Abstract
Background:
Gender distribution of acute stroke patients varies considerably among stroke registries throughout the world, but factors responsible for this phenomenon remained vastly unknown.
Materials and Methods:
Using data from prospective hospital-based stroke registries in China (n = 752 acute stroke patients), Germany (n = 96054), India (n = 1500), and Iran (n = 1392), this descriptive study explored gender distribution of stroke patients and its determinants. In addition, the proportions of males and females to be expected in fictive study populations were calculated, and differences in gender distribution between stroke databases throughout the world were described.
Results:
In the German dataset, a maximum male preponderance was found for patients aged between 55 and 64 years (proportion of male patients 0.67 [95% CI: 0.66-0.67]), whereas patients older than 84 years revealed a strong overbalance of females (0.27 [0.26-0.28]). In Germany, age-specific gender distribution of stroke patients is well explained by the numbers of females and males in the general population and by gender-specific stroke incidence rates. Both in China and India, a strong preponderance of male stroke patients was found for the majority of age categories with a maximum proportion of male patients of 0.82 in the 35-44 years age group. In contrast, the Iranian stroke register revealed an overbalance of females (0.13 [0.11-0.14]) in nearly all age categories. A total of 1392 Iranian ischemic stroke patients (738 female, 654 male) were investigated.
Conclusion:
Gender distribution of acute stroke patients is highly variable. Gender distribution varied considerably between countries. Apart from demographic factors reflecting gender ratio in the general population and gender-specific stroke incidence rates, sociocultural peculiarities may also play an important role in this context.
Keywords: Gender, incidence, stroke
INTRODUCTION
Within the last two decades, a large number of studies on hospital-based stroke registries have been published reporting clinical data on thousands of stroke patients from all over the world.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19] Authors aimed on estimating stroke incidence in well-defined populations or on characterizing stroke subtypes and risk factors. Screening the baseline variables of the study populations, it is remarkable that gender distribution is considerably different between these registries. For example, databases from the USA, from Iran, and from Ireland documented up to 5% more women than men,[4,5,14] whereas registries from China and India reported a vast majority of male patients (66% and 72%, respectively).[8,11] The upper extremity constitutes a subgroup analysis of lacunar infarctions derived from an Indian stroke registry with a male to female ratio as high as 3.5-1.[20] Factors responsible for this huge variance in gender distribution among registries are still unknown and not characterized.
Beyond stroke registries, a disproportional gender distribution can also be found in acute stroke studies. As an example, the large randomized trials on thrombolysis in acute ischemic stroke reported only 41-42% of female patients.[21,22] Apparently, this finding stands in discrepancy to stroke registries from Europe and USA where the number of male and female patients included was shown to be rather similar or even revealed a slight overbalance of females. Thus, what causes the preponderance of male patients in these stroke studies as compared to registries?
There are several conceivable factors that may influence the gender distribution in hospitalized stroke patients. Among them, the gender ratio of the general population and gender-specific stroke incidence rates are likely to be the major determinants, but sociocultural peculiarities may also play a crucial role.
It is both of clinical and sociocultural importance to investigate the causes for gender gap in stroke patients—to guarantee that both female and male patients have equal access to in-hospital medical care[23] and to further enlighten gender-specific differences in stroke. Based on data from a large hospital-based stroke registry in Germany and three stroke registries in China, India, and Iran, respectively, the present study was performed to investigate gender distribution in hospitalized stroke patients with respect to the following topics: (I) to characterize gender distribution with respect to age and stroke subtypes, (II) to identify major determinants of gender distribution, (III) to characterize the gender distribution of fictive study populations, and (IV) to explore differences in gender distribution between stroke databases throughout the world.
MATERIALS AND METHODS
Data from Germany
The German data were derived from a large prospective stroke registry, provided by the “Arbeitsgruppe Schlaganfall Hessen” (for details see: www.gqhnet.de [accessed March 14th, 2007]).[24] This registry is a country-wide quality assurance measure based on state law, where all hospitalized stroke patients should be documented anonymously. At present, more than 100 hospitals participate in enrolling patients with a final diagnosis of transient ischemic attack (TIA, ICD-10: G45), ischemic stroke (IS, ICD-10: I63), or intracerebral hemorrhage (ICH, ICD-10: I61) into this standardized and computerized registry. All parameters relevant to this analysis including sex, age, severity of clinical symptoms at hospital admission (assessed using the modified Rankin Scale (mRS)), and time span from stroke onset to hospital admission (categorized as <3 h vs. >3 h or unknown) were recorded. For the present analysis, we chose all datasets with patient admission dates between January 1st 1998 and December 31, 2005 and excluded those with missing information on sex and those with patient age below 18 years.
Data regarding stroke incidence rates in Germany were derived from a pooled analysis of three prospective population-based stroke registries in Europe (European Registries of Stroke Collaboration including data from France, Great Britain, and Germany[25]). Demographic data on the German population for the year 2005 were obtained from the national statistics bureau (Statistisches Bundesamt Deutschland, www.destatis.de [accessed March 17th, 2007]).
Data from hospital-based stroke registries of other countries
To compare the German dataset to hospital-based stroke registries from other countries, we also analyzed data on gender distribution of hospitalized stroke patients from two Asian nations (China, India) and from one nation of the Middle East (Iran).
The Chinese data were derived from the Nanjing Stroke Registry Program (NSRP).[11] NSRP is the first hospital-based stroke registry program conducted in mainland China, which was inaugurated in July 2002. It is an ongoing, prospective observational project aimed at consecutively assembling demographic, clinical, neuroimaging, and laboratory data of registered patients. NSRP is based in Jinling Hospital, Nanjing University School of Medicine, which is located in Nanjing, a city in southeast China. The detailed protocol for NSRP has been published previously.[11] A total of 752 patients with first-ever stroke had been enrolled by the date of December 31, 2005, which were analyzed in this study.
The data from India were derived from the stroke registry at Nizam΄s Institute of Medical Sciences (NIMS). NIMS is a major neurological center and a University Hospital in Hyderabad, the capital city of south Indian state of Andhra Pradesh. The pilot data consisted of 392 consecutive patients of ischemic stroke enrolled between February 2000 and January 2001.[8] Stroke data bank of NIMS is an ongoing, prospective observational project aimed at consecutively collecting the clinical, neuroimaging, laboratory, and outcome data on all fully investigated cases of acute stroke. At present, datasets from 1500 stroke patients are included which were used for analysis in this study.
The data for Iran were based on the Khorasan Stroke Registry.[5] The province of Southern Khorasan (682 000 inhabitants) is located in eastern Iran, with Birjand as the largest city in the area. Every patient with a possible diagnosis of stroke is routinely admitted to the Valie-Asr tertiary care hospital in Birjand. Patients were examined by an emergency stroke team and admitted to the Department of Neurology. Consecutive patients with each severity of stroke were hospitalized. Data on demographics and clinical findings were prospectively saved in the Khorasan Stroke Registry. Between 2001 and 2005, a total of 1392 ischemic stroke patients were included who were analyzed in the present study.
The numbers of males and females in the general population and the population pyramid graphs for China, India, and Iran were extracted from the International Database of Population Pyramids of the US census bureau (www.census.gov/ipc/www/idbpyr.html, accessed March 14th, 2007). The data refer to the year 2000.
Statistical analysis
From absolute numbers of female and male stroke patients in different age ranges as well as from demographic and incidence data, the proportion of males (pm) was derived. Confidence intervals were obtained using the Wilson method. The Statistical Package for the Social Science version 11.5 (SPSS, Chicago, IL, USA) was used to analyze patient data.
RESULTS
Results based on the German dataset
A total of 96,054 stroke patients were included (50.2% male, mean age 71.8 ± SD: 12.7 years). Table 1 summarizes the baseline variables for both females and males. The pm varies considerably between different age categories [Figure 1, grey line]. A maximum male preponderance was found for patients aged between 55 and 64 years (pm 0.67 [95% CI: 0.66-0.67]), whereas patients older than 84 years revealed a strong overbalance of females (pm 0.27 [0.26-0.28]). Figure 1 also displays the pm in the German general population for the year 2005 (black line). Up to the age of 55 years, the number of women and men is nearly balanced with a slight predominance of males. Above the age of 55, an overbalance of females starts to appear, most likely due to their longer life expectancy. This is highest in the age category >84 years with a pm of only 0.25.
Table 1.
Baseline characteristics of the study population as derived from a large prospective stroke registry in Germany
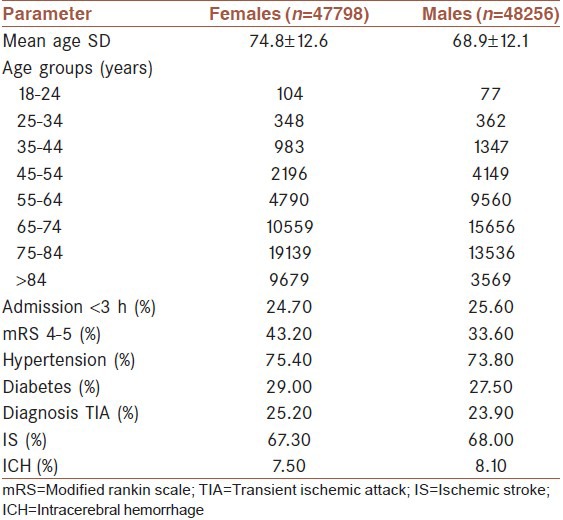
Figure 1.
Gender distribution of stroke patients and major determinants. Black line: Proportion of males in the general German population. Grey line: Proportion of males in the stroke registry. Red line: Proportion of males as derived from European stroke incidence studies (proportion of male stroke patients expected if 100,000 women and 100,000 men are followed up for first ever stroke for 1 year). Dotted line: Proportion of male stroke patients theoretically expected as a function of gender specific stroke incidence rates (red line) and the number of males and females in the general German population (black line). Black line: Proportion of males in the general German population. Grey line: Proportion of males in the stroke registry. Red line: proportion of males as derived from European stroke incidence studies. Dotted line: Proportion of male stroke patients theoretically expected as a function of gender specific stroke incidence rates.
In a next step, we were interested whether the observed gender distribution in our registry is consistent with the gender ratio theoretically expected as a function of gender-specific stroke incidence rates in Germany [Figure 1, red line] and the number of males and females in the general German population (black line). By means of this model, the pm of the registry was accurately predicted for patients aged 65 years or older (model: 0.60 [0.59-0.61] for 65-74 years, 0.39 [0.39-0.40] for 75-84 years, 0.27 [0.27-0.27] for >84 years; registry: 0.60, [0.59-0.60] 0.41, [0.41-0.42] 0.27; [0.26-0.28] respectively). However, in younger stroke patients, the data from the registry suggested a higher pm than calculated (model: 0.58 [0.55-0.59] for 45-54 years, 0.61 [0.59-0.62] for 55-64 years; registry: 0.65, [0.64-0.67] 0.67; [0.66-0.67] respectively; [Figure 1, dotted line].
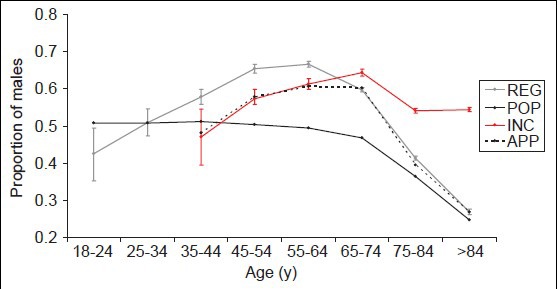
Additionally, we were interested whether the gender distribution of a fictive study population depends on the age span of included patients. We defined 18 years as the fixed lower age limit of this study population and varied the upper age limit [Figure 2]. In IS, the highest pm was found if 60 years was chosen as the upper age limit (0.66 [0.65-0.67]). In contrast, studies with an unlimited upper age revealed the lowest pm (0.51 [0.51-0.51]). In ICH, this distribution is fairly similar (highest pm was 0.64 [0.62-0.67] for a study population aged 18-60 years, lowest pm was 0.52 [0.51-0.53] for a study population without an upper age limit). Figure 2 also displays data on patients admitted within 3 h after stroke onset and on patients with a severe clinical deficit at hospital admission, respectively. Based on this data, excluding older patients from stroke studies will cause a markedly overbalance of male patients compared to studies with an unlimited upper age.
Figure 2.
Graphs showing how the gender distribution of a fictive stroke study population depends on the age span of included patients. 18 years was chosen as a fixed lower age limit, and the upper age limit was varied. <3 h: Hospital admittance within 3 h after stroke onset, mRS: Modified Rankin scale. (a) Patients with ischemic stroke (IS), (b) patients with intracerebral hemorrhage
Results based on stroke registries from other countries
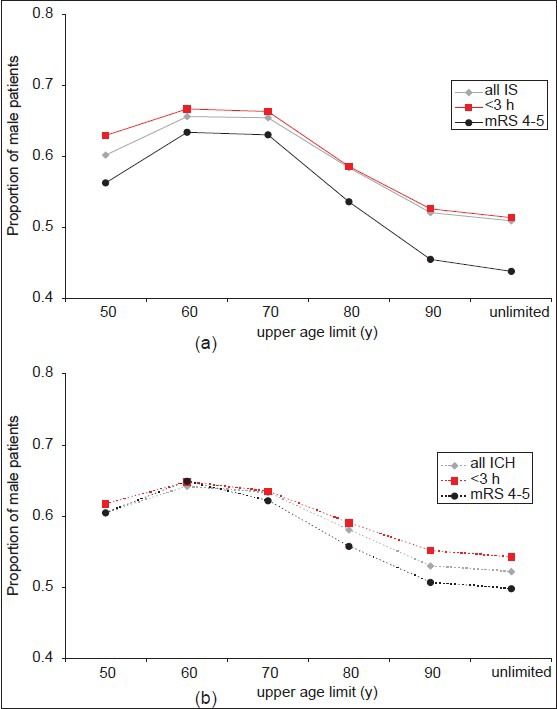
Table 2 summarizes 20 studies on hospital-based stroke registries from different parts of the world. All of them were published within the last 15 years. The pm varied between 0.45 and 0.72, and the mean age of the enrolled patients ranged from 53 to 76 years. We found an inverse correlation between mean age and the pm in the registries [Spearman Rho: −0.547, P = 0.012; Figure 3]. Figure 4 displays the pm for the general population of four different countries (China, India, Iran, and Germany; see black lines). In contrast to the German data, countries like India and Iran show a nearly balanced gender distribution in the general population even at higher ages. Gender distribution of stroke patients is also shown as derived from the corresponding stroke registries (grey lines), and these gender curves revealed a huge variation among the countries. Whereas countries like China and India show a very high preponderance of males for nearly all age categories, the curve of the Iranian stroke register is inversed revealing a higher proportion of female stroke patients aged between 45 and 74 years. In contrast to all other countries, the German registry revealed a striking overbalance of females in elderly stroke patients.
Table 2.
Overview on 20 publications on hospital-based stroke registries from different parts of the world. All of them were published within the last 15 years
Figure 3.
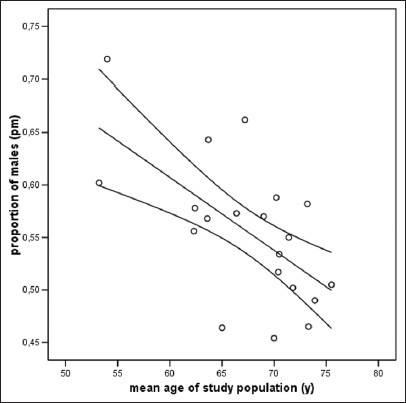
Scatter plot showing the inverse correlation between the proportion of male patients in the respective study population and the mean age of the study population for hospital-based stroke registries from 20 different countries [see also Table 2]
Figure 4.
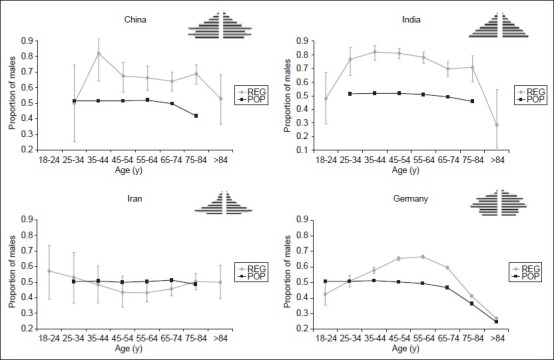
Graphs showing the gender distribution of stroke patients from four different countries. Black lines: Proportion of males in the respective general populations. Grey lines: Proportion of males as derived from stroke registries Black lines: Proportion of males in the respective general populations. Grey lines: Proportion of males as derived from stroke registries
DISCUSSION
The present study focused on characterizing gender distribution in hospitalized stroke patients. In a first step, we analyzed the gender distribution in a large registry from Germany and found a considerable variation of the pm between different age categories. Whereas around two times more male stroke patients are admitted aged 55-64 years, a three-fold overbalance of females can be found in patients older than 84 years.
In a second step, we tried to identify determinants of gender distribution. In patients older than 65 years, the pm in the registry was closely related to the pm theoretically expected as a function of the number of women and men in the general German population and gender-specific stroke incidence rates as reported from prospective population-based studies in Europe.[25] However, for younger stroke patients, the pm was higher in the registry than that one derived from the above-mentioned model. This is most likely caused by imprecise incidence rates for younger stroke patients obtained from prospective incidence studies due to the relatively low numbers of strokes that occurred in the younger age categories. According to our dataset, the true incidence rates of stroke are likely to be higher in young males or lower in young females than previously reported. An alternative explanation would be that younger female stroke patients are less often being admitted to hospital as compared to men. However, this is not likely as younger stroke patients—in comparison to elderly ones—were shown to be particularly alert to stroke signs and symptoms.[24] Our study demonstrates that the pm in the upper age groups (covering for the vast majority of all patients) is nearly fully expla ined by gender distribution in the general population and gender-specific stroke incidence rates. Thus, in Germany, relevant sociocultural factors causing an additional imbalance in gender distribution are unlikely to exist.
In a third step, we described how the upper age limit of a fictive population of stroke patients in Germany influences its gender proportion. In fact, restricting the upper age limit in stroke trials to certain values will increase the number of males included in the trial, which at least partially explains the higher pm in randomized controlled acute stroke trials as compared to stroke registries. This fact is not always negligible when study results should be transferred into the general medical practice. Regarding thrombolytic therapy, several authors reported their experience in extending the upper age limit of 80 years to higher values.[26,27] According to the data from the present study, beyond the age of 84 years much more females will be treated than males (pm for patients >84 years = 0.27; pm in the NINDS trial = 0.58). This needs consideration as therapeutic effectiveness and the risk of complications may not always be identical in both genders.[28]
By comparing registry data from Germany with data from other countries, considerable variations in pm curves were found. Several influencing factors are conceivable for these findings:
Demographic factors: In Germany, the larger number of male patients admitted upon younger stroke patients are balanced by an overweight of females in elderly patients due to higher life expectancy in women, which resulted in a pm of around 0.5 for the overall registered patients. In contrast, India shows a nearly balanced gender ratio in the general population even at higher ages suggesting that life expectancy might not be that different between females and males in this country as it is in Germany. Thus, even at higher ages more male than female stroke patients will be admitted. This is one reason explaining the overall high numbers of males documented in Asian registries.
Differences in stroke incidence rates: The high male preponderance in Asian stroke registries may also be a result of higher stroke incidence rates in men or lower stroke incidence rates in women from these regions as compared to European ones. For example, recently published data from China suggested particularly higher stroke incidence rates in males than in females (141.6 [males] vs. 103.7 [females] cases per 100 000 person-years),[29] whereas in Germany, these numbers tend to differ to a lower extent (200 vs. 170). A noticeable imbalance of stroke risk factors between males and females may in part explain this phenomenon. In China, female cigarette smokers are extremely rare, but as many as 60% of male elderly are regular smokers. In addition, prevalence of hypertension had been show to be considerably higher in males than in females in most age ranges in Chinese population.[30] Another Chinese epidemiologic study on stroke patients showed that female patients were globally older than male patients at stroke onset and having higher of prevalence of diabetes mellitus, heart diseases, and atrial fibrilation, However, females were less likely to drink heavily or smoke than males.[31] Of high interest is the pm curve from Iran as it has a nearly inversed shape than that from other countries. Stroke incidence rates in Iranian women as compared to men seem to be comparably high, which is again most likely a consequence of risk factor distribution. Studies reported hypertension as the most common risk factor in 63% of Iranian women and only in 42% of men with ischemic stroke.[5] Gender distribution of risk factors among Pakistanian stroke patients revealed more prevalence of hypertension and dyslipidemia in females.[32] Investigation on Italian federation of anticoagulation clinics showed gender differences of bleeding and stroke risk in old atrial fibrillation patients.[33] Elderly males showed a higher rate of bleeding complications and females showed a slightly higher rate of stroke.[33]
Differences in the sociocultural situation: Around the world, one can still find females to be in an inferior sociocultural situation, especially in developing countries. Fewer of them have a regular work, and more of them do not have health insurance.[34,35] Their roles as a family or social number may be less important than male's. In some developing countries, the likelihood of a female stroke patient being send to a small clinic or managed at home (by traditional medicine or acupuncture, for example) may be higher than for males.[36] The presence of sociocultural differences between male and female patients is emphatically strengthen by findings from India, where stroke registries reported among the highest pm in the world, but a population-based study on stroke incidence in India suggested a higher incidence rate in females than males (100 vs. 149 cases per 100,000 person years).[37] Austrian health interview survey showed that only among women did the risk for diabetes mellitus and hypertension increase with decreasing educational level, adjusted for age, income, family status, and lifestyle.[38] There is an urgent need for further research clarifying the discrepancy between the high stroke incidence rates in females and the low rates of females registered in the hospital-based registries. At the other side, there is a significant gender difference in stroke outcome for community stroke survivors. In USA, women showed significantly greater deficits on multiple 1 year outcome measures compared to men.[39]
Influence of gender on recurrence of stroke among patients with ischemic cerebrovascular events is another aspect of gender and stroke relationships.[40]
In summary, this descriptive study characterizes gender distribution of acute stroke patients based on data from stroke registries of four different countries. Demographic factors as well as gender-specific stroke incidence rates and sociocultural factors have been identified as major determinants of gender distribution. Further research is necessary to clarify major differences in gender distribution of stroke patients between nations as well as to enlighten discrepancies between gender-specific stroke incidence rates and the gender distribution in stroke registries.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.al-Rajeh S, Larbi EB, Bademosi O, Awada A, Yousef A, al-Freihi H, et al. Stroke register: Experience from the eastern province of Saudi Arabia. Cerebrovasc Dis. 1998;8:86–9. doi: 10.1159/000015823. [DOI] [PubMed] [Google Scholar]
- 2.Bornstein NM, Aronovich BD, Karepov VG, Gur AY, Treves TA, Oved M, et al. The Tel Aviv Stroke Registry. 3600 consecutive patients. Stroke. 1996;27:1770–3. doi: 10.1161/01.str.27.10.1770. [DOI] [PubMed] [Google Scholar]
- 3.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Consoli D, Wolfe CD, et al. Risk factors and outcome of subtypes of ischemic stroke. Data from a multicenter multinational hospital-based registry. The European Community Stroke Project. J Neurol Sci. 2006;244:143–50. doi: 10.1016/j.jns.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Fan CW, McDonnell R, Johnson Z, O’Keeffe S, Crowe MJ. Hospital-based stroke care in Ireland: Results from one regional register. Ir J Med Sci. 2000;169:30–3. doi: 10.1007/BF03170480. [DOI] [PubMed] [Google Scholar]
- 5.Ghandehari K, Izadi Z. Khorasan Stroke Registry. The Khorasan Stroke Registry: Results of a five-year hospital-based study. Cerebrovasc Dis. 2007;23:132–9. doi: 10.1159/000097050. [DOI] [PubMed] [Google Scholar]
- 6.Glader EL, Stegmayr B, Norrving B, Terént A, Hulter-Asberg K, Wester PO, et al. Sex differences in management and outcome after stroke: A Swedish national perspective. Stroke. 2003;34:1970–5. doi: 10.1161/01.STR.0000083534.81284.C5. [DOI] [PubMed] [Google Scholar]
- 7.Jeng JS, Lee TK, Chang YC, Huang ZS, Ng SK, Chen RC, et al. Subtypes and case-fatality rates of stroke: A hospital-based stroke registry in Taiwan (SCAN-IV) J Neurol Sci. 1998;156:220–6. doi: 10.1016/s0022-510x(98)00046-x. [DOI] [PubMed] [Google Scholar]
- 8.Kaul S, Sunitha P, Suvarna A, Meena AK, Uma M, Reddy JM. Subtypes of Ischemic Stroke in a Metropolitan City of South India (One year data from a hospital based stroke registry) Neurol India. 2002;50:S8–14. [Google Scholar]
- 9.Kumral E, Ozkaya B, Sagduyu A, Sirin H, Vardarli E, Pehlivan M. The Ege Stroke Registry: A hospital-based study in the Aegean region, Izmir, Turkey. Analysis of 2,000 stroke patients. Cerebrovasc Dis. 1998;8:278–88. doi: 10.1159/000015866. [DOI] [PubMed] [Google Scholar]
- 10.Lee BI, Nam HS, Heo JH, Kim DI. Yonsei Stroke Team. Yonsei Stroke Registry. Analysis of 1,000 patients with acute cerebral infarctions. Cerebrovasc Dis. 2001;12:145–51. doi: 10.1159/000047697. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Xu G, Wu W, Zhang R, Yin Q, Zhu W. Subtypes and one-year survival of first-ever stroke in Chinese patients: The Nanjing Stroke Registry. Cerebrovasc Dis. 2006;22:130–6. doi: 10.1159/000093241. [DOI] [PubMed] [Google Scholar]
- 12.Martí-Vilalta JL, Arboix A. The Barcelona Stroke Registry. Eur Neurol. 1999;41:135–42. doi: 10.1159/000008036. [DOI] [PubMed] [Google Scholar]
- 13.Moulin T, Tatu L, Vuillier F, Berger E, Chavot D, Rumbach L. Role of a stroke data bank in evaluating cerebral infarction subtypes: Patterns and outcome of 1,776 consecutive patients from the Besançon stroke registry. Cerebrovasc Dis. 2000;10:261–71. doi: 10.1159/000016068. [DOI] [PubMed] [Google Scholar]
- 14.Reeves MJ, Arora S, Broderick JP, Frankel M, Heinrich JP, Hickenbottom S, et al. Acute stroke care in the US: Results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36:1232–40. doi: 10.1161/01.STR.0000165902.18021.5b. [DOI] [PubMed] [Google Scholar]
- 15.Rojas JI, Zurru MC, Patrucco L, Romano M, Riccio PM, Cristiano E. Ischemic stroke registry. Medicina (B Aires) 2006;66:547–51. [PubMed] [Google Scholar]
- 16.Silvestrelli G, Corea F, Paciaroni M, Milia P, Palmerini F, Parnetti L, et al. The Perugia hospital-based Stroke Registry: Report of the 2 nd year. Clin Exp Hypertens. 2002;24:485–91. doi: 10.1081/ceh-120015324. [DOI] [PubMed] [Google Scholar]
- 17.Tu JV, Gong Y. Trends in treatment and outcomes for acute stroke patients in Ontario, 1992-1998. Arch Intern Med. 2003;163:293–7. doi: 10.1001/archinte.163.3.293. [DOI] [PubMed] [Google Scholar]
- 18.Vemmos KN, Takis CE, Georgilis K, Zakopoulos NA, Lekakis JP, Papamichael CM, et al. The Athens stroke registry: Results of a five-year hospital-based study. Cerebrovasc Dis. 2000;10:133–41. doi: 10.1159/000016042. [DOI] [PubMed] [Google Scholar]
- 19.Zenebe G, Alemayehu M, Asmera J. Characteristics and outcomes of stroke at Tikur Anbessa Teaching Hospital, Ethiopia. Ethiop Med J. 2005;43:251–9. [PubMed] [Google Scholar]
- 20.Kaul S, Venketswamy P, Meena AK, Sahay R, Murthy JM. Frequency, clinical features and risk factors of lacunar infarction (data from a stroke registry in South India) Neurol India. 2000;48:116–9. [PubMed] [Google Scholar]
- 21.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 22.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–51. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 23.American Medical Association Commission to End Health Care Disparities, 2005. [Last accessed 2012 Oct 01]. Available from: http://www.ama-assnorg/ama/pub/category/14629 html .
- 24.Foerch C, Misselwitz B, Sitzer M, Berger K, Steinmetz H, Neumann-Haefelin T, et al. Difference in recognition of right and left hemispheric stroke. Lancet. 2005;366:392–3. doi: 10.1016/S0140-6736(05)67024-9. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe CD, Giroud M, Kolominsky-Rabas P, Dundas R, Lemesle M, Heuschmann P, et al. Variations in stroke incidence and survival in 3 areas of Europe. European Registries of Stroke (EROS) Collaboration. Stroke. 2000;31:2074–9. doi: 10.1161/01.str.31.9.2074. [DOI] [PubMed] [Google Scholar]
- 26.Sylaja PN, Cote R, Buchan AM, Hill MD. on behalf of Canadian Alteplase for Stroke Effectiveness Study (CASES) Investigators. Thrombolysis in patients older than 80 years with acute ischaemic stroke: Canadian Alteplase for Stroke Effectiveness Study. J Neurol Neurosurg Psychiatry. 2006;77:826–9. doi: 10.1136/jnnp.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelter ST, Bonati LH, Lyrer PA. Intravenous thrombolysis in stroke patients of > or=80 versus < 80 years of age: A systematic review across cohort studies. Age Ageing. 2006;35:572–80. doi: 10.1093/ageing/afl104. [DOI] [PubMed] [Google Scholar]
- 28.Kent DM, Price LL, Ringleb P, Hill MD, Selker HP. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: A pooled analysis of randomized clinical trials. Stroke. 2005;36:62–5. doi: 10.1161/01.STR.0000150515.15576.29. [DOI] [PubMed] [Google Scholar]
- 29.Jiang B, Wang WZ, Chen H, Hong Z, Yang QD, Wu SP, et al. Incidence and trends of stroke and its subtypes in China: Results from three large cities. Stroke. 2006;37:63–5. doi: 10.1161/01.STR.0000194955.34820.78. [DOI] [PubMed] [Google Scholar]
- 30.Gu D, Reynolds K, Wu X, Chen J, Duan X, Muntner P, et al. Prevalence, awareness, treatment, and control of hypertension in china. Hypertension. 2002;40:920–7. doi: 10.1161/01.hyp.0000040263.94619.d5. [DOI] [PubMed] [Google Scholar]
- 31.Yao XY, Lin Y, Geng JL, Sun YM, Chen Y, Shi GW, et al. Age- and gender-specific prevalence of risk factors in patients with first-ever ischemic stroke in China. Stroke Res Treat. 2012;10:51–5. doi: 10.1155/2012/136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basharat Z, Mumtaz S, Rashid F, Rashid S, Mallam SA, Diljan A, et al. Prevalence of risk factors of ischemic stroke in a local Pakistani population. High-density lipoproteins, an emerging risk factor. Neurosciences (Riyadh) 2012;17:357–62. [PubMed] [Google Scholar]
- 33.Poli D, Antonucci E, Testa S, Ageno W, Palareti G. on the behalf of FCSA (Italian Federation of Anticoagulation Clinics). Gender differences of bleeding and stroke risk in very Old atrial fibrillation patients on VKA treatment: Results of the EPICA study on the behalf of FCSA (Italian Federation of Anticoagulation Clinics) Thromb Res. doi: 10.1016/j.thromres.2012.10.009. Published on line 2012 Nov 12. [DOI] [PubMed] [Google Scholar]
- 34.Henderson G, Jin S, Akin J, Li X, Wang J, Ma H, et al. Distribution of medical insurance in China. Soc Sci Med. 1995;41:1119–30. doi: 10.1016/0277-9536(94)00420-x. [DOI] [PubMed] [Google Scholar]
- 35.Standing H. Gender and equity in health sector reform programmes: A review. Health Policy Plan. 1997;12:1–18. doi: 10.1093/heapol/12.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Wang Y, Collins CD, Tang S. Urban health insurance reform and coverage in China using data from National Health Services Surveys in 1998 and 2003. BMC Health Serv Res. 2007;7:37. doi: 10.1186/1472-6963-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das SK, Banerjee TK, Biswas A, Roy T, Raut DK, Mukherjee CS, et al. A prospective community-based study of stroke in Kolkata, India. Stroke. 2007;38:906–10. doi: 10.1161/01.STR.0000258111.00319.58. [DOI] [PubMed] [Google Scholar]
- 38.Kautzky-Willer A, Dorner T, Jensby A, Rieder A. Women show a closer association between educational level and hypertension or diabetes mellitus than males: A secondary analysis from the Austrian HIS. BMC Public Health. 2012;12:392. doi: 10.1186/1471-2458-12-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roth DL, Haley WE, Clay OJ, Perkins M, Grant JS, Rhodes JD, et al. Race and gender differences in 1-year outcomes for community-dwelling stroke survivors with family caregivers. Stroke. 2011;42:626–31. doi: 10.1161/STROKEAHA.110.595322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen C, Zhao Y, Zhang J, Wang H, Wang X, Ma X, et al. Analysis of multiple risk factors for the recurrence of nondisabling stroke. J Natl Med Assoc. 2012;104:331–5. doi: 10.1016/s0027-9684(15)30173-5. [DOI] [PubMed] [Google Scholar]


