Abstract
In the present study, methanol extracts of Costus speciosus Koen. aerial parts were assessed for antiinflammatory, analgesic and antipyretic activities in experimental animals. The antiinflammatory activity of methanol extract of Costus speciosus (400 and 800 mg/kg, p.o.) was evaluated using carrageenan-induced paw oedema test. Analgesic effect was evaluated using acetic acid-induced writhing and Eddy’s hot-plate models and antipyretic activity was assessed by Brewer’s yeast-induced pyrexia in rats. The methanol extract of aerial parts of Costus speciosus in a dose of 400 and 800 mg/kg showed significant antiinflammatory activity (19.36 and 40.05% reduction) at 5 h postmedication. In analgesic models extract treated animals at (400 and 800 mg/kg) inhibited writhing’s caused by acetic acid by 14.24 and 31.90%, respectively, and it also increased the latency period at both high and low doses which showed the mean reaction time at 16.60±0.355 s and 14.12±0.355 s, respectively, when compared to control in hot-plate test. It also reduces the rectal temperature of the animals at low and high doses significantly 37.03±0.108° and 36.63±0.098°, respectively, in Brewer’s yeast induced pyrexia. The obtained results of the present investigation revealed that methanol extract of Costus speciosus has significant antiinflammatory, analgesic and antipyretic activities.
Keywords: Analgesic, antiinflammatory, antipyretic, carrageenan, Costus speciosus
Inflammation or phlogosis is a pathophysiological response of living tissues to injuries that leads to the local accumulation of plasmatic fluid and blood cells, which involves a complex sequence of bio-chemical events closely associated to the pathogenesis of various diseases such as rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, acute gout, migraine.[1–3]
Now-a-days, the synthetic antiinflammatory drugs are although, dominating the market but the element of toxicity that these drugs entail, cannot be ruled out. Many drugs (both nonsteroidal antiinflamatory drugs (NSAIDs) and corticosteroids) have been developed but their safety profile studies have shown that none of them is clearly safe. Due to adverse reactions of synthetic and chemical medicines i.e., they cause gastrointestinal irritation and reappearance of symptoms after discontinuation being observed round the globe, herbal medicines have made a comeback to improve our basic health needs. Many plants and herbs such as ginger, turmeric and olive oil, have been shown to exhibit potent antiinflammatory effects.[4] Currently available drugs such as opiates and NSAIDs are not useful in all cases due to their adverse effects. In this respect, new compounds with improved pain management capacity and fewer side effects are being searched every nook and corner of the world. Therefore, drugs lacking those effects are being searched all over the world as alternatives to NSAIDs and opiates. During this process, the investigations of the efficacy of plant-based drugs used in the traditional medicine have been paid great attention because they are cheap having little side-effects.[5,6]
Costus speciosus Koen. (Keu, Crape ginger), an Indian ornamental plant, has long been medicinally used in traditional systems of medicine. This plant of Costaceae family is commonly known as Keukand (Hindi), Variegated Crepe Ginger (English). It is an erect, succulent, perennial herb, up to 2.7 m in height, arising from a horizontal rhizome, found in tropical region of India and also cultivated for ornament. The rhizomes and roots are ascribed to be bitter, astringent, acrid, cooling, aphrodisiac, purgative, anthelmintic, depurative, febrifuge, expectorant, tonic, improves digestion and stimulant herb that clears toxins. Juice of the rhizome is applied to head for cooling and relief from headache.[7–12]
According to the available literature on the pharmacological and phytochemical prospective of C. speciosus, no scientific reports are available on the antiinflammatory, analgesic and antipyretic activities of methanol extracts of the aerial parts of the plant. Based on this, an attempt has been made to evaluate these activities of the methanol extract of aerial parts of C. speciosus.
MATERIALS AND METHODS
Fresh aerial parts of C. speciosus Koen. (Costaceae), for the proposed work were collected from the Bahadurpur forests of Kolkata and were authenticated by Birbal Sahni Institute of Palaeobotany, Lucknow, India. All the experiments were carried out using adult albino rats (160-200 g) and Swiss albino mice (24-30 g). All the experimental procedures and protocols used in this study were approved by the Institutional Animal Ethics Committee (1205/c/08/CPCSEA, 21.04.08). All animals were housed in polypropylene cages and maintained under standard laboratory conditions. Animals were housed at a temperature of 24±2° and relative humidity of 60-70%. They were fed with a standard diet and water was given ad libitum. All experiments were conducted after overnight fasting but there was free access to water. A minimum of six animals were used in each group.
The following chemicals procured from various sources were used in this investigation i.e., carrageenan, Brewer’s yeast (Sigma Aldrich, Bangalore, India), petroleum ether, chloroform, ethyl acetate, methanol, acetic acid (Rankem, New Delhi, India), carboxymethyl cellulose (CMC, Loba Chemie, Mumbai, India), diclofenac sodium (Akums Drugs and Pharmaceuticals, Delhi, India) and aspirin (Research Lab, Mumbai, India) were used during the experimental protocol.
Extraction and preparation of sample:
The aerial part of the plant was dried under shade and mechanically reduced to moderate coarse powder. The coarse powder was successively extracted using a soxhlet apparatus with the solvents in increasing polarity starting with petroleum ether, chloroform, ethyl acetate and methanol. The extracts of the aerial parts were then concentrated to 3/4th of its original volume by using rotary evaporator at 40° under reduced pressure. The concentrated extracts were then transferred to a china dish and evaporated on a thermostat-controlled water-bath until they were dried. The methanol extract of Costus speciosus (MECS) was subjected to chemical tests for the detection of phytoconstituents. The dried methanol extract was suspended in 0.5% CMC in distilled water (vehicle) and used for pharmacological investigations.
Carrageenan-induced paw oedema:
Rats of both sexes were randomised into four groups of six animals each. The first group served as control and was given 1% CMC (1 ml/100 g body weight). The second group was kept as the standard, which received diclofenac sodium orally in a dose of 10 mg/kg suspended in CMC. The third and fourth groups received the methanol extract (orally) in doses of 400 and 800 mg/kg, respectively as low and high doses groups. The paw oedema was induced by injection of 0.1 ml of 1% carrageenan in 0.9% saline into subplantar region of the left hind paw of the rats. The test extracts (MECS) at 400 and 800 mg/kg dose, standard (diclofenac sodium; 10 mg/kg) and control (1% CMC) were administered. The volume of injected paw was measured at 1, 2, 3, 4 and 5 h after the injection using a plethysmometer and the size of oedema was expressed by changes in paw volumes.[13–15]
The percentage of inhibition was calculated using the formula, %Inhibition=(X-Y)×100. Where, X=Difference in paw volume of rats in the control group. Y=Difference in paw volume of rats in the drug treated group. The percentage inhibition of inflammation was calculated for standard, test and control groups.
Writhing test:
Mice of both sexes were randomised into four groups of six each. Group I - Control, 1% CMC (1 ml/100 g body weight); Group II - Standard, diclofenac sodium 10 mg/kg suspended in CMC; Group III - Test I, methanol extract in doses of 400 mg/kg orally. Group IV- Test II, methanol extract in doses of 800 mg/kg orally.
The animals of the standard group were administered orally diclofenac (10 mg/kg) suspended in 1% CMC. The control group received 1% CMC (1 ml/100 g b.w.) while the test groups received 400 and 800 mg/kg, respectively. Thirty minutes later each mouse was given 0.1 ml/10 g of 1% acetic acid solution (i.p.). Five minutes after acetic acid injection, the number of writhes was counted for 15 min.[16,17] The percentage inhibition of writhing was calculated according to the following formula, %Inhibition=((WC-WT)/WT)×10. Where, WC=Average writhes in control group, WT=Average writhes in control group.
Hot plate method:
The animals were divided into four groups of six animals each. Group I served as control was given 1% CMC, group II served as standard and was injected with diclofenac sodium (9 mg/kg) intraperitoneally. Group III and IV were treated orally with methanol extract at 400 and 800 mg/kg, respectively. The animals were individually placed on the hot eddy’s plate maintained at 55°. After 1 h of respective treatments, the response time was noted by a stop watch as the time at which animals reacted to the pain stimulus either by paw licking or jump response. The cutoff time for the reaction was 15 s. The latency as recorded before and after 0.5, 1, 2 and 3 h following administration of the drugs to the respective groups.[16,17]
Brewer’s yeast-induced pyrexia in rats:
Rats weighing between 130 and 170 g were divided into groups of six animals each. The first group was kept as control and was given 1% CMC. The second group received acetyl salicylic acid in a dose of 300 mg/kg suspended in 1% CMC. The third and fourth groups received the methanol extract at 400 mg/kg and 800 mg/kg, respectively. Their initial rectal temperature was recorded by insertion of a thermometer at a depth of 2 cm in the rectum. A 20% suspension of Brewer’s yeast in 0.9% saline was injected subcutaneously in back below the nape of the neck in a dose of 20 mg/kg. After 18 h, animals that showed an increase of 0.3-0.5° in rectal temperature were selected. Rectal temperature was recorded by digital thermometer immediately before and 18 h after Brewer’s yeast injection. The food was withdrawn and rectal temperatures were recorded at 0.5, 1, 2, 3, 4 and 5 h after the temperature rise begun. The maximum reduction in average rectal temperature of the treated group animals as compared with the control hyperpyrexic group and standard group was calculated.[13,16]
Statistical analysis:
The results were expressed as the mean±SEM. The results obtained from the present study were analysed using one way ANOVA followed by Bonferroni multiple comparison tests. Data were computed for statistical analysis by using GraphPAd Prism, GraphPad Software, Inc., La Jolla, USA.; version 5.03.
RESULTS AND DISCUSSION
Preliminary phytochemical studies showed the presence of carbohydrates, glycosides, saponins, tannins, flavonoids and alkaloids in methanol extract of CS. The methanol extract of aerial parts of C. speciosus in a dose of 800 mg/kg showed significant antiinflammatory activity (40.05% reduction) and in a dose of 400 mg/kg showed (19.36% reduction) at 5 h postmedication (Table 1 and 2). The standard drug diclofenac sodium at a dose of 10 mg/kg produced significant reduction of carrageenan-induced paw oedema (63.0% reduction), therefore, proving the antiinflammatory efficacy of C. speciosus.
Table 1.
EFFECT OF COSTUS SPECIOSUS METHANOL EXTRACT (AERIAL PARTS) ON CARRAGEENAN INDUCED PAW VOLUME
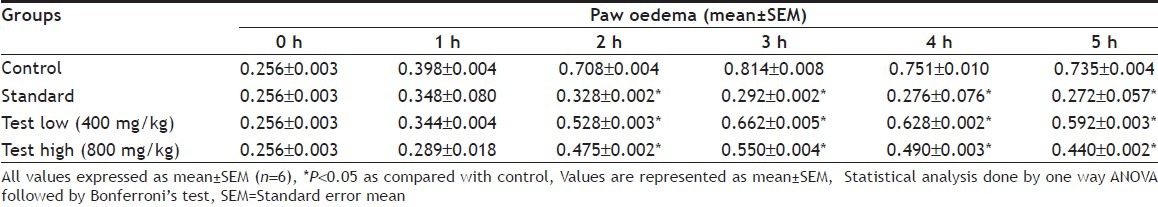
Table 2.
PERCENTAGE INHIBITION OF EDEMA BY METHANOL EXTRACT OF COSTUS SPECIOSUS (AERIAL PARTS) AT DIFFERENT TIME INTERVALS DETERMINED AGAINST DICLOFENAC SODIUM AS REFERENCE
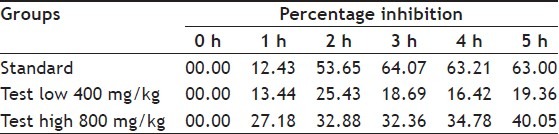
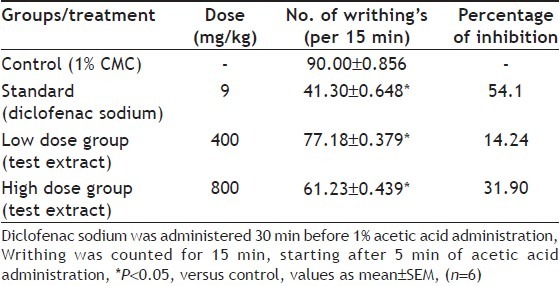
The intraperitoneal injection of acetic acid (1%) caused strong nociceptive response in the control group, with (90.00±0.856) abdominal contortions. At high and low doses (MECS) it showed more number of writhing’s (61.23±0.439 and 77.18±0.379, respectively) as compared to standard (41.30±0.648). Treated animals with MECS (400 and 800 mg/kg) inhibited writhing’s caused by acetic acid by 14.24 and 31.90%, respectively. Diclofenac sodium (9 mg/kg), the standard for this experiment, reduced contortions by 54.1% (Table 3).
Table 3.
EFFECT OF METHANOL EXTRACT OF AERIAL PARTS OF COSTUS SPECIOSUS ON THERMIC STIMULUS INDUCED PAIN (HOT PLATE TEST) IN MICE
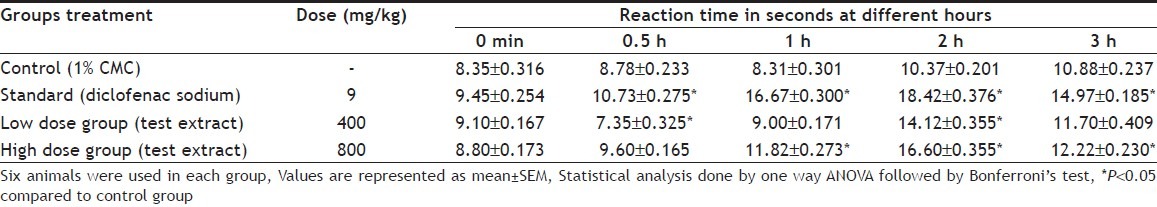
Hot plate result showed significant reduction of pain at 120 min following extracts medication (400 and 800 mg/kg) as compared to control. The animals pretreated with MECS showed a dose dependent increase in latency of response in the hot-plate method. Increase in mean reaction time by diclofenac in the standard group was significantly higher (18.42±0.376 s) at 2 h than both high and low doses, which showed the mean reaction time at 16.60±0.355 s and 14.12±0.355 s, respectively when compared to control at 10.37±0.207 s (Table 4).
Table 4.
EFFECT OF METHANOL EXTRACT OF AERIAL PARTS OF COSTUS SPECIOSUS ON ACETIC ACID INDUCED WRITHING IN MICE
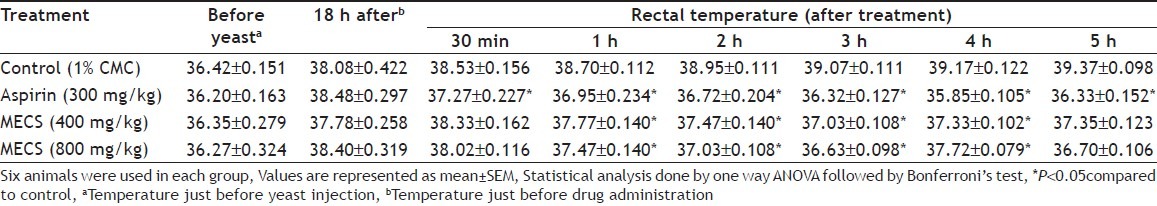
The subcutaneous injection of 20% Brewer’s yeast suspension substantially, increased the rectal temperature of the rats 18 h after administration (38.08±0.422° vs. 36.42±0.151°). MECS at 400 and 800 mg/kg produced significant antipyretic activity reaching peak effect at 3 h with 37.03±0.108° and 36.63±0.098°, respectively. Whereas, aspirin (300 mg/kg) showed consistent antipyretic activity throughout the observation period of 5 h with a temperature of 36.33±0.152°. MECS treatment at low and high doses significantly reduced the rectal temperature of the animals respectively, as compared to the control group (39.07±0.111°).
With the aim of providing, the first scientific evidence for the popular use of aerial parts of C. speciosus, the pharmacological effects of MECS were investigated, particularly those related to the inflammatory process.
The present experimental investigation revealed that methanol extract of aerial parts of C. speciosus possessed significant antiinflammatory, analgesic and antipyretic activities in experimental animals at a dose of 400 and 800 mg/kg. Carrageenan-induced hind paw oedema is the standard experimental model of acute inflammation. Moreover, the experimental model exhibits a high degree of reproducibility.[18] It has a biphasic effect. The first phase is due to release of histamine and serotonin (5-HT) (0-2 h), plateau phase is maintained by a kinin like substance (3 h) and a second accelerating phase of swelling is attributed to PG release (>4 h).[19] MECS produced dose-dependent and significant inhibition of carrageenan-induced paw oedema. The inhibition was however, less than that of the standard drug, diclofenac sodium.
The mechanism for testing analgesic was selected such that both centrally and peripherally mediated effects were investigated. The abdominal constriction response induced by acetic acid is a sensitive procedure to establish peripherally acting analgesics. This response is thought to involve local peritoneal receptors. It causes an increase in the concentration of PGE 2 and PGF 2α in the peritoneal fluid.[20,21] In this method, pain is generated indirectly via endogenous mediators like prostaglandins, which stimulate peripheral nociceptive neurons. These neuronal fibres are sensitive to both narcotics and nonsteroidal antiinflammatory drugs.[21] The local irritation caused by intraperitoneal administration of this agent, unleashes the release of several mediators, such as bradykinin, substance P and PGs, as well as cytokines such as IL-1β, TNF-α and IL-8. These mediators activate chemosensitive nociceptors which contribute to the development of inflammatory pain.[22] The experimental results obtained in this study indicated that the extract dose dependently reduced acetic acid induced writhes. Diclofenac significantly increased the pain threshold throughout the observation period of 1-3 h. The analgesic activity induced by the high dose of MECS was less effective than that induced by the standard drug diclofenac (9 mg/kg). The methanol extract of aerial parts showed analgesic effect in acetic acid-induced writhing probably by inhibiting prostaglandin synthesis.
The hot-plate method has been found to be suitable for evaluation of centrally acting analgesics. Thus, MECS at both the doses significantly exhibited marked central analgesic effect as evident by significant increase in mean reaction time when compared to the control. An increase in reaction time is generally considered and important parameter of central and peripheral analgesic activity by nonselective COX inhibition and nociceptors. Hot-plate result showed significant reduction of pain at 120 min following extracts medication (400 and 800 mg/kg) as compared to control. The animals pretreated with MECS showed a dose dependent increase in latency of response in the hot-plate method.
The brewer’s yeast induced pyrexia in rats was employed to investigate the antipyretic activity of MECS (Table 5). Yeast-induced fever is called pathogenic fever. Its aetiology includes production of prostaglandins, which set the thermoregulatory centre at a lower temperature.[23] In the present study, the effect of both the concentrations of the methanol extract was compared at different times with that of the standard drug and control. It was found that the antipyretic effect was found to be highly significant in maintaining normal body temperature and reducing yeast-induced elevated body temperature in rats in a dose dependent manner and its effect is significantly comparable to that of the standard antipyretic drug aspirin. The extract markedly decreased elevated body temperature but not in normal animals. There are several mediators or multi processes underlining the pathogenesis of fever. Inhibition of any of these mediators may bring about antipyresis.[24]
Table 5.
EFFECT OF METHANOL EXTRACT OF COSTUS SPECIOSUS ON BREWER’S YEAST INDUCED PYREXIA IN RATS
On preliminary phytochemical screening MECS was found to contain flavonoid compounds. Flavonoids are known to target prostaglandins, which are involved in the late phase of acute inflammation and pain perception.[25] Hence, the presence of flavonoids may be contributory to the antiinflammatory and analgesic activities of MECS.
Although, the exact nature of the antiinflammatory, antinociceptive and antipyretic activity mechanisms of the phytoconstituents have not been elucidated, the results of the present study validate from a preclinical point-of-view, the popular use of this medicinal plant in the treatment of inflammatory diseases. These studies are valuable for identifying lead compounds for antiinflammatory drugs, keeping in mind the side-effects of NSAIDs and corticosteroids. Further, human studies are needed to prove the safety and efficacy of long term administration of methanol extract of C. speciosus as potential antiinflammatory, analgesic and antipyretic agent in routine clinical practice.
Footnotes
Srivastava, et al.: Antiinflammatory, Analgesic and Antipyretic potential of Costus speciosus Koen
REFERENCES
- 1.Vazquez AI, Sanchez CM, Delgado NG, Alfonso AM, Ortega YS, Sanchez HC. Antiinflammatory and analgesic activities of red seaweed Dichotomaria obtusata. Braz J Pharm Sci. 2011;47:111–8. [Google Scholar]
- 2.Panda BB, Gaur K, Kori ML, Tyagi LK, Nema RK, Sharma CS, et al. Antiinflammatory and analgesic activity of Jatropha gossypifolia in experimental animal models. Glob J Pharmacol. 2009;3:1–5. [Google Scholar]
- 3.Harvey RA, Champe PC, editors. Lippincott’s Illustrated Reviews: Pharmacology. 4th ed. Philadelphia: Lippincott Williams and Wilkins, Wolters Kluwar Health; 2009. p. 499. [Google Scholar]
- 4.Gomase PV, Shire PS, Nazim S, Choudhari AB. Development and evaluation of polyherbal formulation for antiinflammatory activity. J Nat Prod Plant Resour. 2011;1:85–90. [Google Scholar]
- 5.Dash PR, Nasrin M, Saha MR. Evaluation of analgesic and neuropharmacological activities of methanolic rhizome extract of Hedychium Coronarium. Int J Pharm Sci Res. 2011;2:979–84. [Google Scholar]
- 6.Gomase PV, Shire PS, Nazim S, Choudhari AB, Shaikh S, Khairnar A. Phytochemical evaluation and Analgesic activity of fresh juice of young stem (tender) bark of Azadirachta indica A. Juss. Der Pharmacia Lett. 2011;3:407–15. [Google Scholar]
- 7.Anonymous . The Wealth of India. Second supplement series (Raw materials) Vol. 2. New Delhi: NISCAIR, CSIR; 2007. pp. 211–3. [Google Scholar]
- 8.Gupta RK. Medicinal and Aromatic Plants. 1st ed. New Delhi: CBS Publishers and Distributors; 2010. p. 234.p. 499. [Google Scholar]
- 9.Deni B. Encyclopedia of Herbs. London: The Royal Horticulture Society, Dorling Kindersley; 2008. p. 181. [Google Scholar]
- 10.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. New Delhi: NISCAIR Press; 2006. p. 79. [Google Scholar]
- 11.Khare CP. Indian Medicinal Plants. India: Springer (India) Private Limited; 2007. pp. 181–2. [Google Scholar]
- 12.Nadkarni KM, Nadkarni AK. Indian Materia Medica. Vol. 1. Mumbai: Bombay Popular Prakashan Pvt. Ltd.; 2007. pp. 385–6. [Google Scholar]
- 13.Binny K, Sunil K, Dennis T. Antiinflammatory and antipyretic properties of the rhizome of Costus speciosus (Koen.) Sm. J Basic Clin Pharm. 2010;1:177–81. [PMC free article] [PubMed] [Google Scholar]
- 14.Jothimanivannan C, Kumar RS, Subramanian N. Antiinflammatory and analgesic activity of ethanol extract of aerial parts of Justicia gendarussa Burm. Int J Pharmacol. 2010;6:278–83. [Google Scholar]
- 15.Prabhu K, Karar PK, Hemlatha S, Ponnudurai K. Antiinflammatory, analgesic and antispasmodic activities of three viburnum Linn. species. Int J Curr Trends Sci Technol. 2010;1:175–86. [Google Scholar]
- 16.Purnima A, Koti BC, Thippeswamy AH, Jaji MS, Swamy AH, Kurhe YV, et al. Antiinflammatory, analgesic and antipyretic activities of Mimusops elengi Linn. Indian J Pharm Sci. 2010;72:480–5. doi: 10.4103/0250-474X.73908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikajoo L. Analgesic activity of aqueous and alcohol root extracts of Pergularia daemia (forsk.) Chiov. Int J Pharm Pharm Sci. 2009;1:33–7. [Google Scholar]
- 18.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 19.El-Shenawy SM, Abdel-Salam OM, Baiuomy AR, El-Batran S, Arbid MS. Studies on the antiinflammatory and antinociceptive effects of melatonin in the rat. Pharmacol Res. 2002;46:235–43. doi: 10.1016/s1043-6618(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 20.Bentley GA, Newton SH, Starr J. Studies on the antinociceptive action of alpha-agonist drugs and their interactions with opioid mechanisms. Br J Pharmacol. 1983;79:125–34. doi: 10.1111/j.1476-5381.1983.tb10504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekiya K, Okuda H, Arichi S. Selective inhibition of platelet lipoxygenase by esculetin. Biochim Biophys Acta. 1982;713:68–72. [PubMed] [Google Scholar]
- 23.Moltz H. Fever: Causes and consequences. Neurosci Biobehav Rev. 1993;17:237–69. doi: 10.1016/s0149-7634(05)80009-0. [DOI] [PubMed] [Google Scholar]
- 24.Morimoto A, Nakamori T, Watanabe T, Ono T, Murakami N. Pattern differences in experimental fevers induced by endotoxin, endogenous pyrogen, and prostaglandins. Am J Physiol. 1988;254:R633–40. doi: 10.1152/ajpregu.1988.254.4.R633. [DOI] [PubMed] [Google Scholar]
- 25.Rajnarayana K, Reddy MS, Chaluvadi MR, Krishna DR. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol. 2001;33:2–16. [Google Scholar]


