Abstract
Irradiation from diverse sources is ubiquitous and closely associated with human activities. Radiation therapy (RT), an important component of multiple radiation origins, is a common therapeutic modality for cancer. More importantly, RT provides significant contribution to oncotherapy by killing tumor cells. However, during the course of therapy, irradiation of normal tissues can result in a wide range of side effects, including self-limited acute toxicities, mild chronic symptoms, or severe organ dysfunction. Although numerous promising radioprotective agents have emerged, only a few have successfully entered the market because of various limitations. At present, the widely accepted hypothesis for protection against radiation-caused injury involves the Wnt canonical pathway. Activating the Wnt/β-catenin signaling pathway may protect the salivary gland, oral mucosa, and gastrointestinal epithelium from radiation damage. The underlying mechanisms include inhibiting apoptosis and preserving normal tissue functions. However, aberrant Wnt signaling underlies a wide range of pathologies in humans, and its various components contribute to cancer. Moreover, studies have suggested that Wnt/β-catenin signaling may lead to radioresistance of cancer stem cell. These facts markedly complicate any definition of the exact function of the Wnt pathway.
KEY WORDS : Signaling transduction, canonical Wnt pathway, β-catenin, radioprotection, radiation
Introduction
Irradiation from diverse sources is ubiquitous and is closely associated with human activities. Among all sources of radiation (Figure 11), natural radiation, including radon, thoron, cosmic radiation, and natural radioactivity in soil and food, dominates the average dose of individual radiation adsorption. The dose from natural radiation can not be controlled. However, artificial radiation, which consists primarily of medical exposure of patients, accounts for approximately 14% of individual radiation absorption and has been attracting increasing attention in recent years. As one of the treatments for malignant tumors, radiation therapy (RT) is administered to more than half of all cancer patients during the course of their treatment2, according to the 2001 statistics from the Swedish Council on Technology Assessment in Health Care. Local or regional control of malignancies can be achieved either through RT alone or in combination with other modalities, such as chemotherapy and surgery. However, during the course of therapy, the irradiation of normal tissues can result in a wide range of side effects, including self-limited acute toxicities, mild chronic symptoms, and severe organ dysfunction.
Figure 1.
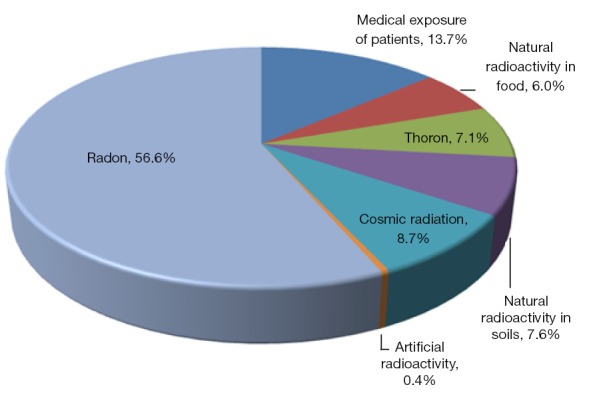
Dose contribution to the individual radiation absorption from all sources of radiation. Artificial radiation consists primarily of medical exposure of patients, accounting for approximately 14% of the individual radiation absorption and has been attracting more and more attention over recent years.
Hence, prevention or treatment of early and late RT effects will improve quality of life and may even increase cancer curability by intensifying the regimens of therapy. Moreover, a large pool of chemical and molecular agents can be effective in protecting and treating radiation damage3. In recent years, technological improvements in radiation delivery and chemical modifiers for radiation injuries have been used to reduce toxicities associated with therapeutic radiation. Although improvements have been achieved in this regard, radiation damage remains a limiting factor in treating numerous diseases with RT. Thus, the search for techniques that can protect normal tissues from radiation has been an area of intense investigation.
Despite the fact that numerous promising radioprotective approaches are emerging, to date, amifostine (WR2721) remains to be the only drug recommended as an effective radioprotective agent against xerostomia resulting from irradiation4,5. However, the use of this drug is limited because of its numerous side effects including nausea, cutaneous reactions, and hypotension, etc. Therefore, the search for radioprotective agents with high potency and low toxicity should be a primary concern for further research.
The Wnt signal transduction pathway (shorten as the Wnt pathway hereafter) is an evolutionarily conserved signaling pathway with an important role in fetal development and central nervous system formation, as well as in cell growth, migration, and differentiation6-8. Recently, an in vivo research9 reported that concurrent transient activation of the Wnt/β-catenin pathway could prevent radiation-induced salivary gland dysfunction. In the study of Thotala et al.10, Wnt signaling was found to play a central role in renewing damaged intestinal epithelium. Furthermore, another research11 demonstrated that upregulation of the Wnt/β-catenin pathway could accelerate repair of radiation oral mucositis.
These pieces of evidence indicate that the Wnt/β-catenin pathway has a significant role in radioprotection. However, the deregulation of the Wnt/β-catenin pathway has been proposed as one of the signaling pathways responsible for carcinogenesis in the hematopoietic systems, the intestines, and the epidermis, as well as for chronic myelogenous leukemia, lung cancer, colorectal cancer and gastrointestinal cancer (GI)12. Studies have also identified overexpression of the Wnt signaling pathway in malignant breast cell lines13. Moreover, Wnt signaling is involved in cancer stem cell radioresistance14. These facts markedly complicate any definition for the exact function of the Wnt pathway. Thus, further investigation regarding the detail of this pathway is highly important to contribute to the development of radioprotection. In this review, our first objective is to describe radioprotective studies regarding the effect of the Wnt/β-catenin pathway to radiation-induced salivary gland dysfunction, oral mucositis, and GI syndrome. Our second objective is to emphasize the potential roles of the Wnt/β-catenin pathway in cancer development. We expect that Wnt signaling in different contexts will facilitate the improvement of RT.
Radiation damage
Tissues are well organized and composed of cells with epithelial and connective tissue origins. Tissue regeneration ability varies with the cell source. First, continuously regenerating cells consist of the epithelial and hematopoietic bone marrow cells. Researchers have shown that the doubling time for regenerating clonogenic cells in the crypts of the small intestine is less than one day, and that the entire intestinal tract epithelial compartment renews completely within a few days15. Second, fibroblasts and the endothelial vascular cells are slow-renewing cells. Studies have reported that the endothelial cell in vivo turnover in conduit blood vessels is approximately one to several years, with only 0.1% of cells actively proliferating in quiescent vessels16. Lastly, common knowledge suggests that neurons never proliferate.
Given such distinct cellular compartmentalization, understanding the events occurring during and shortly after irradiation of tissues and cell is essential. This information is necessary in understanding the mechanisms of radioprotectors and mitigators. Figure 2 shows the sequence of events in cells and tissues following radiation exposure. Tissue exposure to ionizing radiation results in early radiation effects, late radiation effects, and bystander effects. Early radiation effects include cellular depletion17, which involves cellular death and depletion of a tissue followed by a proliferative response of stem cells and reactive gene activation18,19. The latter entails cellular and tissue dysfunction followed by increased vascular permeability, tissue edema, growth factor and cytokine production on behalf of fibroblasts and endothelial cells, and chemoattraction of macrophages and other white cells which leads to radiation inflammation. Chronic tissue dysfunction and disorganization result in late toxicity. Bystander effects20, refer to the radiation damage induced in cells within an organ or the entire body that have not been directly exposed to radiation, have recently become the focus of intensive research to clarify their molecular mechanisms. Various radioprotective policies can disturb destructive processes resulting from cell exposure to radiation, including the occurrence of genetic mutation, cell death, and tissue disorganization.
Figure 2.
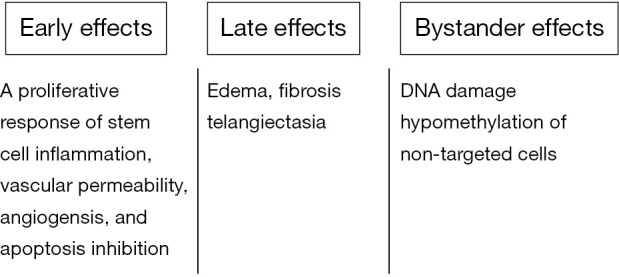
Type of radiation damage.
Death pathway modulators, which regulate early radiation damage, are among such radioprotective policies. This policy involves the activation of the Wnt/β-catenin pathways.
The Wnt signaling pathway
The Wnt signaling pathway was discovered during an embryogenesis experiment on Xenopus laevis. The Wnt1 gene, the first gene of the Wnt protein family, was identified in 1982 as a gene activated by the integration of mouse mammary tumor virus proviral DNA into virally induced breast tumors21. Since then, Wnt signaling has often been reported associated with cancer and cell proliferation.
Wnt ligands are a family of secreted glycoproteins involved in cell-to-cell communication that controls each major develop mental process, including cell-fate determination as well as cell proliferation, polarity, adhesion, motility, and apoptosis, and subsequently, patterning and morphogenesis22. Historically, Wnt ligands activate two major intracellular pathways: the canonical (β-catenin-dependent)23 and non-canonical (β-catenin-independent) pathways24. Β-catenin is the main effector of canonical signaling. In this paper, we will thoroughly review the research progress in the radioprotection of the Wnt canonical pathway.
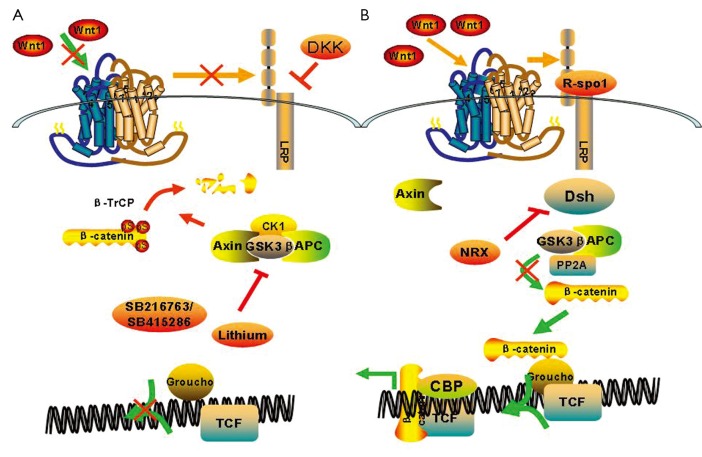
Cytoplasmic β-catenin is degraded by the “destruction complex” in the absence of a Wnt ligand binding to its receptor complex. In the destruction complex, Axin acts as a scaffold protein, in which adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK-3β), and casein kinase 1α (CK1α) bind to facilitate the sequential phophorylation of β-catenin in 45 serine by kinase CK1α and 41’threomine, and in 37’,33’serine by GSK-3β25,26. Accordingly, phosphorylated β-catenin is recognized by β-transducin-repeat-containing protein (β-TrCP) and is constantly degraded by the ubiquitin-proteasome pathway. Wnt signaling is activated via Wnts ligation of the seven transmembrane frizzled (Fz) proteins and the low-density lipoprotein receptors. Cytoplasmic protein disheveled (Dvl) is then recruited, phosphorylated, and activated. Activation of Dvl induces the dissociation of GSK-3β from Axin and leads to GSK-3β inhibition. Phosphorylation and degradation of β-catenin is inhibited as a result of the inactivation of the destruction complex. Subsequently, the stabilized β-catenin translocates into the nucleus. Nuclear β-catenin is the ultimate effector, binding to transcription factor T cell factor and lymphoid-enhancing factor(Tcf/Lef), which leads to changes in different target gene expressions27 (Figure 3).
Figure 3.
Wnt canonical pathway. (A) In the absence of a Wnt ligand, the cytoplasmic β-catenin is degraded by the “destruction complex”, which contains adeomatous polyposis coli (APC) and axin, glycogen synthase 3β (GSK-3β) and casein kinase 1α (CK1α) phosphorylated β-catenin. Accordingly, phosphorylated β-catenin is recognized by β-TrCP and constantly degraded by the ubiquitin-proteasome pathway; (B) The binding of WNTs, such as WNT3A and WNT1, to frizzled (FZD) and LRP5 or LRP6 co-receptors transduces a signal across the plasma membrane that results in the activation of the Dishevelled (DVL) protein. Activation of Dvl induces the dissociation of GSK-3β from Axin and leads to the inhibition of GSK-3β. This results in the accumulation of β-catenin in the cytoplasm and ultimately the nucleus where it displaces cyclic AMP response element-binding protein (CBP) β-catenin and facilitates transcriptional actiation of lymphocyte enhancer binding actor (LEF)/T cell factor (TCF) resulting in altered gene transcription. Ultimately, Wnt canonical pathway modulates changes in cell behaviours such as proliferation, survival and differentiation. DKK: dickkopf homologue; NRX: nucleoredoxin; R-spon1: R-spondin1.
With the increasing interest of researchers in the Wnt/β-catenin pathway, Wnt signaling is revealed to be modulated by numerous evolutionarily conserved inhibitors and activators. The Wnt/β-catenin pathway can be specifically blocked by endogenously secreted inhibitors from the Dickkopf (DKK) family, which interact with LRP5/6 and high-affinity receptors from the Kremen family, thus causing rapid endocytosis of Kremen-Dkk-LRP complex and removal of LRP from the cell membranes28,29. The Wnt activator roof plate-specific spondin (R-spondin)30,31 promotes Wnt signaling by binding to Wnt receptors or reloading a Wnt-inhibitory step. Previously, lithium was found to be an activator of the Wnt signaling pathway by inhibiting GSK-3β from species as diverse as Dictyostelium discoideum and Xenopus laevis32. Moreover, research showed that Wnt/β-catenin signaling is of major importance in controlling lithium-dependent thyrocyte proliferation33 and lithium-enhanced bone formation and improves bone mass in mice by activating Wnt/β-catenin signaling. In this study, we focus on the protective effects of the Wnt/β-catenin pathway on radiation-induced salivary gland dysfunction, oral mucositis, and GI syndrome.
Radioprotection for radiation-induced salivary gland dysfunction
Head and neck cancer (HNC) is the fifth most common cancer, with an estimated annual incidence exceeding 500,000 worldwide34. Given that radiation therapy is the most common form of treatment for HNC, non-cancerous salivary gland cells (SGs) are often exposed to radiation. Because of the enhanced radiosensitivity of SGs, irreversible hyposalivation is common (60% to 90%) among HNC survivors treated with radiotherapy35. Hyposalivation leads to dental caries and periodontal disease. Moreover, it causes mastication and swallowing problems, a burning sensation in the mouth, and dysgeusia, etc, thus significantly impairing the quality of life of patients36. At present, a dry mouth can be temporarily relieved through artificial saliva and saliva secretion stimulators37. Irreversible hyposalivation was previously hypothesized to be caused by the sterilization of the primitive glandular stem/progenitor cells that normally continuously replenish aged saliva producing cells38. By contrast, recent findings suggest that human salivary gland stem/progenitor cells remain dormant even after irradiation39, thus suggesting that the loss of the regenerating capacity after irradiation may be reversible.
To our knowledge, Hakim et al.40 conducted the first in vivo study that investigated the increase of the Wnt-1 expression in irradiated but viable acinar structures along with the membranous upregulation of β-catenin. In accordance with previous in vitro studies, Wnt pathway activation was demonstrated to provide a key radioprotective mechanism in irradiated cancer cells40-42.
To further understand the involvement of Wnt signaling in normal SG development and regeneration after injury. Hai et al.43 used Wnt reporter transgenic mice and discovered that Wnt signaling is active in the ductal regions of postnatal submandibular glands (SMGs) and is upregulated with Hedgehog (Hh) signaling during SMG regeneration induced by the ligation of the main secretor ducts. Inhibiting of epithelial Wnt signaling increases SMG stem/progenitor cell population and activates Hh signaling. The results of this previous study indicated that Wnt/beta-catenin signaling regulates the activity of salivary gland stem/progenitor cells upstream of the Hh pathway, and suggested that manipulating Wnt or Hh signaling is a potential strategy for restoring salivary function in patients with hyposalivation.
To further explore the potential of activating Wnt/β-catenin signaling in preventing radiation damage to salivary glands, Hai et al.9 used Wnt reporter transgenic mice exposed to 15 Gy single-dose radiation in the head and neck area and obtained the following results: (1) radiation damage did not significantly affect the activity of the Wnt/β-catenin pathway compared with physical damage; (2) transient expression of the Wnt1 pathway prevented chronic salivary gland dysfunction following irradiation by suppressing apoptosis and preserving functional salivary stem/progenitor cells; (3) excessive Wnt activation before irradiation failed to inhibit apoptosis probably because of the extensive induction of mitosis and the upregulation of the proapoptosis gene PUMA, whereas activation after irradiation probably missed the critical treatment window.
Radioprotection for radiation-induced oral mucositis
Oral mucositis, which is characterized by mucosal damage and inflammation in the oral cavity, is a common complication of chemotherapy and radiotherapy among cancer patients with head and neck tumors and hematological malignancies44. The impairment of the regenerative capability of the oral mucosal epithelium leads to atrophy and ulceration, and further limits the ability of patients to tolerate optimal anti-tumor treatment regimens43. As such finding an effective prevention method for radiotherapy-induced oral mucositis is urgently needed to enhance treatment effect and improve the quality of life of patients with head and neck tumors. The keratinocyte growth factor (KGF) which is one of the mucosal epithelial growth factors has been shown to be efficient in treating experimental animal models with chemotherapy or radiotherapy-induced oral mucositis. This growth factor is also the first approved biologic therapy for human cancer patients with oral mucositis. However, KGF has been reported to have numerous side effects45,46.
The R-spondin family of proteins comprises novel secreted proteins acting as major agonists and modulators of the Wnt-β-catenin signaling pathway47,48. Four human paralogs49, each containing a lead signal peptide; two cystein-rich, furin-like domains; and one thrombospondin type 1 domain human R-spondin-1 (Rspo1), a 29 kd, 263 amino acid protein, have been shown to augment canonical Wnt/β-catenin signaling in both in vitro and in vivo studies50. Recently, R-spondin has been found to exhibit synergistic cooperation with Wnt ligands in activating canonical Wnt signaling. The Wnt/β-catenin signaling has a pivotal role in both embryonic development and homeostatic self-renewal of adult tissues, including GI and oral mucosa. Zhao et al.11, using transgenic Wnt reporter TOPGAL mice, identified the oral mucosa as a target tissue for Rspo1 by injecting recombinant Rspo1. Their results demonstrated that Rspo1 significantly reduces both chemotherapy- and radiotherapy-induced damage to the oral mucosa by amplifying Wnt/β-catenin signaling and subsequently triggering epithelial cell growth to accelerate mucosal healing in mice. Moreover, Rspo1 decreases the extent of overt tongue ulceration by repairing disintegrated mucosa in mice exposed to high doses of radiation delivered to the head region. Rspo1 is effective in both injury and healing phases of oral mucositis in mice by increasing basal layer epithelial cell density in the tongue. More importantly, using Rspo1 appears to be effective during various stages of experimental oral mucositis in mice.
The LRP5/6 receptor also appears to play a role in activating the Wnt/β-catenin pathway. Wei et al.51 investigated the combination of Rspo1 with LRP6 and Fzd8. Their results showed that Rspo1 combined firmly with LRP6, but hardly combined with Fzd8. Therefore, LRP6 was considered to be an affinity receptor of Rspo1 protein.
In summary, Rspo1 demonstrates a protective effect against radioactive oral mucosa inflammation through the activation of the Wnt/β-catenin pathway. As a receptor protein for the Wnt signaling pathways, the internalization of LRP6 is an essential step in β-catenin activation52. However, factors affecting Rspo1 and LRP6 combination as well as the improvement of their binding ratio still need to be elucidated.
Radioprotection for radiation-induced GI syndrome (RIGS)
Normal homeostasis of the intestinal epithelium is a dynamic balance maintained by an intricate cell replacement process in which terminally differentiated epithelial cells are continuously and rapidly replaced by replication and differentiation of epithelial cells (transit cells) located within the intestinal crypt. RIGS results from a combination of direct cytocidal effect on the intestinal crypt and on endothelial cells, and the subsequent loss of the mucosal barrier. This situation results in electrolyte imbalance, diarrhea, weight loss, infection, and mortality. DNA damage and, presumably, endoplasmic reticulum and mitochondrion damage induced by radiation-free radicals immediately trigger the pro-apoptotic cell response53. GI symptoms, which affect quality of life, can induce change in one or more specific physiological functions in extensively separated parts of the GI tract. Furthermore, these symptoms, which are experienced after pelvic radiotherapy, are substantially widespread and frequently poorly managed54. The intestine exhibits strong sensitivity to radiation, which is, in part, caused by continuously renewing cells within the crypt of Lieberkuhn54. Radiation-induced apoptosis occurs predominantly within the stem cell region. To date, few studies have elaborated on the molecular determinants of intestinal radiosensitivity. In the GI system, ionizing radiation causes a dose-dependent increase in apoptosis in the small intestinal crypts within hours after exposure51. The Wnt/β-catenin pathway has been implicated in regulating radiation-induced apoptosis in crypt cells.
The β-catenin/TCF signal transduction pathway most probably has a significant role in regulating the proliferation and differentiation of intestinal epithelial cells during the regeneratiation and maturation processes along the crypt-villus axis55. Wnt signaling and β-catenin activation are important in the proliferation of the pluripotent stem cell which give rise to crypt epithelial progenitors. The amount of Wnt proteins in intestinal epithelial cells decreases with the progression toward the villus. As Wnt signaling decreases, β-catenin forms a complex with APC and Axin (destruction complex), thus leading to the degradation of β-catenin56. Therefore, Wnt signaling has a critical role in maintaining the undifferentiated state of intestinal crypt progenitor cells57. Barker et al.58 reported that a Wnt target gene, Lgr5 (that is, leucine-rich-repeat-containing G-protein-coupled receptor 5, also known as Gpr49) was identified as a marker for intestinal stem cells because it marks small columnar cells at the base of the crypt interspersed among Paneth cells. Moreover, research showed that Rspo1 was radioprotective against RIGS and that the mechanism behind this action was probably related to induction of the Wnt/β-catenin pathway.
GSK-3 located upstream of Wnt signaling, is involved in numerous important biological signal paths, and has an essential role in the Wnt/β-catenin pathway. In addition, GSK-3β, a type of GSK-3, is a major component of Wnt signaling which has an important role in the development and renewal of the intestinal epithelium by maintaining stem/progenitor cells and controlling migration and localization of epithelial cells along the crypt-villus axis59. Moreover, GSK-3β signaling is a key regulator of radiation-induced injury, and reports have shown that the small molecule inhibitor of GSK-3β has a protective effect against irradiated hippocampal neurons from apoptosis, and consequently, improves cognitive performances in irradiated mice60.
Thotala et al.10 used C57/BL6 mice to evaluate the radioprotective effect of GSK-3β inhibitors. Their results showed that mice pretreated with SB216763 or SB415286 exhibited significant reduction in the number of TUNEL- and Bax-positive cells and an increase in the number of Bcl-2-positive cells in intestinal crypts at 4 h and 12 h after radiation with 4 Gy and 8 Gy, respectively, compared with exposure to radiation alone, without significantly improving the survival rate of mice irradiated with 8 and 12 Gy. Several pathways have been implicated in the targeted molecular radioprotector of GSK-3β inhibitors, including the Bax/caspasae-3 pathway. Alternatively or additively, Wnt signaling transduces the radioprotective effect of GSK-3β inhibitors to perform one of the central roles in renewing damaged intestinal epithelium59. Given that GSK-3β phosphorylates β-catenin and targets it for ubiquitination and subsequent degradation, inhibiting GSK-3β can prevent β-catenin phosphorylation and degradation, whereas β-catenin accumulation and activation can promote cell cycle entry and the progression of stem cells within the intestinal crypts.
The role of Wnt/β-catenin signaling in cancer cells
The Wnt signaling pathway controls myriad biological phenomena throughout the development and adult life of all animals. In parallel, aberrant Wnt signaling underlies an extensive range of pathologies in humans, with its various components contributing to cancer development. Loss of APC function resulting from sporadic colorectal cancer leads to inappropriate stabilization of β-catenin and the formation of constitutive complexes between β-catenin and the intestinal TCF family member TCF461. In addition to APC mutations, factor 7-like 2 (TCF7L2, previously known as TCF4), β-catenin and Wilms tumour gene on the X chromosome have also been predicted to activate Wnt/β-catenin signalling62, as shown by the sequencing of colorectal tumors. In addition, mutations that are predicted to disrupt phosphorylation and degradation of β-catenin are frequent in hepatocellular carcinoma (HCC)63,64, medulloblastoma65, and ovarian cancer66; whereas deletions and truncation mutations in Axin1 are common in HCC and colorectal tumors67,68 (Table 1).
Table 1. Somatic mutation in WNT pathway genes in various cancers types*.
| Gene | Type of mutation | Primary tissues | Number of mutated samples | % mutated | Total samples |
|---|---|---|---|---|---|
| APC | Primarily frameshift and deletion mutations leading to compromised ability to degrade ATNNB1 | Large intestine | 2,152 | 39% | 5,517 |
| Stomach | 129 | 15% | 214 | ||
| Soft tissue | 50 | 12% | 430 | ||
| Small intestine | 34 | 16% | 214 | ||
| Pancreas | 26 | 14% | 184 | ||
| Liver | 11 | 12% | 94 | ||
| CTNNB1 | Mutations in CTNNB1 cluster around the amino-terminus and prevent the phosphorylation amino acids, S33, S37, T41 and S45, resulting in impaired degradation of CTNNB1 | Liver | 907 | 23% | 3,933 |
| Soft tissue | 673 | 42% | 1,601 | ||
| Endometrium | 218 | 20% | 1,098 | ||
| Kidney | 168 | 14% | 1,225 | ||
| Pancreas | 125 | 26% | 476 | ||
| Ovary | 104 | 11% | 913 | ||
| Adrenal gland | 100 | 19% | 534 | ||
| Pituitary | 86 | 24% | 360 | ||
| Biliary tract | 43 | 10% | 433 | ||
| AXIN1 | Many mutations prevent AXIN1 from acting as a scaffold to degrade CTNNB1 | Biliary tract | 10 | 38% | 26 |
| Liver | 49 | 11% | 448 | ||
| WTX (also known as FAM123B) | Predicted to be loss-of-function mutations | Kidney | 125 | 13% | 949 |
| Large intestine | 19 | 13% | 151 | ||
| TCF7L2 | Unknown | Large intestine | 13 | 28% | 47 |
*Cited from the Catalogue of Somatic Mutations in Cancer (COSMIC) database.
Genes mutated in at least 10% of the analysed samples for each cancer type are included in the table: APC, CTNNB1β-catenin, and WTX.
Recently, several studies have investigated the possibility of using the Wnt/β-catenin signaling pathway for overcoming radiation resistance. Che et al.69 described the implication of the Wnt pathway in esophageal cancer. These researchers used a COX-2 inhibitor, that is, NS398, to evaluate its radiosensitizing effect on radioresistant esophageal cancer Eca109R50 Gy cells. NS398 can enhance radiosensitivity of Eca109R50 Gy cells obtained through fractional irradiation from Eca109 cells using several mechanisms, including redistribution of cell cycle, inhibition of DNA-dependent protein kinase catalytic sub-unit expression, and induction of tumor cell apoptosis. The same team reported that NS398 intake could enhance the radiosensitivity of Eca109R50 Gy cells, while decreasing β-catenin expression. In addition, increasing doses was accompanied by an increase in β-catenin70 at the molecular level. Kendeziorra et al.71 reported over-expression of the Wnt transcription factor TCF4 in rectal cancer cell lines that were resistant to chemoradiotherapy.
Wnt/β-catenin signaling and reactive oxygen species
Radiation is widely believed to affect the homeostasis of reactive oxygen species (ROS)72, and that ROS accumulation can cause injury to cells. Numerous studies have indicated that ROS, which are cellular by-products of respiration, can modulate various signaling pathways and have certain physiological roles. Ionizing radiation can induce abnormal levels of ROS, thus exceeding the ability of organisms to clear them and consequently leading to oxidative stress-mediated damaging effects73. Recent studies have revealed that superoxide dismutase is expected to play an indispensable role in treating ROS-mediated normal tissue injuries originating from radiation exposure. Although ROS are widely recognized for their role as intracellular messengers, the mechanism of ROS-dependent signaling remains unknown. Previous research has demonstrated that ROS can modulate signaling by the Wnt/β-catenin pathway74. The canonical Wnt/β-catenin signaling, which is receiving the most attention, is characterized by the translocation of β-catenin to the nucleus as well as the subsequent downregulation and upregulation of epithelial and mesenchymal markers, respectively. Arnold et al.75 showed that ROS increased to a certain extent can stimulate cell proliferation and transformation to a certain extent. Moreover, the study of Funato et al.76 suggested that such enhanced proliferation is at least mediated in part by the release of the nucleoredoxin-dependent inhibition of Wnt/β-catenin signaling. Thus, β-catenin may be a key regulator in determining whether cells will proliferate or arrest to repair oxidative damage.
To our knowledge, the Wnt/β-catenin pathway is an important signaling mechanism for cell proliferation. β-Catenin migrates to the nucleus after the inactivation of GSK-3β, which is followed by the gene expression of cyclin D177. ROS have been known to modulate signaling by the Wnt/β-catenin pathway. Current data show that tempol-elicited downregulation of nuclear β-catenin may indicate that ROS have activated the Wnt signaling/β-catenin pathway in the neural stem/progenitor cells78. Further study needs to be conducted to verify the scavenger role of ROS in Wnt signaling pathway.
Conclusion
The Wnt/β-catenin pathway evidently has an important role in preventing damage induced by RT. The activation of the Wnt/β-catenin pathway, which provides a key radioprotective mechanism in irradiated cancer cells, exhibits protective effect against RT-induced oral mucositis and xerostomia. Moreover, activating Wnt/β-catenin signaling is a potential strategy for treating GI radiation syndrome. As a common signal pathway in the development and adult life of all animals, the Wnt signal transduction cascade, particularly the canonical Wnt pathway, is expected to occupy an indispensable position in treating normal tissue injuries, resulting from radiation exposure. Wnt/β-catenin signaling is associated with the development, renewal, and regeneration of numerous endoderm-derived organs; and research on this field has been ongoing for almost 40 years. Although a number of positive outcomes regarding the activation of the Wnt/β-catenin signaling pathway of antiradiation have been obtained, studies on the activation of the Wnt/β-catenin pathway related to antiradiation research remain extremely few to date. No drug based on Wnt/β-catenin signaling has been approved for radioprotective use in the clinical setting. The canonical Wnt pathway has been linked to cell proliferation in a variety of tissues and systems. Rspo1 is the only protein that can increase the mucosal thickness and reduce ulceration in the oral mucosa after irradiation and chemotherapy, presumably by increasing the proliferation of the mucosal epithelium in the basal layer of the tongue through the activation of the Wnt/β-catenin signaling pathway. The inhibitor of GSK3β SB216763 or SB415286 is studied only as a radioprotector primarily in animals because of its inclination to induce untoward effects in humans. The scientific community should further elucidate the side effects of the Wnt/β-catenin pathway to provide a potential therapeutic approach for preventing radiation-induced damage.
From the preceding discussion, we firmly believe that the Wnt/β-catenin signaling pathway activator should be developed as a potential therapeutic approach to prevent radiation-induced damage in the future. At present, confirming such protective effects is essential, particularly in the clinical setting (such as the approach, treatment window, and dosage of the inhibitor derived from the Wnt/β-catenin signaling pathway). However, considering the critical role of Wnt signaling in various cancer developments, the issue on specifically targeting the side effects of Wnt pathway-based drugs without increasing cancer risk should be addressed urgently.
Footnotes
No potential conflicts of interest are disclosed.
References
- 1.Radiological Protection Institute of Ireland. Your radiation exposure: Sources of radiation. Available online: http://www.rpii.ie/Your-Health/Your-radiation-exposure.aspx (accessed October 16, 2011)
- 2.Liu SZ. Biological effects of low level exposures to ionizing radiation: theory and practice. Hum Exp Toxicol 2010;29:275-281 [DOI] [PubMed] [Google Scholar]
- 3.Brown DQ, Pittock JW, 3rd, Rubinstein JS. Early results of the screening program for radioprotectors. Int J Radiat Oncol Biol Phys 1982;8:565-570 [DOI] [PubMed] [Google Scholar]
- 4.Cassatt DR, Fazenbaker CA, Bachy CM, Hanson MS. Preclinical modeling of improved amifostine (Ethyol) use in radiation therapy. Semin Radiat Oncol 2002;12:97-102 [DOI] [PubMed] [Google Scholar]
- 5.Wasserman TH, Brizel DM. The role of amifostine as a radioprotector. Oncology (Williston Park)2001;15:1349-1354; discussion 1357-60 [PubMed] [Google Scholar]
- 6.Klaus A, Birchmeier W.Wnt signalling and its impact on development and cancer. Nat Rev Cancer 2008;8:387-398 [DOI] [PubMed] [Google Scholar]
- 7.Tan LP, Ng BK, Balraj P, Lim PK, Peh SC. No difference in the occurrence of mismatch repair defects and APC and CTNNB1 genes mutation in a multi-racial colorectal carcinoma patient cohort. Pathology 2007;39:228-234 [DOI] [PubMed] [Google Scholar]
- 8.Huang D, Du X.Crosstalk between tumor cells and microenvironment via Wnt pathway in colorectal cancer dissemination. World J Gastroenterol 2008;14:1823-1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hai B, Yang Z, Shangguan L, Zhao Y, Boyer A, Liu F.Concurrent transient activation of Wnt/β-catenin pathway prevents radiation damage to salivary glands. Int J Radiat Oncol Biol Phys 2012;83:e109-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thotala DK, Geng L, Dickey AK, Hallahan DE, Yazlovitskaya EM. A new class of molecular targeted radioprotectors: GSK-3beta inhibitors. Int J Radiat Oncol Biol Phys 2010;76:557-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Kim KA, De Vera J, Palencia S, Wagle M, Abo A. R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/beta-catenin pathway. Proc Natl Acad Sci U S A 2009;106:2331-2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruffner H, Sprunger J, Charlat O, Leighton-Davies J, Grosshans B, Salathe A, et al. R-Spondin potentiates Wnt/β-catenin signaling through orphan receptors LGR4 and LGR5. PLoS One 2012;7:e40976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A 2003;100:15853-15858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen MS, Woodward WA, Behbod F, Peddibhotla S, Alfaro MP, Buchholz TA, et al. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci 2007;120:468-477 [DOI] [PubMed] [Google Scholar]
- 15.Schepers A, Clevers H.Wnt signaling, stem cells, and cancer of the gastrointestinal tract. Cold Spring Harb Perspect Biol 2012;4:a007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luis TC, Ichii M, Brugman MH, Kincade P, Staal FJ. Wnt signaling strength regulates normal hematopoiesis and its deregulation is involved in leukemia development. Leukemia 2012;26:414-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Xing L.Optimization of radiotherapy dose-time fractionation with consideration of tumor specific biology. Med Phys 2005;32:3666-3677 [DOI] [PubMed] [Google Scholar]
- 18.Boerma M, van der Wees CG, Vrieling H, Svensson JP, Wondergem J, van der Laarse A, et al. Microarray analysis of gene expression profiles of cardiac myocytes and fibroblasts after mechanical stress, ionising or ultraviolet radiation. BMC Genomics 2005;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys 2006;66:1281-1293 [DOI] [PubMed] [Google Scholar]
- 20.Baskar R.Emerging role of radiation induced bystander effects: Cell communications and carcinogenesis. Genome Integr 2010;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982;31:99-109 [DOI] [PubMed] [Google Scholar]
- 22.Vidya Priyadarsini R, Senthil Murugan R, Nagini S.Aberrant activation of Wnt/β-catenin signaling pathway contributes to the sequential progression of DMBA-induced HBP carcinomas. Oral Oncol 2012;48:33-39 [DOI] [PubMed] [Google Scholar]
- 23.Schlessinger K, Hall A, Tolwinski N.Wnt signaling pathways meet Rho GTPases. Genes Dev 2009;23:265-277 [DOI] [PubMed] [Google Scholar]
- 24.Sugimura R, Li L.Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res C Embryo Today 2010;90:243-256 [DOI] [PubMed] [Google Scholar]
- 25.Orme MH, Giannini AL, Vivanco MD, Kypta RM. Glycogen synthase kinase-3 and Axin function in a beta-catenin-independent pathway that regulates neurite outgrowth in neuroblastoma cells. Mol Cell Neurosci 2003;24:673-686 [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Bower KA, Xu M, Ding M, Shi X, Ke ZJ, et al. Cyanidin-3-glucoside reverses ethanol-induced inhibition of neurite outgrowth: role of glycogen synthase kinase 3 Beta. Neurotox Res 2009;15:321-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang H, He X.Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol 2008;20:119-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 2007;13:156-163 [DOI] [PubMed] [Google Scholar]
- 29.Niehrs C.Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006;25:7469-7481 [DOI] [PubMed] [Google Scholar]
- 30.Carmon KS, Lin Q, Gong X, Thomas A, Liu Q. LGR5 interacts and cointernalizes with Wnt receptors to modulate Wnt/β-catenin signaling. Mol Cell Biol 2012;32:2054-2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao AS, Kremenevskaja N, Resch J, Brabant G. Lithium stimulates proliferation in cultured thyrocytes by activating Wnt/beta-catenin signalling. Eur J Endocrinol 2005;153:929-938 [DOI] [PubMed] [Google Scholar]
- 32.Wang JS, Wang CL, Wen JF, Wang YJ, Hu YB, Ren HZ. Lithium inhibits proliferation of human esophageal cancer cell line Eca-109 by inducing a G2/M cell cycle arrest. World J Gastroenterol 2008;14:3982-3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94:153-156 [DOI] [PubMed] [Google Scholar]
- 34.Wijers OB, Levendag PC, Braaksma MM, Boonzaaijer M, Visch LL, Schmitz PI. Patients with head and neck cancer cured by radiation therapy: a survey of the dry mouth syndrome in long-term survivors. Head Neck 2002;24:737-747 [DOI] [PubMed] [Google Scholar]
- 35.Kagami H, Wang S, Hai B.Restoring the function of salivary glands. Oral Dis 2008;14:15-24 [DOI] [PubMed] [Google Scholar]
- 36.Rhodus NL, Bereuter J. Clinical evaluation of a commercially available oral moisturizer in relieving signs and symptoms of xerostomia in postirradiation head and neck cancer patients and patients with Sjögren’s syndrome. J Otolaryngol 2000;29:28-34 [PubMed] [Google Scholar]
- 37.Konings AW, Coppes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys 2005;62:1187-1194 [DOI] [PubMed] [Google Scholar]
- 38.Tatsuishi Y, Hirota M, Kishi T, Adachi M, Fukui T, Mitsudo K, et al. Human salivary gland stem/progenitor cells remain dormant even after irradiation. Int J Mol Med 2009;24:361-366 [DOI] [PubMed] [Google Scholar]
- 39.Chang HW, Roh JL, Jeong EJ, Lee SW, Kim SW, Choi SH, et al. Wnt signaling controls radiosensitivity via cyclooxygenase-2-mediated Ku expression in head and neck cancer. Int J Cancer 2008;122:100-107 [DOI] [PubMed] [Google Scholar]
- 40.Rødningen OK, Overgaard J, Alsner J, Hastie T, Børresen-Dale AL. Microarray analysis of the transcriptional response to single or multiple doses of ionizing radiation in human subcutaneous fibroblasts. Radiother Oncol 2005;77:231-240 [DOI] [PubMed] [Google Scholar]
- 41.Bhagat R, Premalata CS, Shilpa V, Pallavi VR, Ramesh G, Vijay CR, et al. Altered expression of β-catenin, E-cadherin, and E-cadherin promoter methylation in epithelial ovarian carcinoma. Tumour Biol. 2013 doi: 10.1007/s13277-013-0797-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Serra R, Easter SL, Jiang W, Baxley SE. Wnt5a as an effector of TGFβ in mammary development and cancer. J Mammary Gland Biol Neoplasia 2011;16:157-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hai B, Yang Z, Millar SE, Choi YS, Taketo MM, Nagy A, et al. Wnt/β-catenin signaling regulates postnatal development and regeneration of the salivary gland. Stem Cells Dev 2010;19:1793-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonis ST. Oral mucositis in cancer therapy. J Support Oncol 2004;2:3-8 [PubMed] [Google Scholar]
- 45.Treister N, Sonis S.Mucositis: biology and management. Curr Opin Otolaryngol Head Neck Surg 2007;15:123-129 [DOI] [PubMed] [Google Scholar]
- 46.Farrell CL, Rex KL, Chen JN, Bready JV, DiPalma CR, Kaufman SA, et al. The effects of keratinocyte growth factor in preclinical models of mucositis. Cell Prolif 2002;35Suppl 1:78-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braun S, Krampert M, Bodó E, Kümin A, Born-Berclaz C, Paus R, et al. Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV irradiation, chemotherapeutic or cytotoxic agents. J Cell Sci 2006;119:4841-4849 [DOI] [PubMed] [Google Scholar]
- 48.Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell 2008;19:2588-2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem 2006;281:13247-13257 [DOI] [PubMed] [Google Scholar]
- 50.Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 2005;309:1256-1259 [DOI] [PubMed] [Google Scholar]
- 51.Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J Biol Chem 2007;282:15903-15911 [DOI] [PubMed] [Google Scholar]
- 52.Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci U S A 2007;104:14700-14705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fei P, El-Deiry WS. P53 and radiation responses. Oncogene 2003;22:5774-5783 [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Matsudaira P, Gong Z.STORM: a general model to determine the number and adaptive changes of epithelial stem cells in teleost, murine and human intestinal tracts. PLoS One 2010;5:e14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008;320:226-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gregorieff A, Clevers H.Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 2005;19:877-890 [DOI] [PubMed] [Google Scholar]
- 57.Pinto D, Gregorieff A, Begthel H, Clevers H.Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 2003;17:1709-1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003-1007 [DOI] [PubMed] [Google Scholar]
- 59.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology 2008;134:849-864 [DOI] [PubMed] [Google Scholar]
- 60.Thotala DK, Hallahan DE, Yazlovitskaya EM. Glycogen synthase kinase 3β inhibitors protect hippocampal neurons from radiation-induced apoptosis by regulating MDM2-p53 pathway. Cell Death Differ 2012;19:387-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002;111:241-250 [DOI] [PubMed] [Google Scholar]
- 62.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laurent-Puig P, Zucman-Rossi J.Genetics of hepatocellular tumors. Oncogene 2006;25:3778-3786 [DOI] [PubMed] [Google Scholar]
- 64.Breuhahn K, Longerich T, Schirmacher P.Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene 2006;25:3787-3800 [DOI] [PubMed] [Google Scholar]
- 65.Zurawel RH, Chiappa SA, Allen C, Raffel C. Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res 1998;58:896-899 [PubMed] [Google Scholar]
- 66.Palacios J, Gamallo C.Mutations in the beta-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res 1998;58:1344-1347 [PubMed] [Google Scholar]
- 67.Salahshor S, Woodgett JR. The links between axin and carcinogenesis. J Clin Pathol 2005;58:225-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet 2000;24:245-250 [DOI] [PubMed] [Google Scholar]
- 69.Che SM, Zhang XZ, Hou L, Song TB. Cyclooxygenase-2 inhibitor NS398 enhances radiosensitivity of radioresistant esophageal cancer cells by inhibiting AKT activation and inducing apoptosis. Cancer Invest 2010;28:679-688 [DOI] [PubMed] [Google Scholar]
- 70.Che SM, Zhang XZ, Liu XL, Chen X, Hou L. The radiosensitization effect of NS398 on esophageal cancer stem cell-like radioresistant cells. Dis Esophagus 2011;24:265-273 [DOI] [PubMed] [Google Scholar]
- 71.Kendziorra E, Ahlborn K, Spitzner M, Rave-Fränk M, Emons G, Gaedcke J, et al. Silencing of the Wnt transcription factor TCF4 sensitizes colorectal cancer cells to (chemo-) radiotherapy. Carcinogenesis 2011;32:1824-1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugamura K, Keaney JF., Jr Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 2011;51:978-992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 2010;15:360-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tien Kuo M, Savaraj N.Roles of reactive oxygen species in hepatocarcinogenesis and drug resistance gene expression in liver cancers. Mol Carcinog 2006;45:701-709 [DOI] [PubMed] [Google Scholar]
- 75.Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, et al. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A 2001;98:5550-5555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Funato Y, Michiue T, Asashima M, Miki H.The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol 2006;8:501-508 [DOI] [PubMed] [Google Scholar]
- 77.Pradeep A, Sharma C, Sathyanarayana P, Albanese C, Fleming JV, Wang TC, et al. Gastrin-mediated activation of cyclin D1 transcription involves beta-catenin and CREB pathways in gastric cancer cells. Oncogene 2004;23:3689-3699 [DOI] [PubMed] [Google Scholar]
- 78.Yoneyama M, Kawada K, Gotoh Y, Shiba T, Ogita K.Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem Int 2010;56:740-746 [DOI] [PubMed] [Google Scholar]