to suppress T cell activation and proliferation (9). Compared to BM, adipose tissue is a rich source of MSCs with up to 10-fold higher yield of MSCs (10). More recent publications demonstrate that AD-MSCs might also have higher immunomodulatory and immunosuppressive potential in vitro as compared to BM-MSCs (11–20). The ease of procurement of large volumes of AD-MSCs through techniques such as liposuction is an important benefit because of expeditious approach and minimal morbidity. Depending on time considerations for cell expansion, the overall duration of cell retrieval to cell infusion could be significantly shortened for AD-MSCs as compared to MSCs from other sources.
There is a growing complement of first in human studies addressing potential of MSC based cell therapies in autoimmune diseases, facilitation of hematopoietic stem cell engraftment in BM transplantation and in solid organ transplantation (4, 21, 22). Recent experimental and clinical studies highlight their potential for immunomodulation, tolerance induction, and prophylaxis and treatment of graft versus host disease (GvHD) (23–25). In VCA, the efficacy and effectiveness as well as mechanisms and outcomes of such therapies may be affected by the antigenicity of the skin component (26), as or by the differential tissue composition of these grafts.
Immunological Function of MSCs
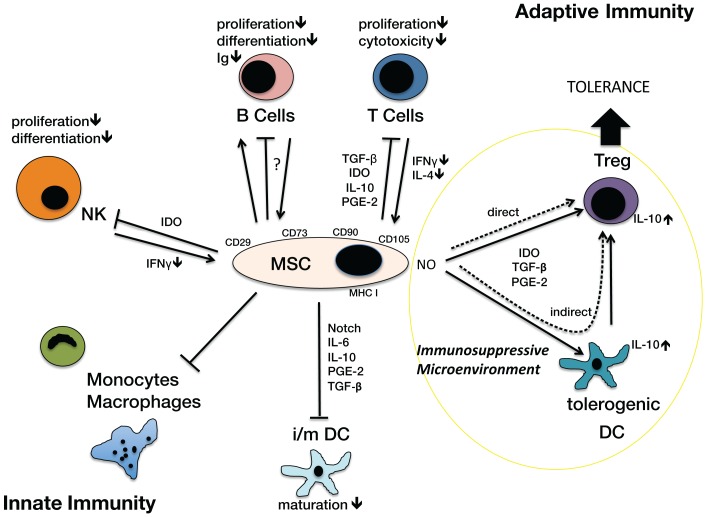
The effects of MSCs on innate and adaptive immunity have been reported in the literature (4, 5). MSCs modulate the innate function of monocytes, macrophages, natural killer (NK) cells, and dendritic cells (DCs). They are capable of modifying the maturation of DC, thereby inhibiting their antigen-presenting function and inducing the generation of tolerogenic DCs. This results in downregulated MHC II and chemokine expression (27–30). MSCs show intermediate expression of MHC I and do not express MHC II on their surface, which reduces their antigenicity. However the intracellular MHC II can become relevant when NK lyse transplanted MSCs (31, 32). In addition, MSCs also affect innate immunity through HLA-G expression leading to inhibition of NK cells and reduction of IFN-g expression (33, 34). English et al. (35) have shown that MSCs can directly induce regulatory T cell (Treg) generation. Mediators of Treg generation include indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), and IFN-g (4). However, MSCs inhibit the activation of cytotoxic lymphocytes and immunoglobulin production through activated and proliferating B cells (36). The nitric oxide (NO) system as well as factors involved in stem cell recruitment like SDF-1, growth factors like VEGF and cytokines like IL-6, IL-8, and IL-10 have been shown to be important players in MSC dependent effects on adaptive immune responses (4, 5) (Figure 1).
Figure 1.
Immunological Function of MSCs on different cell types of the innate and adaptive immunosystem. MSC, mesenchymal stromal cell, characterized by surface antigens CD29, CD73, CD90 (CD105), MHC I; cell types: B cells, T Cells, Treg; regulatory T cells; DC, dendritic cells (tolerogenic, i/m, immature/mature), monocytes, macrophages, NK, natural killer cells. Arrows indicate activation or induction, T-bars indicate blockade of function or activation, in particular inhibition of proliferation, differentiation, cytotoxicity, maturation. The NO system is closely involved in creating an immunosuppressive microenvironment. Via an indirect way the generation of tolerogenic DCs induces Tregs. MSCs can also directly activate Treg generation. These Tregs play a significant role in the development of tolerance.
MSCs Promote Nerve Regeneration
The functional advantages of VCA over prosthetic fitting rely not only on motor recovery but also sensory recovery following regeneration of peripheral nerves and reintegration of neuronal pathways into the premotor cortex of the brain. MSCs have shown promise in improving clinical and electrophysiological outcomes in animal models of peripheral nerve injury (37–39). In comparison to local delivery, some groups have demonstrated significantly accelerated functional neuronal recovery following systemic administration of MSCs (40). The postulated mechanisms for such outcomes could include direct and paracrine effects (38, 41).
MSC Use in Allotransplantation
Bartholomew et al. (9) first described prolongation of skin graft survival after allotransplantation in primates with a single application of expanded BM-MSCs at day 0. Sbano et al. (42) also reported similar results with cultured BM-MSC administered on day 0 and 3 in a rat model. Skin graft survival was prolonged, although grafts were rejected without supplementary immunosuppression.
Solari et al. (43) demonstrated long-term islet allograft survival in rats using syngeneic MSCs. In this study, repetitive application of syngeneic BM-MSCs was superior to allogeneic BM-MSCs. However, long-term tolerance could only be achieved in 40%, and cells were expanded up to P8. Their inherent immunomodulatory function has promoted use of MSCs in BM transplantation, solid organ transplantation, and treatment of GvHD (30). BM-MSCs have been used in experimental animal heart, liver, kidney, and islet transplantation (14, 30, 43–49). Immunomodulation was related to the suppression of alloreactive effector lymphocytes (50) through expression of a variety of cytokines (51–53) (Figure 1) and generation of CD4/CD25/FoxP3 Tregs that achieved long-term tolerance in animal studies via adaptive immune mechanisms (31, 50). Data on AD-MSCs in solid organ transplantation is scarce; however sources for MSCs other than BM have been supported/investigated (54). In addition, studies have confirmed the multifaceted properties of MSCs including their potential as part of induction therapy (55), in GvHD (56), tolerance induction (55, 57), and facilitating engraftment of BM transplants (58, 59). Several clinical trials have demonstrated their immunosuppressive function that could have potential in autoimmune disease (32, 60, 61). Such properties could offer potential for MSCs in acute and chronic rejection after VCA. MSCs have been successfully used in pediatric patients as rescue therapy of GVHD and repetitive rejection of BM transplants (62).
Critical Issues for MSC Use in VCA
The success of organ transplantation and VCA is dependent on graft acceptance in the absence of GvHD. Various induction and maintenance regimens have been successful in controlling acute rejection. All these drugs have their role in VCA for specific immunosuppressive functions, but their unwarranted collateral effects on MSCs are less well investigated.
Several studies in solid organs have reported pre-transplant, peri-transplant, or post-transplant use of MSCs for immunomodulation (63–65). However, the negative effects of the depletion regimens that include irradiation, and polyclonal (antithymocyte globulin/serum), or monoclonal antibodies (e.g., alemtuzumab) on MSC recruitment, homing, and function remain to be clarified.
Interaction with immunosuppressive drugs
Several immunosuppressive drugs significantly affect in vitro activity of MSCs. Hoogduijn et al. (66) investigated the in vitro effect of tacrolimus, rapamycin, and MPA on human MSCs and vice versa. Exposure of MSCs to high dosages of tacrolimus was toxic and reduced cell viability. While tacrolimus did not inhibit proliferation rates of MSCs, rapamycin, and MPA led to a dose-dependent reduction of proliferation and differentiation at therapeutic dosage levels. Unlike rapamycin, tacrolimus did not affect the osteogenic differentiation potential of MSCs (67). The effects of Cyclosporin A (CsA) on MSCs were insignificant. Some authors demonstrated a synergistic immunosuppressive effect of MSCs and MPA (68, 69). However, the immunomodulatory properties of MSCs were antagonized by rapamycin and tacrolimus. In vivo experiments support these findings of synergistic or opposing effects between MSC and pharmacological immunosuppression (68, 70). Hoogduijn’s et al (66) reported that MSCs inhibited the suppressive effect of tacrolimus and rapamycin on alloreactive lymphocytes but had no effect on MPA.
Timing and dosage of MSCs
Currently, there is no consensus on dosing and timing of administration of MSCs in cell-based VCA protocols. Experimental studies indicate that the dosage of MSCs showing beneficial results ranges from 5 × 105 to 5 × 107 cells/kg body weight and time point (Table 1). In rats, the total amount of BM-MSCs administered over time was 6−10 × 106 cells, whereas in pigs total amounts from 5 to 12.5 × 107. BM-MSCs were used. In a study utilizing AD-MSCs, as many as 24 × 106 cells/kg bodyweight were administered over three time points (58, 59, 71). MSCs were administered as early as day −30 to as late as +21 relative to transplantation or at different frequency in the interim (57–59). The time point was chosen with regards to the desired effect such as induction, immunomodulation, or support of BM engraftment.
Table 1.
Overview of the currently available experimental literature on MSC based cellular therapy for immunomodulation in VCA.
| Reference | Type of graft | Species | MSCs amount/type | MSC application | Induction regimen | Immuno-suppression | Main outcome |
|---|---|---|---|---|---|---|---|
| Pan et al. (59) | Hindlimb | Rat | 107 BM-MSCs (allogeneic) | Day −30 | Irradiation, ALS, BMT | Rapamycin | Chimerism in peripheral blood |
| Tolerance (>100 days) | |||||||
| Protection against GvHD | |||||||
| Kuo et al. (58) | Hindlimb | Pig | 107 BM-MSCs (allogeneic) | Day −1, +3, +7, +14, +21 | Irradiation, BMT | Cyclosporin | Perivascular MSC engraftment (graft) |
| Chimerism in peripheral blood | |||||||
| Tolerance (>200 days) | |||||||
| Protection against GvHD | |||||||
| Treg ⇑ | |||||||
| Kuo et al. (87) | Hindlimb | Pig | 107 BM-MSCs (allogeneic) | Day −1, +3, +7, +14, +21 | Irradiation | Cyclosporin | Tolerance (>120 days) |
| Treg ⇑ | |||||||
| Kuo et al. (57) | Hindlimb | Rat | 2 × 106 AD-MSCs (allogeneic) | Day +7, +14, +21 | ALS | Cyclosporin | Tolerance (>150 days) |
| Chimerism peripheral blood | |||||||
| Treg ⇑ | |||||||
| IL-10 ⇑ | |||||||
| Kuo et al. (88) | Face | Pig | 2.5 × 107 BM-MSCs (allogeneic) | Day −1, +3, +7, +14, +21 | N/A | Cyclosporin | Treg ⇑ |
| IL-10 ⇑ | |||||||
| Aksu et al. (71) | Skin flap | Rat | 2–3 × 106 (repetitive) BM-MSCs (syngeneic) | Days 0, +7, +14, and +21 | Irradiation BMT (repetitive) | Cyclosporin | Syngeneic MSCs limit toxicity of allogeneic BMT |
| Prolongation of tolerance | |||||||
| Enhanced mixed chimerism |
Homing of MSCs/chimerism
Eggenhofer et al. (72) recently reported a murine study reduced life span of intravenously delivered cultured MSCs due to entrapment in the lung capillaries or liver sinusoids. Others have shown distribution to other organs like kidney and spleen, BM, and peripheral blood (73). Kuo et al. (58) demonstrated recruitment and homing of MSCs to perivascular sites with long-term survival in VCA models. In addition peripheral blood chimerism after VCA with MSC-co-transplantation was demonstrated in pig and rat models (57–59). Indeed, the first-pass clearance of MSCs in lung and liver may be an obstacle for these cell therapies (74, 75). Several strategies including vasodilation, co-transplantation, and repetitive infusions have been suggested to increase cell passage. Freshly isolated MSCs have been shown to be superior to culture-expanded MSCs in terms of lower entrapment potential due to smaller size as well as better homing potential (76). They are found in high number in sites of trauma and ischemia after several days (77).
Cultured MSCs
For therapeutic indications, MSCs need to be expanded in culture to achieve sufficient numbers for transplantation. Karp and Leng (78) reported that the culture medium and conditions are likely to influence the properties of MSCs as well as their morphology (79, 80). The number of passages inversely correlates with the number of surface antigens and homing potential (81–84). Cultured MSCs may be morphologically indistinguishable from fibroblasts and even show similar cell-surface markers, differentiation potential, and immunologic properties (80). Multiple cell passages not only affect proliferation and differentiation but also alter cell-surface antigens and cytokine production (85, 86). To our knowledge, there is no study to date on the immunomodulatory potential of long-term-cultured MSCs. In our view, short-term culture of MSCs (<P3) and freshly isolated MSCs must be given preference in cell therapy protocols until we gain more insights into the properties of expanded MSCs.
MSCs in Vascularized Composite Allotransplantation
Only a few groups have advocated the use of BM-MSCs or AD-MSCs for immunomodulatory strategies in VCA (Table 1). Despite growing enthusiasm, the basic experience is limited to few experimental studies with MSCs. Aksu et al. (71) reported that co-administration of host BM-MSCs with unmodified donor BM and immunosuppression (CsA + irradiation) enabled prolonged survival of full MHC mismatched rodent vascularized skin grafts with generation of mixed chimerism and absence of GvHD. Outcomes positively correlated with number of times the BM-MSCs were administered. Pan et al. (59) reported a rat hindlimb VCA model where limb transplants were performed a month after conditioning with total body irradiation, and anti-lymphocyte-serum followed by allogeneic BM-MSC and BM infusion. This resulted in stable chimerism, donor specific tolerance, and no GvHD. Allogeneic BM-MSC transplantation with or without co-transplantation of BM has been shown to be successful in prolongation (>200 days) of pig limb allograft survival after irradiation and CsA treatment (58, 87). Repetitive high dose BM-MSC treatment was also successful in prolonging survival in a pig hemi-facial transplantation model without conditioning therapy (88). The authors reported only mild rejection of the graft (Grade I–II), improved under CsA treatment. The positive effects of BM-MSCs on rejection grades were correlated to IL-10 upregulation and Treg induction. Despite the prolongation, all grafts succumbed within 90 days. In a different approach, Kuo et al. (57) administered three fold numbers of AD-MSCs under temporary immunosuppression in a rat hindlimb allotransplantation model. After cessation of immunosuppression, this regimen prolonged allograft survival significantly with stable tolerance in 89% for>150 days. Treg populations were significantly increased and elevated donor lymphoid cell counts (RT1n) resulted in stable peripheral blood chimerism until endpoint. The same group conducted a study in a swine hindlimb allotransplantation model using BM-MSCs, irradiation, and short-term CsA. They were able to demonstrate prolongation of the survival (>100 days in 67%) and an increase of the Treg population.
Conclusion
Taken together, emerging literature evidence highlights the potential promise of MSC based cellular therapies for immunomodulation and neurodegeneration in VCA. Extensive experimental and clinical studies in the areas of solid organ transplantation, hematopoietic stem cell co-transplantation and autoimmune disease underscore the relevance and impact of MSC based therapies in VCA. Traditionally, the chief obstacle hampering application of BM-MSCs has been the limited cell yield and requirement for donor cell expansion. The high cell yields of AD-MSCs from adipose sources obtained through easy, fairly non-invasive techniques have enabled expeditious cell processing, thus expanding the clinical feasibility of these therapies. These advantages, combined with the insights supporting the superior immunomodulatory potential of AD-MSCs versus BM-MSCs truly advocate adipose-based cellular therapies. The higher cell yields also facilitate repetitive infusion both systemically and locally in the graft. Freshly isolated AD-MSCs can overcome the loss of viability and entrapment during the first-pass phenomenon in the capillary systems of the lung. Importantly, MSCs mediate paracrine effects on remote tissues through specific cytokines, chemokines, and growth factors. It still remains to be defined if such paracrine effects mediate also tolerogenic or immunomodulatory effects in VCA or other applications. Future protocols should carefully address the dosing and timing of MSC administration and the effects of conditioning regimens and maintenance immunosuppression on function of these cells. Most notably, the effect of MSC based strategies on nerve regeneration, critical for functional outcomes in VCA offers an uncharted area of investigation. Thus far, only one donor BM cell-based protocol has been clinically evaluated in human upper extremity VCA (Pittsburgh Protocol) and involves megadose donor derived BM infusion after at day 11–14 after surgery (89). Insights into the impact of such protocols in minimizing the need for dosing, frequency, and duration of immunosuppression are just emerging. Meanwhile, we must continue to explore the multifaceted potential of MSCs in experimental VCA to further fine tune standard protocols as used in conventional VCA or solid organ transplantation. The true clinical scope and impact of disparate VCA strategies will only be realized when such protocols enable optimization of the functional and immunological benefits of these novel reconstructive procedures while reducing long-term risk of lifelong immunosuppression.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors have no financial interests. Jan A. Plock and Jonas T. Schnider are recipients of Swiss National Science foundation funding.
References
- 1.Petruzzo P, Lanzetta M, Dubernard JM, Landin L, Cavadas P, Margreiter R, et al. The international registry on hand and composite tissue transplantation. Transplantation (2010) 90:1590–4 10.1097/TP.0b013e3181ff1472 [DOI] [PubMed] [Google Scholar]
- 2.Petruzzo P, Kanitakis J, Badet L, Pialat JB, Boutroy S, Charpulat R, et al. Long-term follow-up in composite tissue allotransplantation: in-depth study of five (hand and face) recipients. Am J Transplant (2011) 11:808–16 10.1111/j.1600-6143.2011.03469.x [DOI] [PubMed] [Google Scholar]
- 3.Petruzzo P, Testelin S, Kanitakis J, Badet L, Lengele B, Girbon JP, et al. First human face transplantation: 5 years outcomes. Transplantation (2012) 93:236–40 10.1097/TP.0b013e31823d4af6 [DOI] [PubMed] [Google Scholar]
- 4.Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol (2011) 6:457–78 10.1146/annurev-pathol-011110-130230 [DOI] [PubMed] [Google Scholar]
- 5.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol (2013) 91:19–26 10.1038/icb.2012.56 [DOI] [PubMed] [Google Scholar]
- 6.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem (2006) 99:1285–97 10.1002/jcb.20904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells (2007) 25:750–60 10.1634/stemcells.2006-0394 [DOI] [PubMed] [Google Scholar]
- 8.Noel D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res (2008) 314:1575–84 10.1016/j.yexcr.2007.12.022 [DOI] [PubMed] [Google Scholar]
- 9.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, Mcintosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol (2002) 30:42–8 10.1016/S0301-472X(01)00769-X [DOI] [PubMed] [Google Scholar]
- 10.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs (2003) 174:101–9 10.1159/000071150 [DOI] [PubMed] [Google Scholar]
- 11.Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med (2013) 2:455–63 10.5966/sctm.2012-0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng (2007) 13:1185–95 10.1089/ten.2006.0315 [DOI] [PubMed] [Google Scholar]
- 13.Fang B, Song Y, Lin Q, Zhang Y, Cao Y, Zhao RC, et al. Human adipose tissue-derived mesenchymal stromal cells as salvage therapy for treatment of severe refractory acute graft-vs.-host disease in two children. Pediatr Transplant (2007) 11:814–7 10.1111/j.1399-3046.2007.00780.x [DOI] [PubMed] [Google Scholar]
- 14.Wan CD, Cheng R, Wang HB, Liu T. Immunomodulatory effects of mesenchymal stem cells derived from adipose tissues in a rat orthotopic liver transplantation model. Hepatobiliary Pancreat Dis Int (2008) 7:29–33 [PubMed] [Google Scholar]
- 15.Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells (2009) 27:2624–35 10.1002/stem.194 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum (2009) 60:1006–19 10.1002/art.24405 [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut (2009) 58:929–39 10.1136/gut.2008.168534 [DOI] [PubMed] [Google Scholar]
- 18.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Pescatori M, Stubbs AP, et al. Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin Exp Immunol (2010) 162:474–86 10.1111/j.1365-2249.2010.04256.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Rey E, Gonzalez MA, Varela N, O’Valle F, Hernandez-Cortes P, Rico L, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis (2010) 69:241–8 10.1136/ard.2008.101881 [DOI] [PubMed] [Google Scholar]
- 20.Engela AU, Baan CC, Dor FJ, Weimar W, Hoogduijn MJ. On the interactions between mesenchymal stem cells and regulatory T cells for immunomodulation in transplantation. Front Immunol (2012) 3:126 10.3389/fimmu.2012.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones BJ, Mctaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp Hematol (2008) 36:733–41 10.1016/j.exphem.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 22.Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther (2010) 21:1641–55 10.1089/hum.2010.156 [DOI] [PubMed] [Google Scholar]
- 23.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng (2010) 12:87–117 10.1146/annurev-bioeng-070909-105309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant (2010) 45:1732–40 10.1038/bmt.2010.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernardo ME, Ball LM, Cometa AM, Roelofs H, Zecca M, Avanzini MA, et al. Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant (2011) 46:200–7 10.1038/bmt.2010.87 [DOI] [PubMed] [Google Scholar]
- 26.Lee WP, Yaremchuk MJ, Pan YC, Randolph MA, Tan CM, Weiland AJ. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg (1991) 87:401–11 10.1097/00006534-199103000-00001 [DOI] [PubMed] [Google Scholar]
- 27.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells (2007) 25:2025–32 10.1634/stemcells.2006-0548 [DOI] [PubMed] [Google Scholar]
- 28.English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett (2008) 115:50–8 10.1016/j.imlet.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 29.Li M, Sun K, Welniak LA, Murphy WJ. Immunomodulation and pharmacological strategies in the treatment of graft-versus-host disease. Expert Opin Pharmacother (2008) 9:2305–16 10.1517/14656566.9.13.2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Jiao C, Zhao S. Role of mesenchymal stem cells in immunological rejection of organ transplantation. Stem Cell Rev (2009) 5:402–9 10.1007/s12015-009-9076-y [DOI] [PubMed] [Google Scholar]
- 31.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells (2008) 26:212–22 10.1634/stemcells.2007-0554 [DOI] [PubMed] [Google Scholar]
- 32.De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med (2012) 12:574–91 10.2174/156652412800619950 [DOI] [PubMed] [Google Scholar]
- 33.Nasef A, Mathieu N, Chapel A, Frick J, Francois S, Mazurier C, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation (2007) 84:231–7 10.1097/01.tp.0000267918.07906.08 [DOI] [PubMed] [Google Scholar]
- 34.Selmani Z, Naji A, Gaiffe E, Obert L, Tiberghien P, Rouas-Freiss N, et al. HLA-G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation (2009) 87:S62–6 10.1097/TP.0b013e3181a2a4b3 [DOI] [PubMed] [Google Scholar]
- 35.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol (2009) 156:149–60 10.1111/j.1365-2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franquesa M, Hoogduijn MJ, Bestard O, Grinyo JM. Immunomodulatory effect of mesenchymal stem cells on B cells. Front Immunol (2012) 3:212 10.3389/fimmu.2012.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu G, Cheng Y, Guo S, Feng Y, Li Q, Jia H, et al. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int J Mol Med (2011) 28:565–72 10.3892/ijmm.2011.725 [DOI] [PubMed] [Google Scholar]
- 38.Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, et al. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS ONE (2011) 6:e17899 10.1371/journal.pone.0017899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carriel V, Garrido-Gomez J, Hernandez-Cortes P, Garzon I, Garcia-Garcia S, Saez-Moreno JA, et al. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J Neural Eng (2013) 10:026022 10.1088/1741-2560/10/2/026022 [DOI] [PubMed] [Google Scholar]
- 40.Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S, Farinazzo A, et al. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A (2012) 18:1264–72 10.1089/ten.TEA.2011.0491 [DOI] [PubMed] [Google Scholar]
- 41.Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY, Wu CW, et al. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol (2007) 204:443–53 10.1016/j.expneurol.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 42.Sbano P, Cuccia A, Mazzanti B, Urbani S, Giusti B, Lapini I, et al. Use of donor bone marrow mesenchymal stem cells for treatment of skin allograft rejection in a preclinical rat model. Arch Dermatol Res (2008) 300:115–24 10.1007/s00403-007-0827-9 [DOI] [PubMed] [Google Scholar]
- 43.Solari MG, Srinivasan S, Boumaza I, Unadkat J, Harb G, Garcia-Ocana A, et al. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun (2009) 32:116–24 10.1016/j.jaut.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 44.Wu GD, Nolta JA, Jin YS, Barr ML, Yu H, Starnes VA, et al. Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation (2003) 75:679–85 10.1097/01.TP.0000048488.35010.95 [DOI] [PubMed] [Google Scholar]
- 45.Inoue S, Popp FC, Koehl GE, Piso P, Schlitt HJ, Geissler EK, et al. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation (2006) 81:1589–95 10.1097/01.tp.0000209919.90630.7b [DOI] [PubMed] [Google Scholar]
- 46.Zhou HP, Yi DH, Yu SQ, Sun GC, Cui Q, Zhu HL, et al. Administration of donor-derived mesenchymal stem cells can prolong the survival of rat cardiac allograft. Transplant Proc (2006) 38:3046–51 10.1016/j.transproceed.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 47.Itakura S, Asari S, Rawson J, Ito T, Todorov I, Liu CP, et al. Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am J Transplant (2007) 7:336–46 10.1111/j.1600-6143.2006.01643.x [DOI] [PubMed] [Google Scholar]
- 48.Hong ZF, Huang XJ, Yin ZY, Zhao WX, Wang XM. Immunosuppressive function of bone marrow mesenchymal stem cells on acute rejection of liver allografts in rats. Transplant Proc (2009) 41:403–9 10.1016/j.transproceed.2008.10.020 [DOI] [PubMed] [Google Scholar]
- 49.Popp FC, Renner P, Eggenhofer E, Slowik P, Geissler EK, Piso P, et al. Mesenchymal stem cells as immunomodulators after liver transplantation. Liver Transpl (2009) 15:1192–8 10.1002/lt.21862 [DOI] [PubMed] [Google Scholar]
- 50.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Weimar W, Hoogduijn MJ. Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev (2010) 19:1843–53 10.1089/scd.2009.0368 [DOI] [PubMed] [Google Scholar]
- 51.Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol (2008) 36:309–18 10.1016/j.exphem.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 52.Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther (2011) 2:34 10.1186/scrt75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casiraghi F, Azzollini N, Todeschini M, Cavinato RA, Cassis P, Solini S, et al. Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transplant (2012) 12:2373–83 10.1111/j.1600-6143.2012.04115.x [DOI] [PubMed] [Google Scholar]
- 54.De Girolamo L, Lucarelli E, Alessandri G, Avanzini MA, Bernardo ME, Biagi E, et al. Mesenchymal stem/stromal cells: a new “cells as drugs” paradigm. Efficacy and critical aspects in cell therapy. Curr Pharm Des (2013) 19:2459–73 10.2174/1381612811319130015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA (2012) 307:1169–77 10.1001/jama.2012.316 [DOI] [PubMed] [Google Scholar]
- 56.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet (2008) 371:1579–86 10.1016/S0140-6736(08)60690-X [DOI] [PubMed] [Google Scholar]
- 57.Kuo YR, Chen CC, Goto S, Lee IT, Huang CW, Tsai CC, et al. Modulation of immune response and T-cell regulation by donor adipose-derived stem cells in a rodent hind-limb allotransplant model. Plast Reconstr Surg (2011) 128:661e–72 10.1097/PRS.0b013e318230c60b [DOI] [PubMed] [Google Scholar]
- 58.Kuo YR, Goto S, Shih HS, Wang FS, Lin CC, Wang CT, et al. Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model. Transplantation (2009) 87:1769–77 10.1097/TP.0b013e3181a664f1 [DOI] [PubMed] [Google Scholar]
- 59.Pan H, Zhao K, Wang L, Zheng Y, Zhang G, Mai H, et al. Mesenchymal stem cells enhance the induction of mixed chimerism and tolerance to rat hind-limb allografts after bone marrow transplantation. J Surg Res (2010) 160:315–24 10.1016/j.jss.2008.09.027 [DOI] [PubMed] [Google Scholar]
- 60.Bernardo ME, Fibbe WE. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann N Y Acad Sci (2012) 1266:107–17 10.1111/j.1749-6632.2012.06667.x [DOI] [PubMed] [Google Scholar]
- 61.Figueroa FE, Carrion F, Villanueva S, Khoury M. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol Res (2012) 45:269–77 10.1590/S0716-97602012000300008 [DOI] [PubMed] [Google Scholar]
- 62.Lawitschka A, Ball L, Peters C. Nonpharmacologic treatment of chronic graft-versus-host disease in children and adolescents. Biol Blood Marrow Transplant (2012) 18:S74–81 10.1016/j.bbmt.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 63.Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol (2008) 181:3933–46 [DOI] [PubMed] [Google Scholar]
- 64.Ge W, Jiang J, Baroja ML, Arp J, Zassoko R, Liu W, et al. Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant (2009) 9:1760–72 10.1111/j.1600-6143.2009.02721.x [DOI] [PubMed] [Google Scholar]
- 65.Oh JY, Lee RH, Yu JM, Ko JH, Lee HJ, Ko AY, et al. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther (2012) 20:2143–52 10.1038/mt.2012.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoogduijn MJ, Crop MJ, Korevaar SS, Peeters AM, Eijken M, Maat LP, et al. Susceptibility of human mesenchymal stem cells to tacrolimus, mycophenolic acid, and rapamycin. Transplantation (2008) 86:1283–91 10.1097/TP.0b013e31818aa536 [DOI] [PubMed] [Google Scholar]
- 67.Isomoto S, Hattori K, Ohgushi H, Nakajima H, Tanaka Y, Takakura Y. Rapamycin as an inhibitor of osteogenic differentiation in bone marrow-derived mesenchymal stem cells. J Orthop Sci (2007) 12:83–8 10.1007/s00776-006-1079-9 [DOI] [PubMed] [Google Scholar]
- 68.Buron F, Perrin H, Malcus C, Hequet O, Thaunat O, Kholopp-Sarda MN, et al. Human mesenchymal stem cells and immunosuppressive drug interactions in allogeneic responses: an in vitro study using human cells. Transplant Proc (2009) 41:3347–52 10.1016/j.transproceed.2009.08.030 [DOI] [PubMed] [Google Scholar]
- 69.Eggenhofer E, Steinmann JF, Renner P, Slowik P, Piso P, Geissler EK, et al. Mesenchymal stem cells together with mycophenolate mofetil inhibit antigen presenting cell and T cell infiltration into allogeneic heart grafts. Transpl Immunol (2011) 24:157–63 10.1016/j.trim.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 70.Eggenhofer E, Renner P, Soeder Y, Popp FC, Hoogduijn MJ, Geissler EK, et al. Features of synergism between mesenchymal stem cells and immunosuppressive drugs in a murine heart transplantation model. Transpl Immunol (2011) 25:141–7 10.1016/j.trim.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 71.Aksu AE, Horibe E, Sacks J, Ikeguchi R, Breitinger J, Scozio M, et al. Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin Immunol (2008) 127:348–58 10.1016/j.clim.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 72.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol (2012) 3:297 10.3389/fimmu.2012.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs (2001) 169:12–20 10.1159/000047856 [DOI] [PubMed] [Google Scholar]
- 74.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc (2007) 39:573–6 10.1016/j.transproceed.2006.12.019 [DOI] [PubMed] [Google Scholar]
- 75.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev (2009) 18:683–92 10.1089/scd.2008.0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia (2003) 17:160–70 10.1038/sj.leu.2402763 [DOI] [PubMed] [Google Scholar]
- 77.Schlosser S, Dennler C, Schweizer R, Eberli D, Stein JV, Enzmann V, et al. Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc Res (2012) 83:267–75 10.1016/j.mvr.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 78.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell (2009) 4:206–16 10.1016/j.stem.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 79.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE (2008) 3:e2213 10.1371/journal.pone.0002213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy (2012) 14: 516–21 10.3109/14653249.2012.677822 [DOI] [PubMed] [Google Scholar]
- 81.Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood (2004) 104:2643–5 10.1182/blood-2004-02-0526 [DOI] [PubMed] [Google Scholar]
- 82.Ruster B, Gottig S, Ludwig RJ, Bistrian R, Muller S, Seifried E, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood (2006) 108:3938–44 10.1182/blood-2006-05-025098 [DOI] [PubMed] [Google Scholar]
- 83.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells (2007) 25:2896–902 10.1634/stemcells.2007-0637 [DOI] [PubMed] [Google Scholar]
- 84.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med (2008) 14:181–7 10.1038/nm1703 [DOI] [PubMed] [Google Scholar]
- 85.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol (2005) 205:194–201 10.1002/jcp.20376 [DOI] [PubMed] [Google Scholar]
- 86.Wagner W, Ho AD, Zenke M. Different facets of aging in human mesenchymal stem cells. Tissue Eng Part B Rev (2010) 16:445–53 10.1089/ten.TEB.2009.0825 [DOI] [PubMed] [Google Scholar]
- 87.Kuo YR, Chen CC, Shih HS, Goto S, Huang CW, Wang CT, et al. Prolongation of composite tissue allotransplant survival by treatment with bone marrow mesenchymal stem cells is correlated with T-cell regulation in a swine hind-limb model. Plast Reconstr Surg (2011) 127:569–79 10.1097/PRS.0b013e318200a92c [DOI] [PubMed] [Google Scholar]
- 88.Kuo YR, Chen CC, Goto S, Huang YT, Wang CT, Tsai CC, et al. Immunomodulatory effects of bone marrow-derived mesenchymal stem cells in a swine hemi-facial allotransplantation model. PLoS ONE (2012) 7:e35459 10.1371/journal.pone.0035459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schneeberger S, Gorantla VS, Brandacher G, Zeevi A, Demetris AJ, Lunz JG, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg (2013) 257:345–51 10.1097/SLA.0b013e31826d90bb [DOI] [PMC free article] [PubMed] [Google Scholar]