Abstract
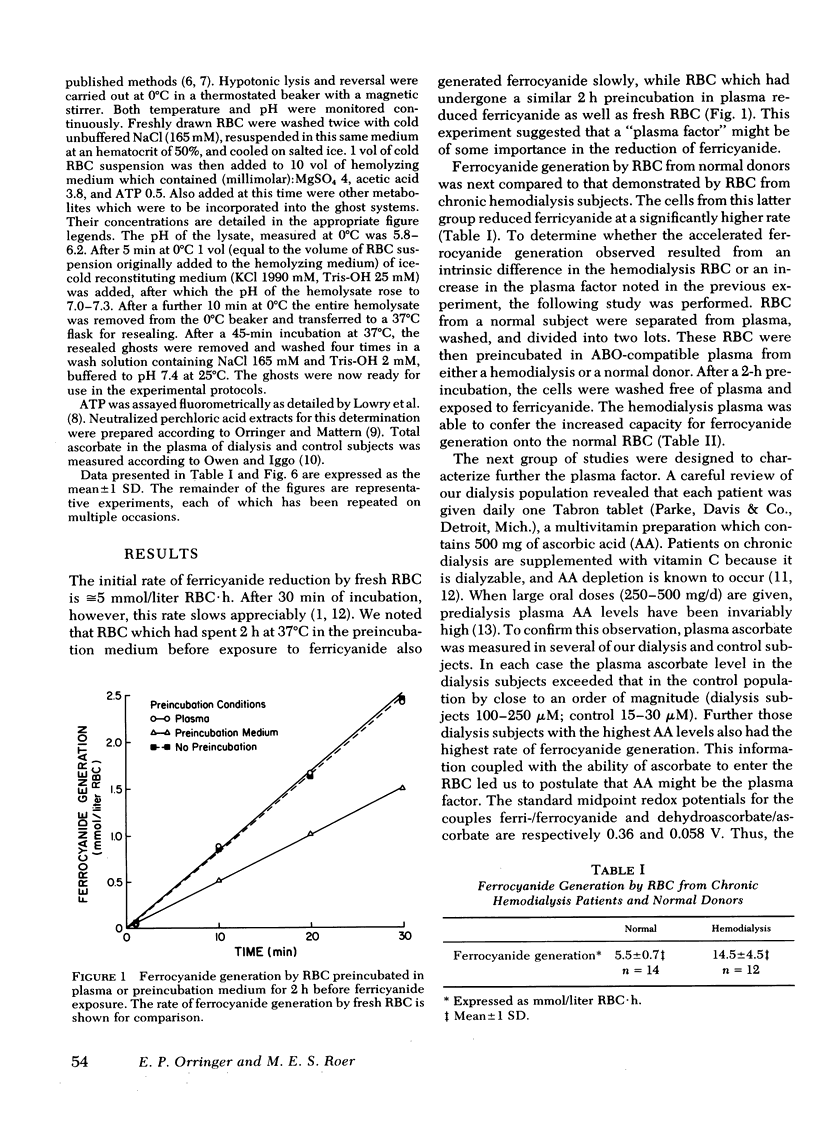
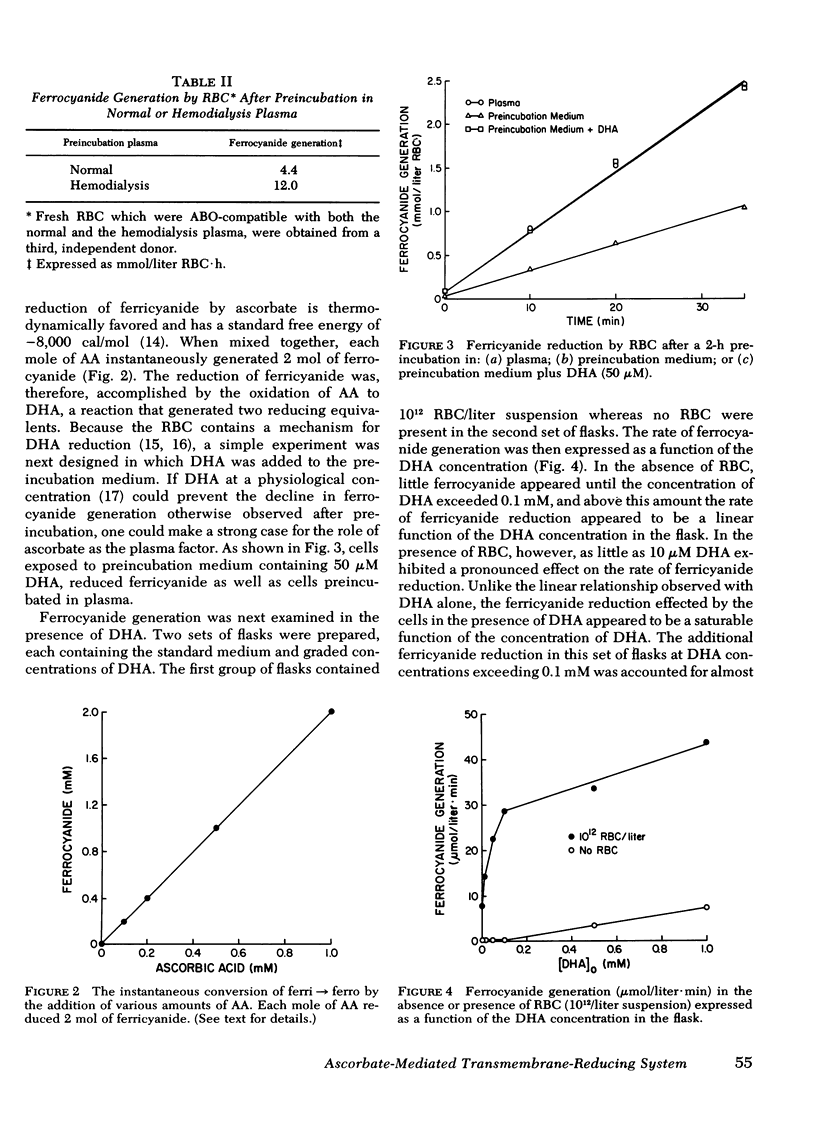
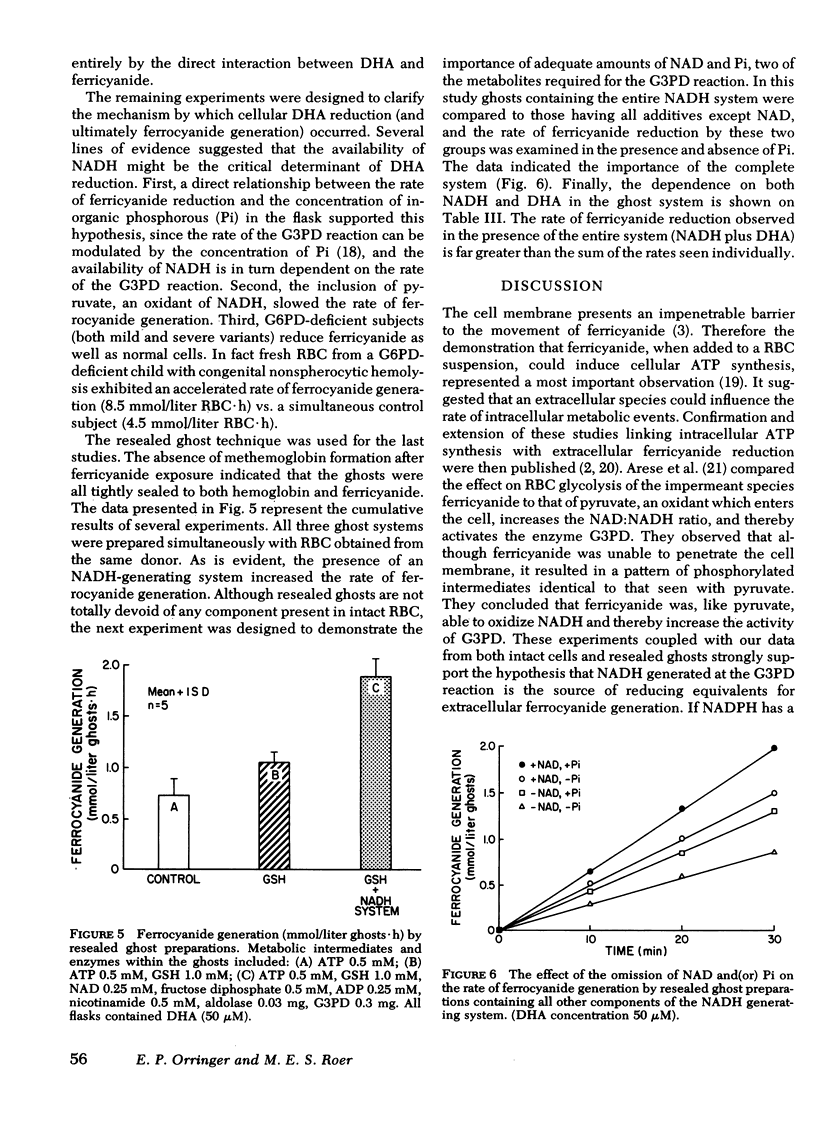
Actively metabolizing human erythrocytes catalyze the extracellular reduction of ferricyanide to ferrocyanide. Because neither of these anions can enter the cell, reducing equivalents generated in the course of glycolysis must in some manner be transferred across the cell membrane, thereby resulting in ferricyanide reduction. Work described in this paper suggests that the transmembrane reduction is effected by ascorbic acid. This compound in its oxidized form (dehydroascorbate) rapidly enters the cell. Here it obtains reducing equivalents which appear to come from NADH made available at the level of glyceraldehyde 3-phosphate dehydrogenase. Once reduced, it leaves the cell as ascorbic acid and accomplishes the non-enzymatic reduction of ferricyanide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., SHAVIT N. A SENSITIVE AND SIMPLE METHOD FOR DETERMINATION OF FERROCYANIDE. Anal Biochem. 1963 Dec;6:549–554. doi: 10.1016/0003-2697(63)90149-0. [DOI] [PubMed] [Google Scholar]
- Bodemann H., Passow H. Factors controlling the resealing of the membrane of human erythrocyte ghosts after hypotonic hemolysis. J Membr Biol. 1972;8(1):1–26. doi: 10.1007/BF01868092. [DOI] [PubMed] [Google Scholar]
- Degkwitz E., Walsch S., Dubberstein M., Winter J. Ascorbic acid and cytochromes. Ann N Y Acad Sci. 1975 Sep 30;258:201–208. doi: 10.1111/j.1749-6632.1975.tb29280.x. [DOI] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- Glover J. M., Jones P. R., Greenman D. A., Hughes R. E., Jacobs A. Iron absorption and distribution in normal and scorbutic guinea pigs. Br J Exp Pathol. 1972 Jun;53(3):295–300. [PMC free article] [PubMed] [Google Scholar]
- HUGHES R. E. REDUCTION OF DEHYDROASORBIC ACID BY ANIMAL TISSUES. Nature. 1964 Sep 5;203:1068–1069. doi: 10.1038/2031068a0. [DOI] [PubMed] [Google Scholar]
- Hughes R. E., Maton S. C. The passage of vitamin C across the erythrocyte membrane. Br J Haematol. 1968 Mar;14(3):247–253. doi: 10.1111/j.1365-2141.1968.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Khan M. M., Martell A. E. Metal ion and metal chelate catalyzed oxidation of ascorbic acid by molecular oxygen. I. Cupric and ferric ion catalyzed oxidation. J Am Chem Soc. 1967 Aug 2;89(16):4176–4185. doi: 10.1021/ja00992a036. [DOI] [PubMed] [Google Scholar]
- Kopple J. D., Swendseid M. E. Vitamin nutrition in patients undergoing maintenance hemodialysis. Kidney Int Suppl. 1975 Jan;(2):79–84. [PubMed] [Google Scholar]
- LOWY B. A., WILLIAMS M. K., LONDON I. M. Enzymatic deficiencies of purine nucleotide synthesis in the human erythrocyte. J Biol Chem. 1962 May;237:1622–1625. [PubMed] [Google Scholar]
- Lipschitz D. A., Bothwell T. H., Seftel H. C., Wapnick A. A., Charlton R. W. The role of ascorbic acid in the metabolism of storage iron. Br J Haematol. 1971 Feb;20(2):155–163. doi: 10.1111/j.1365-2141.1971.tb07024.x. [DOI] [PubMed] [Google Scholar]
- Lynch S. R., Seftel H. C., Torrance J. D., Charlton R. W., Bothwell T. H. Accelerated oxidative catabolism of ascorbic acid in siderotic Bantu. Am J Clin Nutr. 1967 Jun;20(6):641–647. doi: 10.1093/ajcn/20.6.641. [DOI] [PubMed] [Google Scholar]
- MANYAI S., SZEKELY M. Die Wirkung von Natriumfluorid und Monojodessigsäure auf die Glykolyse von menschlichen roten Blutkörperchen. Acta Physiol Acad Sci Hung. 1954;5(1-2):7–18. [PubMed] [Google Scholar]
- Mann G. V., Newton P. The membrane transport of ascorbic acid. Ann N Y Acad Sci. 1975 Sep 30;258:243–252. doi: 10.1111/j.1749-6632.1975.tb29285.x. [DOI] [PubMed] [Google Scholar]
- Morgan E. H., Baker E. The effect of metabolic inhibitors on transferrin and iron uptake and transferrin release from reticulocytes. Biochim Biophys Acta. 1969 Jul 30;184(2):442–454. doi: 10.1016/0304-4165(69)90048-8. [DOI] [PubMed] [Google Scholar]
- OWEN J. A., IGGO B. The use of p-chloromercuribenzoic acid in the determination of ascorbic acid with 2:6-dichlorophenolindophenol. Biochem J. 1956 Apr;62(4):675–680. doi: 10.1042/bj0620675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orringer E. P., Mattern W. D. Formaldehyde-induced hemolysis during chronic hemodialysis. N Engl J Med. 1976 Jun 24;294(26):1416–1420. doi: 10.1056/NEJM197606242942602. [DOI] [PubMed] [Google Scholar]
- SZEKELY M., MANYAI S., STRAUB F. B. Uber den mechanismus der osmotischen hämolyse. Acta Physiol Acad Sci Hung. 1952;3(3-4):571–584. [PubMed] [Google Scholar]
- Schwoch G., Passow H. Preparation and properties of human erythrocyte ghosts. Mol Cell Biochem. 1973 Dec 15;2(2):197–218. doi: 10.1007/BF01795474. [DOI] [PubMed] [Google Scholar]
- Stankova L., Rigas D. A., Bigley R. H. Dehydroascorbate uptake and reduction by human blood neutrophils, erythrocytes, and lymphocytes. Ann N Y Acad Sci. 1975 Sep 30;258:238–242. doi: 10.1111/j.1749-6632.1975.tb29284.x. [DOI] [PubMed] [Google Scholar]
- Sullivan J. F., Eisenstein A. B. Ascorbic acid depletion during hemodialysis. JAMA. 1972 Jun 26;220(13):1697–1699. [PubMed] [Google Scholar]
- Sullivan J. F., Eisenstein A. B. Ascorbic acid depletion in patients undergoing chronic hemodialysis. Am J Clin Nutr. 1970 Oct;23(10):1339–1346. doi: 10.1093/ajcn/23.10.1339. [DOI] [PubMed] [Google Scholar]
- TSUBOI K. K., FUKUNAGA K. INORGANIC PHOSPHATE AND ENHANCED GLUCOSE DEGRADATION BY THE INTACT ERYTHROCYTE. J Biol Chem. 1965 Jul;240:2806–2810. [PubMed] [Google Scholar]
- Tolbert B. M., Downing M., Carlson R. W., Knight M. K., Baker E. M. Chemistry and metabolism of ascorbic acid and ascorbate sulfate. Ann N Y Acad Sci. 1975 Sep 30;258:48–69. doi: 10.1111/j.1749-6632.1975.tb29267.x. [DOI] [PubMed] [Google Scholar]
- Wapnick A. A., Bothwell T. H., Seftel H. The relationship between serum ion levels and ascorbic acid stores in siderotic Bantu. Br J Haematol. 1970 Aug;19(2):271–276. doi: 10.1111/j.1365-2141.1970.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Weiner J. H. The localization of glycerol-3-phosphate dehydrogenase in Escherichia coli. J Membr Biol. 1974;15(1):1–14. doi: 10.1007/BF01870078. [DOI] [PubMed] [Google Scholar]
- Weis W. Ascorbic acid and biological systems. Ascorbic acid and electron transport. Ann N Y Acad Sci. 1975 Sep 30;258:190–200. doi: 10.1111/j.1749-6632.1975.tb29279.x. [DOI] [PubMed] [Google Scholar]
- Workman E. F., Jr, Graham G., Bates G. W. The effect of serum and experimental variables on the transferrin and reticulocyte interaction. Biochim Biophys Acta. 1975 Aug 13;399(2):254–264. doi: 10.1016/0304-4165(75)90256-1. [DOI] [PubMed] [Google Scholar]
- Zamudio I., Canessa M. Nicotinamide-adenine dinucleotide dehydrogenase activity of human erythrocyte membranes. Biochim Biophys Acta. 1966 May 12;120(1):165–169. doi: 10.1016/0926-6585(66)90290-1. [DOI] [PubMed] [Google Scholar]
- Zamudio I., Cellino M., Canessa-Fischer M. The relation between membrane structure and NADH: (acceptor) oxidoreductase activity of erythrocyte ghosts. Arch Biochem Biophys. 1969 Jan;129(1):336–345. doi: 10.1016/0003-9861(69)90184-2. [DOI] [PubMed] [Google Scholar]


