Abstract
Trigonella foenum-graecum, commonly called fenugreek, is a leguminous plant native to many Asian, Middle Eastern and European countries. Fenugreek oil is very effective in digestion. Identification of fenugreek genotype rich in saponins and fixed oil will be useful for pharmaceutical industries. In the present study, steroidal saponin and fixed oil content was analysed in 46 diverse fenugreek genotypes on dry weight basis. Significant differences were observed in the total saponin and fixed oil content among the genotypes. Saponin and fixed oil content ranged from 0.92 g to 1.68 g and 3.25 to 6.88 g with corresponding mean value of 1.34 g and 5.19 g/100 g dw, respectively.
Keywords: Fenugreek, fixed oil, sapogenin, steroidal saponin, Trigonella foenum-graecum
Fenugreek, Trigonella foenum-graecum also known as methi, Greek hayseed, and bird’s foot is a member of the legume family. The seeds of fenugreek have long been known as a traditional medicine, having hypocholesterolemic and antidiabetic effects.[1,2] The hypocholesterolemic activity is related to the defatted part of the seed extract[3] and involves saponin-rich subfractions.[4] In addition, fenugreek seeds are assumed to have restorative and nutritive properties and to stimulate the digestive process.[5] In India, they are known to be an important constituent of a traditional food (MethiPak) consumed during pregnancy and lactation. Moreover, fenugreek seeds are well-known for their pungent aromatic properties; as a spice, they are a component of many curry preparations and are often used to flavour food and stimulate appetite.[6]
Saponins, widely distributed in the plant kingdom, include a diverse group of compounds characterized by their structure containing a steroidal or triterpenoid aglycone and one or more sugar chains. Their structural diversity is reflected in their physicochemical and biological properties, which are exploited in a number of traditional and industrial applications.[7] Food and nonfood sources of saponins have come into renewed focus in recent years due to increasing evidence of their health benefits. Saponins can impact the immune system through their adjuvant activity, their ability to improve effectiveness of orally administered vaccines by facilitating the absorption of large molecules, and their immune-stimulatory effects. The ability of saponins to act as immunological adjuvants by enhancing the immune response to antigens has been recognized since 1940’s.[8,9]
Cholesterol-lowering activity of saponins, which has been demonstrated in animal and human trials, has been attributed to inhibition of the absorption of cholesterol from the small intestine, or the reabsorption of bile acids.[10–12] The cholesterol lowering effect of dietary saponins in humans is also supported by ecological studies.[13] Anticancer activity has been reported for a number of triterpene and steroid saponins[14,15] and diosgenin.[16]
These compounds are very useful in pharmaceutical industries as a natural source of steroidal hormones. Since its discovery, diosgenin is the single main precursor in the manufacture of synthetic steroids in the pharmaceutical industry. To date, diosgenin and related steroidal saponins were commercially obtained from the tubers of Dioscorea species; however, it is crucial to discover new and alternative sources of these compounds due to decreasing plant resources as well as increasing demand. One such alternative is fenugreek.
Considering the high market potential of steroidal saponins and vivid reports of steroidal saponins and fixed oil content in fenugreek seeds, the present study was carried out to identify fenugreek genotype rich in steroidal saponins and fixed oil content. We believe that identifying fenugreek genotype with high levels of steroidal saponins and fixed oil content might help to make this plant species economically, utilized as a source of steroidal saponins used for the synthesis of steroid drugs in pharmaceutical industries.
The experimental materials comprising 46 accessions of fenugreek genotypes were obtained from National Gene Bank, National Bureau of Plant Genetic Resources, New Delhi, India. The seed samples (3 replicates of each genotype) were milled and passed through USA Standard Test Sieve No. 20 with American Society for Testing and Materials (ASTM) E11 specifications (Sieve Size 0.850 mm) and dried for 3 h in a hot air oven at 60° and stored in desiccators and kept in dark.
All the chemicals used in the present study were of analytical grade. Diosgenin (~95%) was procured from Sigma Chemical Co., St. Louis, MO, USA. p-anisaldehyde (4-methoxybenzaldehyde) (≥98%) and sulphuric acid (~98%) were obtained from Merck, Hohenbrunn, Germany. All other organic solvents were purchased from Fisher Scientific, Nepean, ON, Canada.
The samples were defatted in a Soxhlet apparatus for 6 h with hexane as solvent and double-thickness cellulose extraction thimbles (Whatman). After completion of extraction n-hexane was evaporated using a rotary evaporator and weighed. The total fixed oil % (total crude fat) was calculated using the formula. Total fixed oil (in %)=100×(W2-W3)/(W2-W0), where W0=Weight of the thimble, W1=Weight of the original sample with a thimble before drying at the pre-extraction stage, W2=Weight of sample with thimble after drying at the pre-extraction stage, W3=Weight of sample with thimble after drying at the postextraction stage.
The material in the thimble was air-dried, ground with a mortar and pestle, and then oven-dried at 60° for 2 h. Samples were stored in desiccators at room temperature at dark before analysis for steroidal sapogenins content.
About 100 mg of the defatted cake (residue after oil extraction) was taken in a centrifuge tube and 3 ml methanol was added to the tubes and left on the shaker overnight, followed by centrifugation. The extraction was repeated again with 3 ml methanol and centrifuged. At the end, all supernatants of methanol extracts were pooled and the methanol was evaporated using rotary evaporator. Finally, a yellowish crystal powder of crude saponins was obtained. Upon acid hydrolysis saponin gives sapogenin which was then estimated using UV/Vis spectrophotometer.
Total steroidal saponin in defatted fenugreek was determined as per the reported procedure[17–19] with some modifications. Dry samples containing steroidal saponins was dissolved in 2 ml of ethyl acetate, to which 1 ml of 0.5% (v/v) p-anisaldehyde in ethyl acetate and 1 ml of 50% (v/v) H2SO4 in ethyl acetate was added. Reaction mixtures were then incubated at 60° for 10 m for colour development. Saponin present in samples is deglycosylated via acid hydrolysis, such that chromophore development arises from total saponin+sapogenin in a sample. After 10 min incubation, each tube was placed in a cold tap water bath. An aliquot of 0.5 ml of distilled water was added to each tube. The absorbance of the colour developed solution was measured in a spectrophotometer (UV 5704 SS, Electronics Corporation of India Limited (ECIL), India) at 430 nm. Ethyl acetate was used as a control for the measurement of absorbance. For reagent blank, 2 ml ethyl acetate was placed in a tube and assayed in similar manner. For the calibration curve, 8-40 μg standard diosgenin in 2 ml ethyl acetate was used.
The content of total fixed oil and total steroidal saponins for the 46 accessions of fenugreek genotypes is presented in Table 1. All mean values are expressed per 100 g of dry weight with standard deviation. A wide range of total steroidal saponin content was observed among the 46 accessions of fenugreek genotypes, ranging from 0.92 g (UM279) to 1.68 g (AM316) diosgenin equivalent per 100 g dw with the mean value of 1.34 g. There was approximately 2-fold difference between the lowest and the highest ranking genotype. Analysis of variance revealed that there was a significant difference among the genotypes with respect to total steroidal saponins at 5% level of significance.
Table 1.
TOTAL FIXED OIL AND STEROIDAL SAPONIN CONTENT IN FENUGREEK GENOTYPES STUDIED
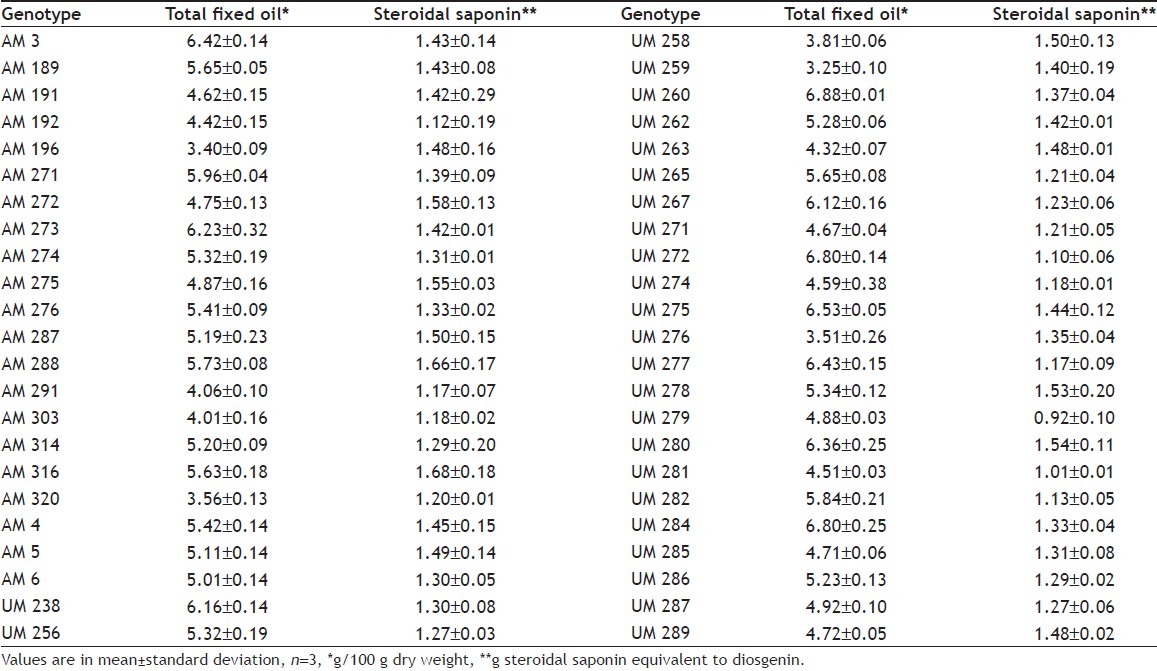
The levels in the total fixed oil content varied greatly among 46 accessions of fenugreek genotypes analysed. It ranged from 3.25 to 6.88 g per 100 g dw with the mean value of 5.19 g per 100 g of dry weight. The maximum fixed oil content present in the genotype UM260 was twice than in the lowest ranking genotype UM259.
The present study revealed wide variability among the fenugreek genotypes with respect to steroidal saponin and fixed oil content. Genotypes, viz. UM278, UM280, AM287, AM288 and AM316 contain more than 1.5 g and 5.0 g of steroidal saponin and fixed oil, respectively. Fenugreek genotypes rich in steroidal saponin could be utilized as alternative sources for the synthesis of steroid drugs in pharmaceutical industries.
ACKNOWLEDGEMENTS
We are grateful to Director, National Bureau of Plant Genetic Resources, New Delhi, India for constant support and encouragement in conducting this study.
Footnotes
Arivalagan, et al.: Steroidal Saponins and Fixed Oil in Fenugreek
REFERENCES
- 1.Singhal PC, Gupta RK, Joshi LD. Hypocholesterolemic effect of Trigonella foenum graecum. Curr Sci. 1982;51:136–7. [Google Scholar]
- 2.Mishkinsky JS, Goldschmied A, Joseph B, Ahronson Z, Sulman FG. Hypoglycaemic effect of Trigonella foenum graecum and Lupinus termis (leguminosae) seeds and their major alkaloids in alloxan-diabetic and normal rats. Arch Int Pharmacodyn Ther. 1974;210:27–37. [PubMed] [Google Scholar]
- 3.Valette G, Sauvaire Y, Baccou JC, Ribes G. Hypocholesterolaemic effect of fenugreek seeds in dogs. Atherosclerosis. 1984;50:105–11. doi: 10.1016/0021-9150(84)90012-1. [DOI] [PubMed] [Google Scholar]
- 4.Sauvaire Y, Ribes G, Baccou JC, Loubatieères-Mariani MM. Implication of steroid saponins and sapogenins in the hypocholesterolemic effect of fenugreek. Lipids. 1991;26:191–7. doi: 10.1007/BF02543970. [DOI] [PubMed] [Google Scholar]
- 5.Fazli FR, Hardman R. The spice, fenugreek (Trigonella foenum graecum L.): Its commercial varieties of seed as a source of diosgenin. Trop Sci. 1968;10:66–78. [Google Scholar]
- 6.Max B. This and that: The essential pharmacology of herbs and spices. Trends Pharmacol Sci. 1992;13:15–20. doi: 10.1016/0165-6147(92)90010-4. [DOI] [PubMed] [Google Scholar]
- 7.Hostettmann K, Marston A. Saponins. Cambridge, New York: Cambridge University Press; 2005. pp. 310–25. [Google Scholar]
- 8.Bomford R, Stapleton M, Winsor S, Beesley JE, Jessup EA, Price KR, et al. Adjuvanticity and ISCOM formation by structurally diverse saponins. Vaccine. 1992;10:572–7. doi: 10.1016/0264-410x(92)90435-m. [DOI] [PubMed] [Google Scholar]
- 9.Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: A review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 10.Oakenfull D, Sidhu GS. Could saponins be a useful treatment for hypercholesterolaemia? Eur J Clin Nutr. 1990;44:79–88. [PubMed] [Google Scholar]
- 11.Matsuura H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J Nutr. 2001;131:1000S–5. doi: 10.1093/jn/131.3.1000S. [DOI] [PubMed] [Google Scholar]
- 12.Kim SW, Park SK, Kang SI, Kang HC, Oh HJ, Bae CY, et al. Hypocholesterolemic property of Yucca schidigera and Quillaja saponaria extracts in human body. Arch Pharm Res. 2003;26:1042–6. doi: 10.1007/BF02994756. [DOI] [PubMed] [Google Scholar]
- 13.Chapman L, Johns T, Mahunnah RL. Saponin-like in vitro characteristics of extracts from selected non-nutrient wild plant food additives used by Masaai in meat and milk based soups. Ecol Food Nutr. 1997;36:1–22. [Google Scholar]
- 14.Berhow MA, Wagner ED, Vaughn SF, Plewa MJ. Characterization and antimutagenic activity of soybean saponins. Mutat Res. 2000;448:11–22. doi: 10.1016/s0027-5107(99)00225-0. [DOI] [PubMed] [Google Scholar]
- 15.Kerwin SM. Soy saponins and the anticancer effects of soybeans and soy-based foods. Curr Med Chem Anticancer Agents. 2004;4:263–72. doi: 10.2174/1568011043352993. [DOI] [PubMed] [Google Scholar]
- 16.Raju J, Patlolla JM, Swamy MV, Rao CV. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2004;13:1392–8. [PubMed] [Google Scholar]
- 17.Baccou JC, Lambert F, Sauvaire Y. Spectrophotometric method for the determination of total steroidal sapogenin. Analyst. 1977;102:458–65. doi: 10.1039/an9770200458. [DOI] [PubMed] [Google Scholar]
- 18.Uematsu Y, Hirata K, Saito K, Kudo I. Spectrophotometric determination of saponin in Yucca extract used as food additive. J AOAC Int. 2000;83:1451–4. [PubMed] [Google Scholar]
- 19.Wang Y, McAllister TA. A modified spectrophotometric assay to estimate deglycosylation of steroidal saponin to sapogenin by mixed ruminal microbes. J Sci Food Agric. 2010;90:1811–8. doi: 10.1002/jsfa.4019. [DOI] [PubMed] [Google Scholar]


