Abstract
Background
There is no consensus regarding optimal position of external ventricular drain (EVD) with regard to clearance of intraventricular hemorrhage (IVH).
Objective
To assess the hypothesis that EVD laterality may influence the clearance of blood from the ventricular system, with and without administration of thrombolytic agent.
Methods
The EVD location was assessed in 100 patients in two CLEAR Phase II trials assessing the safety, and dose optimization of thrombolysis through the EVD to accelerate the clearance of obstructive IVH. Laterality of catheter was correlated with clearance rates.
Results
Clearance of IVH over the first 3 days was significantly greater when thrombolytic was administered as compared to placebo, regardless of catheter laterality (p < 0.005, CI −14.0, −4.14 for contralateral EVD and CI −24.7, −5.44 for ipsilateral EVD, respectively). When thrombolytic was administered, there was a trend of more rapid clearance of total IVH through an EVD placed on the side of dominant intraventricular blood as compared to an EVD on the side with lesser blood (P = 0.09; CI −9.62, 0.69). This was not true when placebo was administered. Clearance of 3rd and 4th ventricular blood was unrelated to EVD laterality.
Conclusion
It is possible that placement of EVD may be optimized to enhance the clearance of total IVH if lytic agents are used. Catheters on either side can clear the 3rd and 4th ventricles with equal efficiency.
Keywords: external ventricular drain, intraventricular hemorrhage, thrombolysis
Introduction
Intraventricular hemorrhage (IVH) is frequently associated with intracerebral hemorrhage1, and the volume of associated IVH a significant and independent predictor of mortality and disability in this disease1–8. An external ventricular drain (EVD) is commonly used to control intracranial pressure, and to divert cerebrospinal fluid in the setting of hydrocephalus, but also to help clear ventricular blood11–12. It is not known whether EVD placement in the lateral ventricle with greater or lesser blood encasement results in greater clearance of IVH, and whether this is different when administering intraventricular thrombolysis. These questions are retrospectively addressed in patients who underwent EVD placement and intraventricular treatment with placebo or recombinant tissue plasminogen activator (rt-PA) in the setting of the recently completed CLEAR IVHPhase II trials, in which results have recently been published13.
Patients and Methods
The CLEAR IVHtrials included patients with obstructive 3rd or 4th ventricular IVH with small associated ICH (< 30 ml on initial eligibility CT scan), excluding patients with known or suspected vascular anomaly, tumor, infratentorial hemorrhagic source, or uncorrected coagulopathy. Mean Glasgow Coma Score on admission of enrolled patients was 8.42 (range 3 to 15), mean volume of IVH at stability was 46.95ml (range 1.53 to 164.8 ml), and mean ICH volume was 9.50 ml at stability (range 0 to 42.5 ml; five cases expanded their ICH to > 30 ml after enrollment). All enrolled patients received EVD placementwith the aim of ICP control. Side of EVD placement was at the discretion of the treating neurosurgeon. The initial safety study (n = 48) used a randomization scheme of 1:1 and the remaining 52 patients were part of the dose-finding tiers, so all patients in those tiers received t-PA at varying doses and intervals. 78 patients ultimately received0.3 to 3 mg of rt-PA doses every 8 to 12 hours, while 22 patientsreceived saline placebo through the EVD at similar volume/frequency. After each injection of the study agent and a standardized saline flush, the EVD was closed for 1 hour, following which the EVD was reopened to resume ventricular drainage. Drainage criteria (intermittent versus continuous), and particular threshold for drainage were left to the investigators and treating physician’s prerogative, as per local practice standards. Thetrial protocolwas approved by the Institutional Review Boards of all participating centers.
Laterality of EVD location was assessed after the conclusion of the trials by review of the CT scan thatwas required after placement of each new EVD catheter, including bone windows to better visualize catheter perforations. Review was conducted by two investigators (JJ and IAA) blinded to treatment status, IVH volume clearance data, or other clinical information. Categorization of EVD laterality was determined by consensus among the two investigators according to prospectively articulated criteria, as ipsilateral (catheter perforations are in the lateral ventricle with greater IVH distribution, with or without extension of some perforations into the third ventricle), contralateral (catheter perforations are in the lateral ventricle with lesser IVH distribution, with or without extension of some perforations into the third ventricle), or other (including cases with bilateral symmetric IVH, catheter perforations crossing into both lateral ventricles, or cases who underwent placement of dual or bilateral catheters in the first three days).
Prospective, protocolized imagingallowed assessment of total IVH volume on daily computed tomographic (CT) scansin all cases, blinded to the treatment assignment. In addition, volume of 3rd and 4th ventricular blood was prospectively assessed in cases receiving thrombolytic (as part of dose escalation pharmacokinetics study). The volumetric assessmentswere calculated by the trial’s Imaging Center staff using free-hand tracing with medical imaging software (Alice®, Perceptive Informatics - Boston, Massachusetts), and methods similar to those reported in other studies14. Clearance rates of IVH were determined using daily IVH volume, relative to initial IVH volume (defined on stability CT scan approximately 6 hours after EVD placement). Population-averaged models were fit for each cohort, and predictors and their interactions includedtime course of clearance and the variables of treatment status (thrombolysis or placebo) and EVD location (ipsilateral or contralateral to dominant IVH). The two variables of treatment status and catheter laterality were assumed to be independent of each other. These models were fit using generalized estimating equations (GEE), with exchangeable correlation, robust standard error, no intercept, and an offset of 100. The offset was used since each clearance is assumed to be 100% at stability (time 0) 15. Beta coefficients represented the average clearance rates (slopes) for each group, and these were plotted for 3 days after IVH stability, and compared using Wald t-tests, with confidence intervals and significance. The 3-day analysis was chosen because all patients had EVDs in place during this time and were receiving active treatment and daily neuroimaging unless death occurred. EVD laterality was determined by the presence of catheter perforations in the respective lateral ventricle.
Results
Figure 1 illustrates the distribution ofEVD placements in relation to dominant lateral ventricle IVH, and examples of typical ipsilateral and contralateral placements. There was no significant difference in CSF volume drained during the index period among cohorts with ipsilateral versus contralateral catheter locations (mean: 128ml versus 165 ml, respectively; p = 0.139). Nine of 66 (14%) cases with initial contralateral EVDplacement, and 1 of 15 (6%) cases with initial ipsilateral placement resulted in clogging or malfunction of EVD or decision to place a second catheter in the contralateral ventricle (difference not statistically significant, p = 0.679). There were no significant differences between the ipsilateral and contralateral groups in terms of gender, age, baseline Glasgow Coma Score, initial IVH volume, initial ICH volume, or in the CSF drained during the 3-day index period of drainage (Table 1). The mean total rt-PA dose administered during the 3-day index period of drainage was significantly larger in the cases with EVD contralateral to dominant IVH as compared to ipsilateral cases (11.8 mg and 5.21 mg, respectively, p = 0.02).
Figure 1.
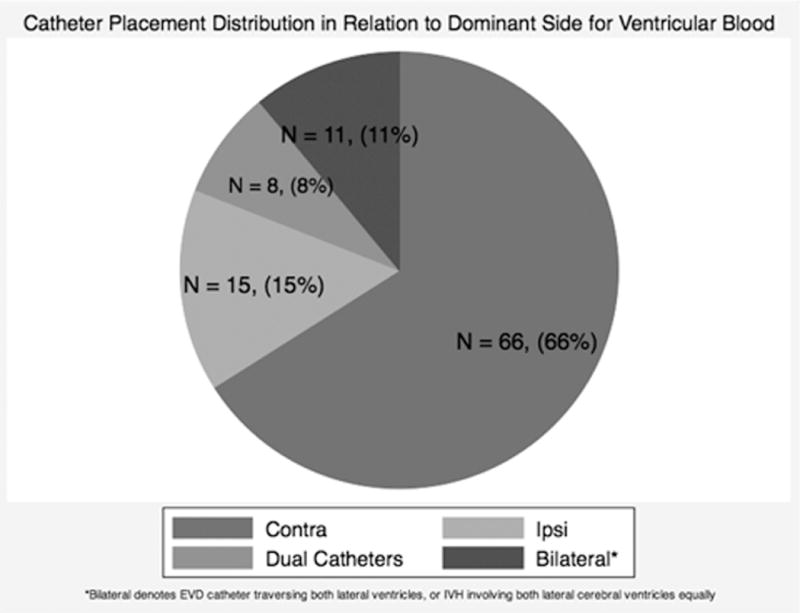
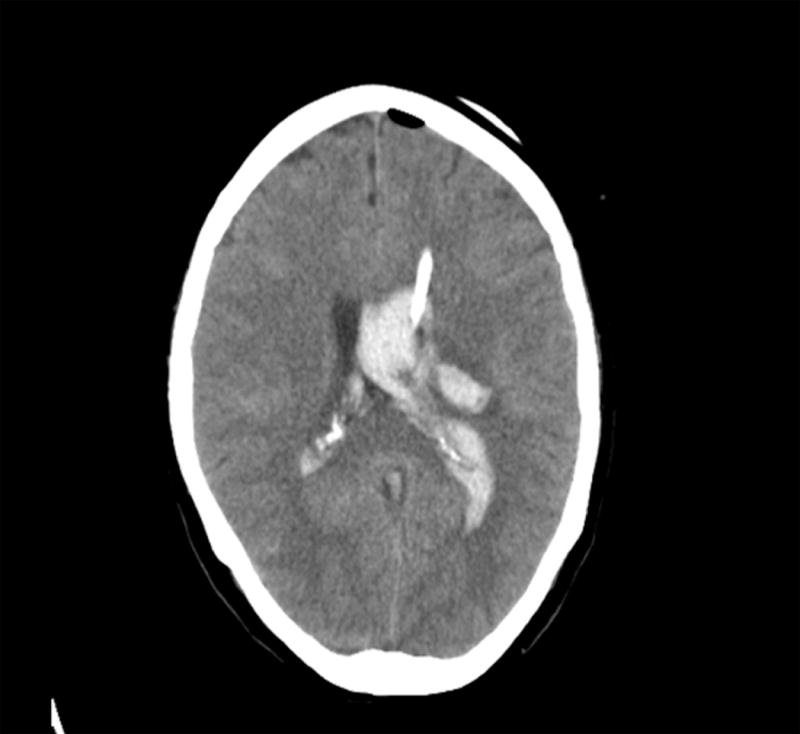
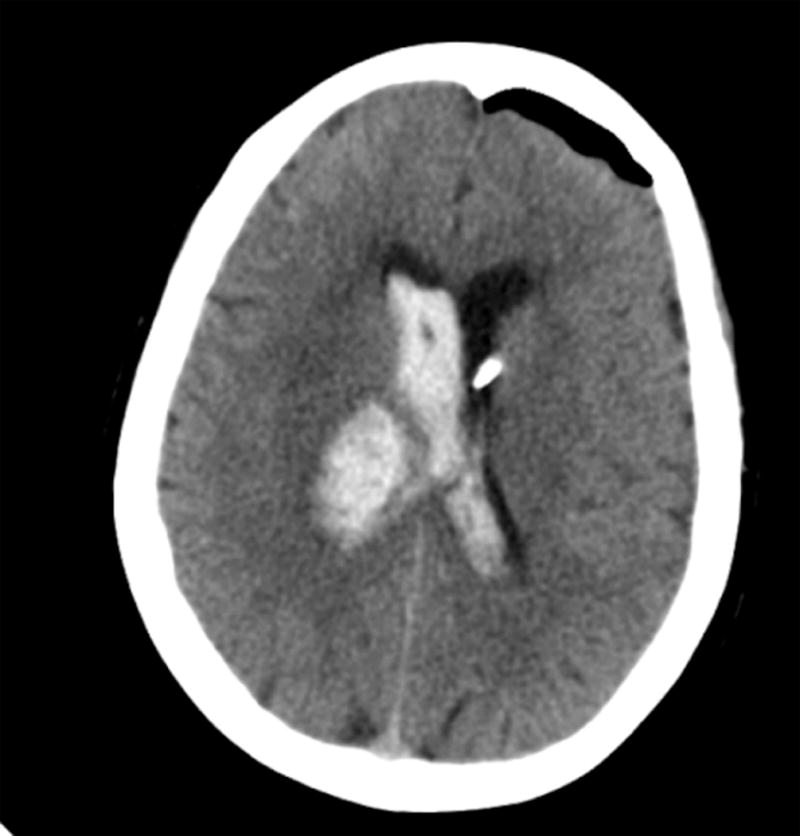
Distribution of initial catheter placements for CLEAR Phase II enrolled subjects (A). CT scan of typical ipsilateral (B) or contralateral (C) EVD placement.
Table 1.
Baseline Characteristics in Cohorts with Ipsilateral and Contralateral EVD Placements
| Contralateral | Ipsilateral | P-value | |
|---|---|---|---|
|
| |||
| Gender (M/F) | 37/29 | 12/3 | 0.09 |
| Mean Age (years) | 55.09 | 55.67 | 0.84 |
| Mean IVH Volume at Stability (mL) | 44.51 | 37.36 | 0.29 |
| Mean ICH Volume at Stability (mL) | 10.76 | 8.25 | 0.35 |
| Mean Baseline GCS | 8.4 | 9.6 | 0.18 |
| Mean CSF drainage (mL)* | 165.5 | 128.8 | 0.14 |
| Mean total rt-PA dose (mG) in patients receiving rt-PA* | 11.78 | 5.21 | 0.02 |
During the 3-day index period of drainage
For clearance comparisons, we limited our analysis to the 81 cases where EVD was clearly ipsilateral or contralateral to the dominant lateral ventricle clot during the index period of drainage. We hence excluded cases where the catheter crossed into both lateral ventricles (n = 11), where EVD catheter was changed to the opposite side (n = 10), and cases with more than a single catheter during the 3-day index period of drainage (n = 8) (Tables 2 and 3). Figure 2 illustrates best-fit total IVH clearance rates, in cohorts receiving placebo or rt-PA through the EVD. There was significantly greater total IVH clearance in rt-PA-treated compared to placebo patients for both contralateral (P < 0.001; CI −14.01, −4.14) and ipsilateral (p = 0.002; CI −24.7, −5.44) placements, respectively. There was a trend of more rapid total IVH clearance in ipsilateral versus contralateral placement in the rt-PA cohort, which did not reach statistical significance (P = 0.09; CI −9.62, 0.69). This trend was not observed in the in placebo patients. Figure 3 illustrates best-fit clearance rates of 3rd and 4th ventricular IVH, with no significant difference with ipsilateral and contralateral EVD locations.
Table 2.
Total IVH Clearance Rates in Ipsilateral and Contralateral EVD Placements Comparing Thrombolytic to Placebo
| Catheter Laterality and Treatment Cohorts | Total IVH Clearance | Significant P Value | Confidence Interval |
|---|---|---|---|
| Thrombolytic Ipsilateral (n = 12) | -22.32 | < 0.001 | (-27.12, -17.52) |
| Thrombolytic Contralateral (n = 52) | -17.89 | < 0.001 | (-19.74, -15.98) |
| Placebo Ipsilateral (n = 3) | -7.24 | 0.089 | (-15.6, 1.12) |
| Placebo Contralateral (n = 14) | -8.78 | < 0.001 | (-13.35, -4.22) |
| Total IVH Clearance in Thrombolytic versus Placebo | |||
|---|---|---|---|
| Ipsilateral Thrombolytic versus Placebo (n = 15) | -15.08 | 0.002 | (-24.72, -5.44) |
| Contralateral Thrombolytic versus Placebo (n = 66) | -9.07 | < 0.001 | (-14.01, -4.14) |
| Total IVH Clearance in Ipsilateral versus Contralateral EVD Location | |||
|---|---|---|---|
| Ipsilateral versus Contralateral Thrombolytic (n = 64) | -4.47 | 0.09 | (-9.62, 0.69) |
| Ipsilateral versus Contralateral Placebo (n = 17) | 1.54 | 0.751 | (-11.06, 7.99) |
Table 3.
3rd and 4th ventricle clearance rates comparing ipsilateral and contralateral EVD placements among cases receiving thrombolytic
| Catheter Laterality Cohorts | 3rd and 4th Ventricular Clearance | Significant P Value | Confidence Interval |
|---|---|---|---|
| Clearance in 3rd Ventricle Among Ipsilateral and Contralateral EVD Placements | |||
| Ipsilateral 3rd Ventricle (n = 8) | −27.22 | < 0.001 | (−34, −20.44) |
| Contralateral 3rd Ventricle (n = 28) | −27.36 | < 0.001 | (−33.72, −20.99) |
| Clearance in 4th Ventricle Among Ipsilateral and Contralateral EVD Placements | |||
| Ipsilateral 4th Ventricle (n = 7) | −31.08 | < 0.001 | (−35.4, −26.77) |
| Contralateral 4th Ventricle (n = 28) | −31.35 | < 0.001 | (−33.9, −28.79) |
| 3rd and 4th Ventricle Clearance Among Ipsilateral versus Contralateral EVD Placements | |||
| 3rd Ventricle Contralateral versus Ipsilateral (n = 36) | 0.14 | 0.98 | (−9.16, 9.44) |
| 4th Ventricle Contralateral versus Ipsilateral (n = 35) | 0.27 | 0.92 | (−4.75, 5.28) |
Figure 2.
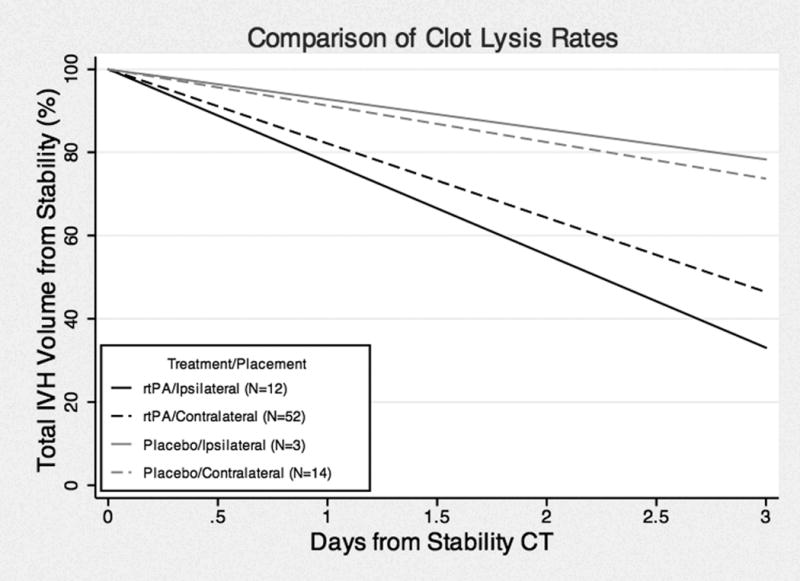
Best fit curves of total IVH clearance rates among ipsilateral and contralateralcatheter placement cohorts, with placebo or thrombolytic, from 100% volume at stability.
Figure 3.
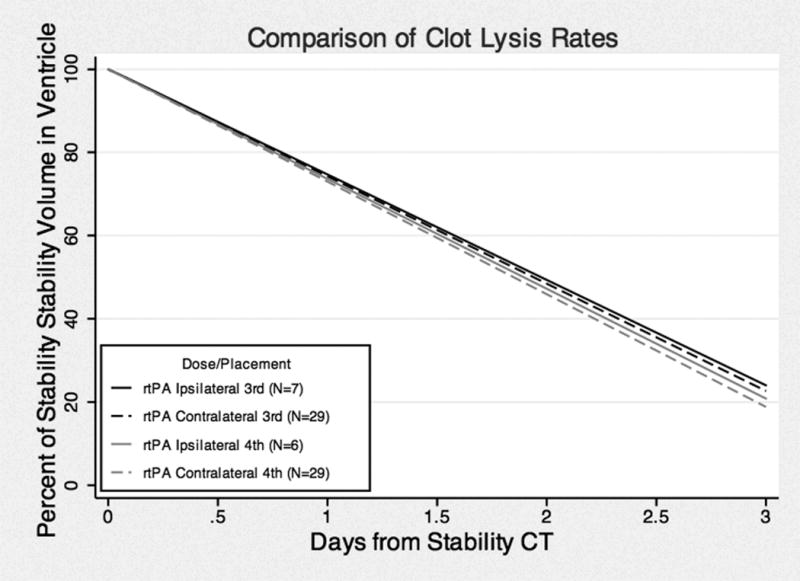
Best fit curves of 3rd and 4th ventricular clearance rates among ipsilateral and contralateral catheter placement cohorts with thrombolytic from 100% volume at stability.
Discussion
Cases enrolled in theCLEAR IVH trials achieved more rapid clearance of IVH and improved outcome in rt-PA treated cases13. Dose optimization, safety profile, and estimates of outcome have motivated a recently launched Phase III trial for more definitive assessment of the potential benefits of thrombolysis16. However, there is no consensus in these trials or in current neurosurgical practice about optimal EVD placement laterality to enhance IVH clearance.
Our analysis suggests that cases with EVD placement ipsilateral to dominant IVH may exhibit a trend toward more rapidtotal clearance of IVHover the first 3 days when receiving thrombolysis, likely related to the access of rt-PA to the dominant ventricular clot. While this trend did not reach statistical significance, it was noted despite a significantly lower rt-PA dose administered in the ipsilateral cases. Such trend toward different clearance ratesbetween ipsilateral and contralateral groups was not noted in the placebo cases, possibly because of obstruction by recalcitrant clot when EVD is placed in the dominant lateral ventricular clot. Third and fourth ventricular blood cleared at equal rates with EVD locations ipsilateral and contralateral to dominant IVH distribution.
Limitations of these data relate to the non-random assignment of catheter placement, with neurosurgeons more typically choosing the less involved ventricle a priori, but without definite consensus on this choice. This was not an intention-to-treat analysis, as there was never an articulated intention about laterality. Comparisons were limited to the cohorts with EVD locationsthatwere clearly ipsilateral or contralateral to the dominant IVH during the 3-day index period of clearance. And two cohorts were comparable in terms of ICH volume, IVH volume, age, sex and initial GCS. The rate of catheter obstructions leading to switching laterality from ipsilateral to contralateral and vice versa was not significantly different. Cases where catheters crossed into both lateral ventricles and cases where laterality of catheter placement was changed during the period of drainage were excluded from analysis. We also excluded cases that required bilateral catheters at any time during the 3-day index period of drainage. Such cases requiring bilateral catheters were more likely associated with larger casted IVH, hence introducing greater bias if they were included in this analysis. The numbers of cases in each variable stratum were too small to allow comprehensive multivariate modeling of clearance rates in relation to catheter placement.
The cases included varying doses of rt-PA as part of dose optimization in Phase II study, and all thrombolysis cases were analyzed as a group, in comparison to placebo cases. There were too few cases in each dose stratum to allow a comparison of impact of catheter laterality at lower versus higher rt-PA doses. The lack of multivariate analysis is acknowledged as an important limitation of the study. But in fact the total dose of rt-PA used during the index period of drainage was greater in contralateral than ipsilateral EVD groups. This difference may reflect an imbalance in randomization in different dose strata, or possibly a dependant outcome, due to stopping rt-PA dose earlier in the ipsilateral group because of more rapid IVH clearance. Regardless, this would not be expected to introduce a bias in favor of greater clearance of IVH in the ipsilateral group, and would not account for the observed trend toward more rapid clearance in theipsilateral group.
Various practice patterns including drainage thresholds were allowed that may have affected IVH clearance, but we have no reason to suspect that these were applied differently of ipsilateral versus contralateral EVD cases. In fact, we present data on CSF drainage, indicating no significant difference between the cohorts during the index period of drainage. This is the best available information to date regarding impact of EVD lateralityon IVH clearance, with randomization of thrombolysis versus placebo, and adjudicated assessment of IVH clearance. Finally, it is possible that any differences of IVH clearance rates could equalize after the acute period, so this information is noted to apply to acute IVH clearance, as defined, in the first three days. The possible impact of EVD localization on ICP control and outcome are beyond the scope of this report. These and analysis of the cohort of CLEAR cases with dual catheters are planned in another communication. And we certainly cannot correlate clearance rates with clinical benefit, although this hypothesis is being addressed in the ongoing CLEAR III trial.
The currently favored practice of placing initial EVD contralateral to the dominant lateral ventricular IVH would still seem to be justified. Other analysis of intracranial pressure control also appears to favor this location (Ziai W, et al. manuscript in preparation). We also provide evidence of equal effectiveness of ipsilateral and contralateral catheter locations in clearing third and fourth ventricular blood, often the main cause of obstructive hydrocephalus. Hence, the placement of initial EVD contralateral to dominant lateral ventricle IVH remains the recommended strategy in the ongoing CLEAR Phase III trial protocol, since the primary aim of EVD placement is control of intracranial pressure and relief of hydrocephalus, andhalf the patients in that trial will be randomized to receive placebo, and the investigator would not know if rt-PA is being administered. But in clinical practice where thrombolysis may be planned in exceptional or compassionate use outside the scope of a trial, or in ineligible cases for the trial, it is possible that an EVD location ipsilateral to dominant IVH may enhance total IVH clearance, with greater access of thrombolytic agent to the recalcitrant clot cast. This hypothesis is illustrated in a case example from the trial, showing prompt clearance with thrombolysis through ipsilateral EVD in the casted ventricle (Figure 4). Similarly, it is possible that a second catheter might be justified in the casted ventricle to help enhance the benefits of thrombolysis, if the initial EVD was placed in contralateral location. This is illustrated in a second case example, where thrombolytic agent in contralateral EVD resulted in slow clearance of major casted blood in the opposite ventricle during the index period, and a subsequent second catheter was placed in the dominant clot, resulting in prompt clearance (Figure 5). This was also suggested in a recent report of greater IVH clearance with dual catheters, compared to volume matched IVH cases treated with a single catheter17.
Figure 4.
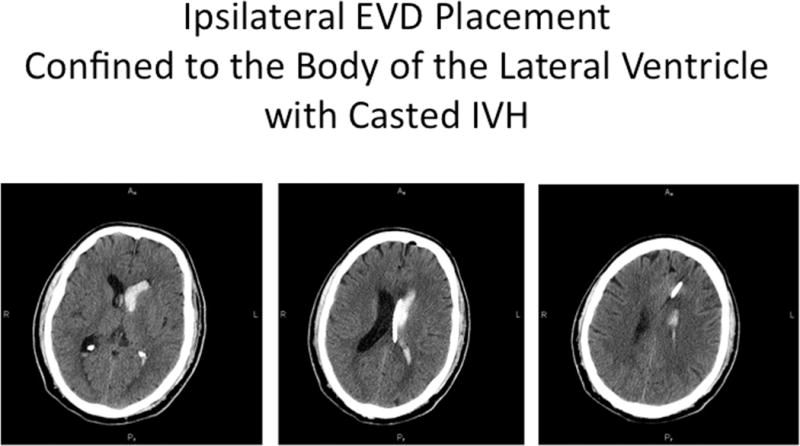
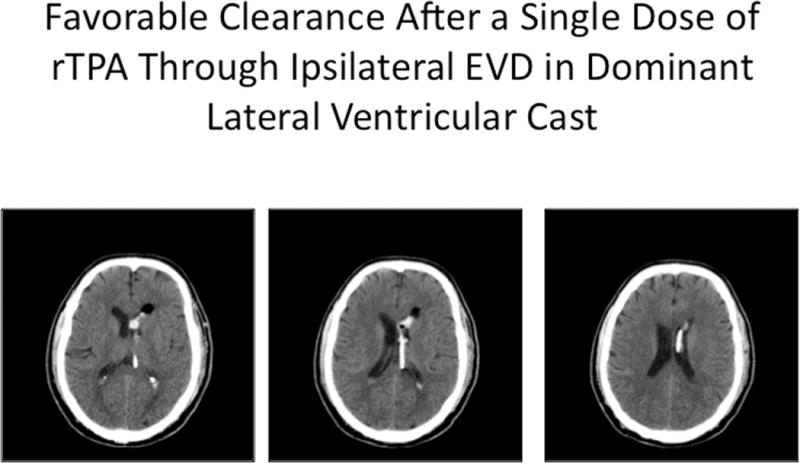
Illustrative case, where EVD was placed ipsilateral to dominant lateral ventricular IVH, with catheter deflected by the septum pellucidum and achieving maximal longitudinal contact with the intraventricular blood cast (A). Excellent clearance of IVH was accomplished after a single dose of rt-PA (B).
Figure 5.
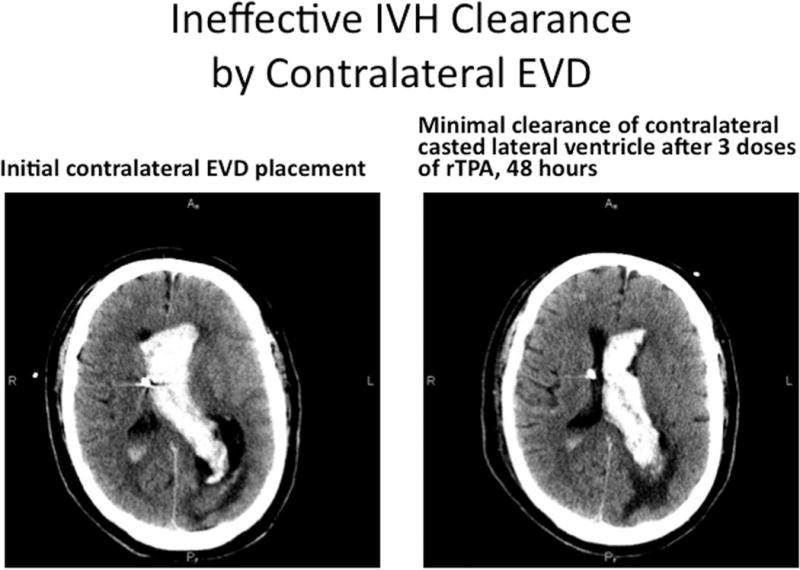
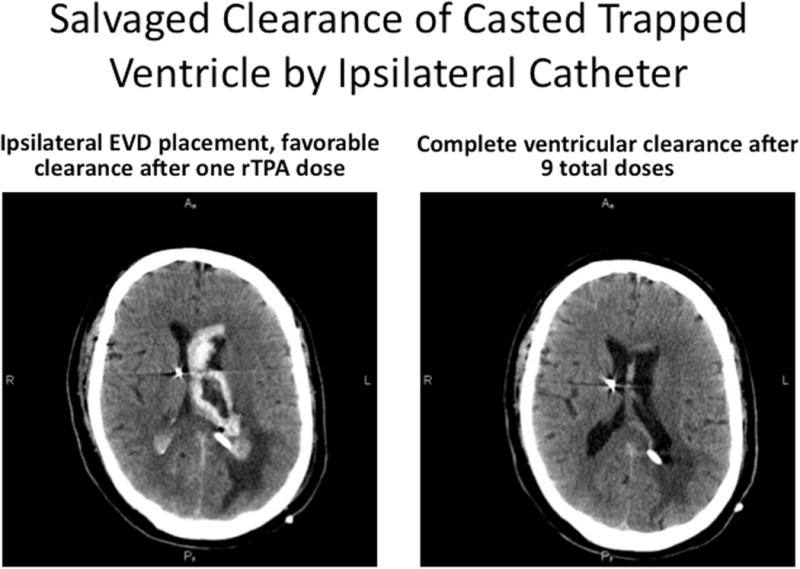
Illustrative case, where initial EVD was placed contralateral to dominant IVH, and poor clearance of the blood was noted in the trapped lateral ventricle, despite multiple doses of thrombolytic over 48 hours (A). With patient in poor neurologic condition, presumed from ventricular trapping by casted blood, a second EVD was placed using a posterior approach, into the lateral ventricular blood cast, and this accomplished prompt clearance of the residual IVH, relief of the trapping and shift, and improved neurologic condition (B).
It may be possible to optimize the placement of EVD to enhance the clearance of total IVH if lytic agents are used. Catheters on either side can clear the 3d and 4th ventricles with equal efficiency. Further research, with a larger sample size, is required to make definitive conclusions concerning ipsilateral vs. contralateral EVD placement.
Acknowledgments
This work was supported in part by the Eleanor Naylor Dana Fellowship (DFH), NIH/NINDS U01 NS062851-01-A1 (DFH, IAA), Grant FD-R-001693-01-1 from the Food and Drug Administration Orphan Products Development Agency (DFH), NIN/NINDS 2R01 NS046309-05 and the France Merrick Foundation Grant (DFH). WZ has received an honorarium for an unrelated publication on intraventricular hemorrhage. The drug used in the CLEAR trial was donated by Genentech.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Steiner T, Diringer MN, Schneider D, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: Risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor vii. Neurosurgery. 2006;59:767–773. doi: 10.1227/01.NEU.0000232837.34992.32. discussion 773–764. [DOI] [PubMed] [Google Scholar]
- 2.Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: Results from the stich trial. Acta Neurochir Suppl. 2006;96:65–68. doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- 3.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the american heart association/american stroke association stroke council, high blood pressure research council, and the quality of care and outcomes in research interdisciplinary working group. Stroke. 2007;38:2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 4.Holloway RG, Benesch CG, Burgin WS, Zentner JB. Prognosis and decision making in severe stroke. JAMA. 2005;294:725–733. doi: 10.1001/jama.294.6.725. [DOI] [PubMed] [Google Scholar]
- 5.Ozdemir O, Calisaneller T, Hasturk A, Aydemir F, Caner H, Altinors N. Prognostic significance of third ventricle dilation in spontaneous intracerebral hemorrhage: A preliminary clinical study. Neurol Res. 2008;30:406–410. doi: 10.1179/174313208X276240. [DOI] [PubMed] [Google Scholar]
- 6.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–621. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 7.Young WB, Lee KP, Pessin MS, Kwan ES, Rand WM, Caplan LR. Prognostic significance of ventricular blood in supratentorial hemorrhage: A volumetric study. Neurology. 1990;40:616–619. doi: 10.1212/wnl.40.4.616. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe J, AlKhawam L, Du H, et al. Outcome predictors and spectrum of treatment eligibility with prospective protocolized management of intracerebral hemorrhage. Neurosurgery. 2009;64:436–445. doi: 10.1227/01.NEU.0000330402.20883.1B. discussion 445–436. [DOI] [PubMed] [Google Scholar]
- 9.Hanley DF. Intraventricular hemorrhage: Severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40:1533–1538. doi: 10.1161/STROKEAHA.108.535419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roos YB, Hasan D, Vermeulen M. Outcome in patients with large intraventricular haemorrhages: A volumetric study. J Neurol Neurosurg Psychiatry. 1995;58:622–624. doi: 10.1136/jnnp.58.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: A systematic review of the literature. J Neurol. 2000;247:117–121. doi: 10.1007/pl00007792. [DOI] [PubMed] [Google Scholar]
- 12.Naff NJ, Hanley DF, Keyl PM, et al. Intraventricular thrombolysis speeds blood clot resolution: Results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery. 2004;54:577–583. doi: 10.1227/01.neu.0000108422.10842.60. discussion 583–574. [DOI] [PubMed] [Google Scholar]
- 13.Naff N, Williams M, Keyl PM, et al. Low-dose rt-PA enhances clot resolution in brain hemorrhage: The intraventricular hemorrhage thrombolysis trial. Stroke. 2011 doi: 10.1161/STROKEAHA.110.610949. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman RD, Maldjian JA, Brun NC, Horvath B, Skolnick BE. Radiologic estimation of hematoma volume in intracerebral hemorrhage trial by CT scan. Am J of Neurorad. 2006;27:666–670. [PMC free article] [PubMed] [Google Scholar]
- 15.Zeger SL, Liang KY, Albert PS. Models for Longitudinal Data: A Generalized Estimating Equation Approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 16.Johns Hopkins University. Division of Brain Injury Outcomes. Clear iii intraventricular thrombolysis trial webpage. http://www.cleariii.com. Accessed November 6, 2009.
- 17.Hinson HE, Melnychuk E, Muschelli J, Hanley DF, Awad IA, Ziai WC. Drainage Efficiency with Dual Versus Single Catheters in Severe Intraventricular Hemorrhage. Neurocrit Care. 2011 Jun 17; doi: 10.1007/s12028-011-9569-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


