Abstract
Cholic acid biosynthesis is defective in individuals with cerebrotendinous xanthomatosis (CTX) and is associated with the excretion of 5β-cholestane-3α,7α, 12α,25-tetrol, an intermediate in the 25-hydroxylation pathway of cholic acid in CTX. To define the enzymatic defect in CTX, two suspected precursors of cholic acid, namely 5β-[7β-3H]cholestane-3α,7α, 12α-triol and 5β-[24-14C]cholestane-3α,7α, 12α,24S,25-pentol were examined by both in vivo and in vitro experiments. A third precursor, 5β-[7β-3H]-cholestane-3α,7α, 12α,25-tetrol, was compared with them in vitro.
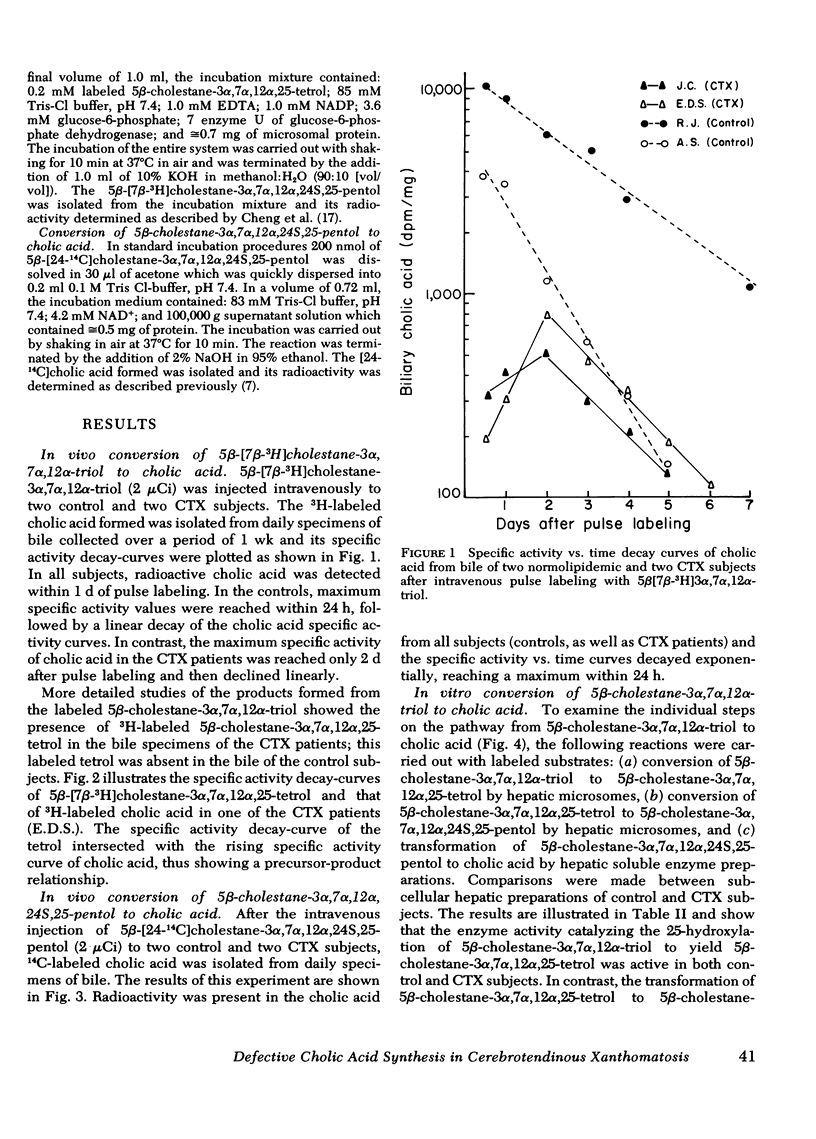
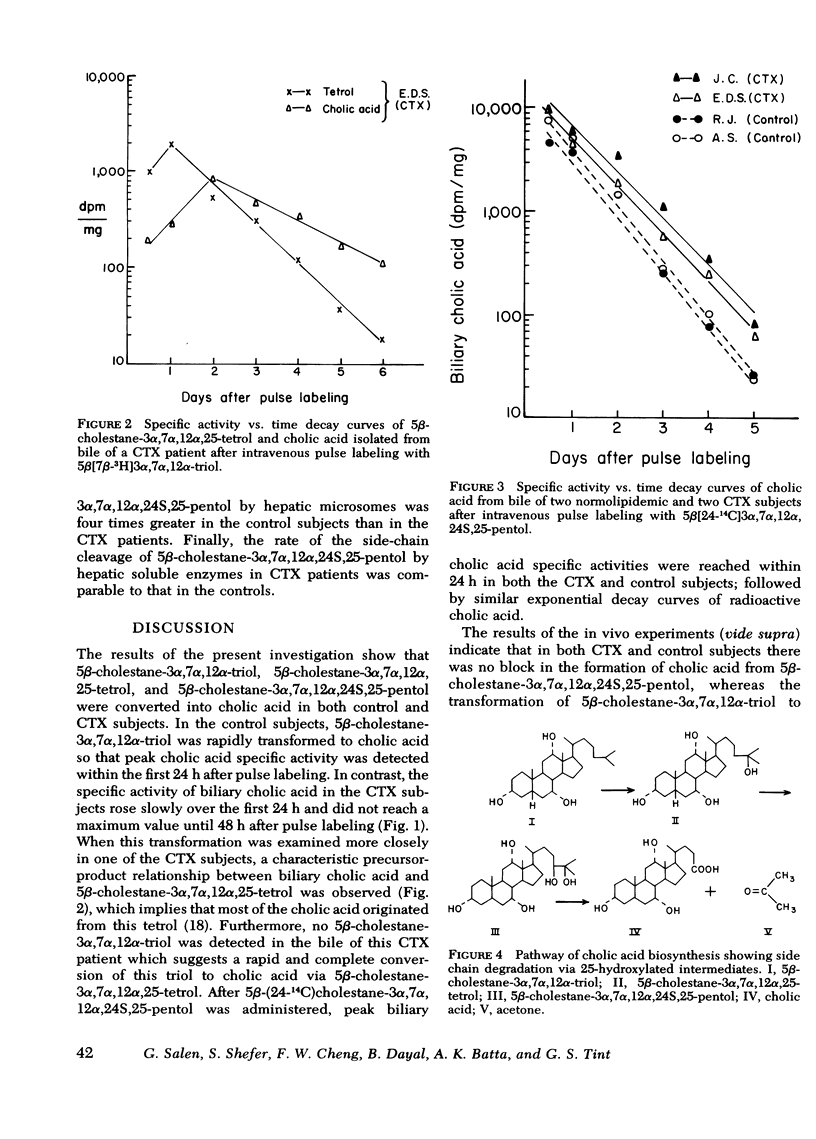
In the in vivo experiments, each one of the labeled precursors was administered intravenously to two CTX and two control subjects. In the controls, 5β-[7β-3H]cholestane-3α,7α, 12α-triol as well as 5β-[24-14C]-cholestane-3α,7α, 12α,24S,25-pentol were rapidly converted to labeled cholic acid. Maximum specific activity values were reached within 1 d after pulse labeling, followed by exponential decay of the cholic acid specific activity curves. In contrast, these two precursors differed widely when administered to two CTX patients. While 5β-[24-14C]cholestane-3α,7α, 12α,24S,25-pentol was rapidly converted to [24-14C]cholic acid and yielded identical decay curves with those obtained in the control subjects, maximum specific activity values in [7β-3H]cholic acid were much lower and peaked only on the second day after the injection of 5β-[7β-3H]cholestane-3α,7α, 12α-triol. Furthermore, an appreciable amount of 3H label was present in the 5β-cholestane-3α,7α, 12α,25-tetrol isolated from the bile of the subjects with CTX.
In the in vitro experiments, three enzymes on the 25-hydroxylation pathway of cholic acid were examined in both control and CTX subjects. The rate of the 25-hydroxylation of 5β-cholestane-3α,7α, 12α-triol in CTX patients was comparable to that in the controls. Similarly, the transformation of 5β-cholestane-3α,7α, 12α,24S,25-pentol to cholic acid, catalyzed by soluble enzymes, proceeded at approximately equal rates in CTX and in control individuals. On the other hand, the rate of 5β-cholestane-3α,7α, 12α,24S,25-pentol formation was about four times greater in the control subjects than in the CTX patients.
The results of the in vivo as well as the in vitro experiments suggest that the site of the enzymatic defect in CTX is at the 24S-hydroxylation of 5β-cholestane-3α,7α, 12α,25-tetrol. The relative deficiency of this hydroxylase in CTX patients, accompanied by the accumulation of its substrate in bile and feces, probably accounts for the subnormal production of bile acids in CTX patients.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkhem I., Gustafsson J., Johansson G., Persson B. Biosynthesis of bile acids in man. Hydroxylation of the C27-steroid side chain. J Clin Invest. 1975 Mar;55(3):478–486. doi: 10.1172/JCI107954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J. Omega-hydroxylation of steriod side-chain in biosynthesis of bile acids. Eur J Biochem. 1973 Jul 2;36(1):201–212. doi: 10.1111/j.1432-1033.1973.tb02902.x. [DOI] [PubMed] [Google Scholar]
- Cheng F. W., Shefer S., Dayal B., Tint G. S., Setoguchi T., Salen G., Mosbach E. H. Cholic acid biosynthesis: conversion of 5beta-cholestane-3alpha,7alpha,12alpha,25-tetrol into 5beta-cholestane-3alpha,7alpha, 12alpha,24beta,25-pentol by human and rat liver microsomes. J Lipid Res. 1977 Jan;18(1):6–13. [PubMed] [Google Scholar]
- Danielsson H., Einarsson K. On the conversion of cholesterol to 7-alpha,12-alpha-dihydroxycholest-4-en-3-one. Bile acids and steroids 168. J Biol Chem. 1966 Apr 10;241(7):1449–1454. [PubMed] [Google Scholar]
- Dayal B., Shefer S., Tint G. S., Salen G., Mosbach E. H. Synthesis of 5beta-cholestane-3alpha, 7alpha, 12alpha, 25-tetrol and 5beta-cholestane-3alpha, 7alpha, 245, 25-pentol. J Lipid Res. 1976 Jan;17(1):74–77. [PubMed] [Google Scholar]
- LANDING B. H. Hereditary metabolic diseases-general considerations. Metabolism. 1960 Mar;9:198–207. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masui T., Staple E. The formation of bile acids from cholesterol. The conversion of 5-beta-cholestane-3-alpha,7-alpha-triol-26-oic acid to cholic acid via 5-beta-cholestane-3-alpha,7-alpha,12-alpha, 24-xi-tetraol-26-oic acid I by rat liver. J Biol Chem. 1966 Sep 10;241(17):3889–3893. [PubMed] [Google Scholar]
- Reiner J. M. Recent advances in molecular pathology. Isotopic analysis of metabolic systems. I. Exp Mol Pathol. 1974 Feb;20(1):78–108. doi: 10.1016/0014-4800(74)90045-8. [DOI] [PubMed] [Google Scholar]
- Salen G. Cholestanol deposition in cerebrotendinous xanthomatosis. A possible mechanism. Ann Intern Med. 1971 Dec;75(6):843–851. doi: 10.7326/0003-4819-75-6-843. [DOI] [PubMed] [Google Scholar]
- Salen G., Grundy S. M. The metabolism of cholestanol, cholesterol, and bile acids in cerebrotendinous xanthomatosis. J Clin Invest. 1973 Nov;52(11):2822–2835. doi: 10.1172/JCI107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G., Shefer S., Setoguchi T., Mosbach E. H. Bile alcohol metabolism in man. Conversion of 5beta-cholestane-3alpha, 7alpha,12alpha, 25-tetrol to cholic acid. J Clin Invest. 1975 Jul;56(1):226–231. doi: 10.1172/JCI108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G., Shefer S., Zaki F. G., Mosbach E. H. Inborn errors of bile acid synthesis. Clin Gastroenterol. 1977 Jan;6(1):91–101. [PubMed] [Google Scholar]
- Setoguchi T., Salen G., Tint G. S., Mosbach E. H. A biochemical abnormality in cerebrotendinous xanthomatosis. Impairment of bile acid biosynthesis associated with incomplete degradation of the cholesterol side chain. J Clin Invest. 1974 May;53(5):1393–1401. doi: 10.1172/JCI107688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Cheng F. W., Dayal B., Hauser S., Tint G. S., Salen G., Mosbach E. H. A 25-hydroxylation pathway of cholic acid biosynthesis in man and rat. J Clin Invest. 1976 Apr;57(4):897–903. doi: 10.1172/JCI108366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Dayal B., Tint G. S., Salen G., Mosbach E. H. Identification of pentahydroxy bile alcohols in cerebrotendinous xanthomatosis: characterization of 5beta-cholestane-3alpha, 7alpha, 12alpha, 24xi, 25-pentol and 5beta-cholestane-3alpha, 7alpha, 12alpha, 23xi, 25-pentol. J Lipid Res. 1975 Jul;16(4):280–286. [PubMed] [Google Scholar]


