Abstract
Background:
Some epidemiological and interventional studies have shown the role of vitamin D on insulin secretion and resistance. A previous study in our center showed that intramuscular vitamin D decreases insulin sensitivity in pre-diabetic patients. We investigated the role of oral vitamin D on the insulin sensitivity index and insulin resistance in pre-diabetic patients.
Materials and Methods:
In a randomized clinical trial, we divided 45 people with pre-diabetes aged 47.4 ± 6.6 (range 33-61) years into three groups: group A subjects treated with 50,000 IU oral vitamin D and 500 mg calcium carbonate (n = 21), group B subjects treated with a single 300,000 IU intramuscular vitamin D and 500 mg calcium carbonate (n = 9), and group C subjects treated with 500 mg calcium carbonate alone (n = 15). Serum 25-hydroxyvitamin D [25(OH) D] was measured at baseline. If it was less than 75 nmol/l, 50,000 IU vitamin D was given weekly, and if serum 25(OH) D was more than that, vitamin D was administered every 2 weeks. Before and after 12 weeks of treatment, a 75-g glucose tolerance test was performed. We used paired t-test and analysis of variance (ANOVA) to analyze the data. P values less than 0.05 were considered significant.
Results:
Mean (SD) of serum vitamin D increased from 77.5 ± 39.2 to 118.8 ± 56.3 nmol/l (P = 0.009) in group A and from 80 ± 36 to 102.8 ± 43.3 nmol/l (P = 0.053) in group B, and decreased from 44.8 ± 18.3 to 34.6 ± 13.9 nmol/l (P = 0.06) in group C. Insulin sensitivity index (Matsuda) decreased from 11.4 ± 3 to 9.9 ± 3.2 (P = 0.046) in group A, but in comparison with other groups, it was not significant.
Conclusion:
Oral vitamin D had no effect on insulin sensitivity in pre-diabetes patients in 12 weeks treatment. A randomized double-blind study with a longer duration of treatment is suggested to investigate the effect of vitamin D on insulin resistance.
Keywords: Glucose tolerance test, insulin resistance, pre-diabetes, vitamin D, vitamin D deficiency
INTRODUCTION
In recent years, the relationship between vitamin D and its effect on insulin secretion and insulin resistance has been shown. In the pancreas, from a local process, vitamin D becomes active through 1α-hydroxylase enzyme. The active form of vitamin D through its receptors directly or through regulation of intracellular calcium helps to secrete insulin.[1,2] Vitamin D increases insulin sensitivity through the effect on its muscle cell receptors by increasing insulin receptor or increasing the sensitivity of insulin receptor to insulin and the effect on peroxisome proliferator-activated receptor (PPAR) δ and the influence on regulation of extracellular calcium.[3,4,5,6,7] Therefore, vitamin D may affect insulin secretion and insulin resistance, the two pathogenesis of type II diabetes. The prevalence of vitamin D deficiency in patients with diabetes and pre-diabetes is high.[7,8] As the treatment of vitamin D deficiency is cheap and safe, it may be an effective method in preventing diabetes.[9] Some cross-sectional and epidemiological studies have shown the role of vitamin D deficiency on increasing the incidence of diabetes.[10,11,12] However, the results of interventional studies with vitamin D on insulin sensitivity were inconstant.[13,14] The reports have shown a positive, no effect, or even a negative effect of vitamin D administration on insulin sensitivity.
In a previously done study in our center, injection of vitamin D to vitamin D deficient pre-diabetic patients, who were the first-degree relatives of type II diabetes mellitus (T2DM) patients, decreased the insulin sensitivity. However, in comparison with control group, it was not significant.[15] Various explanations for these conflicting results are noted, including the dosage, the route of prescription, and race. To examine the effect of the route of medication administration on insulin resistance, we investigated the effect of oral vitamin D on insulin resistance and sensitivity indexes and compared the results with intramuscular injection and control group.
MATERIALS AND METHODS
This study was conducted on pre-diabetic patients admitted to the Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, during May to November 2011. Pre-diabetic patients were individuals who participated in the research project “Does the intramuscular injection of vitamin D increase insulin resistance?”[15] At least 1 year had passed since the end of the previous study, and a new group of pre-diabetic patients was selected as control. Exclusion criteria included type I diabetes mellitus (T1DM) or T2DM based on the American Diabetes Association (ADA) 2011 criteria,[16] cigarette smoking, history of hyperparathyroidism, pregnancy, lactation, drugs (glucocorticoid, hydrochlorothiazide, bisphosphonate, metformin, and anticonvulsants), psychiatric illness, and a history of kidney disease. Written informed consent was obtained from all participants.
Anthropometric measurement was performed in participants with light clothing and shoes removed. Height in centimeters, weight in tenth of kilogram, and body mass index (BMI) calculated as weight in kilograms divided by the square of height in meters (kg/m2) were measured. Systolic and diastolic blood pressure was measured in the right arm with the patient in a sitting position after 5 min rest. Waist circumference was measured in standing position at the top of iliac crest in horizontal plane and in normal expiration.
Fasting morning blood was drawn for measurement of glucose, insulin, calcium, phosphate, albumin, HbA1c, and 25-hydroxyvitamin D [25(OH) D], and glucose tolerance test with 75 g glucose was performed. Blood samples were drawn after 30, 60, and 120 min for glucose and insulin measurement. Insulin was measured by Sandwich chemiluminescent immunoassay (DIAPLUS insulin-96 test; Diasorin, Saluggia, Italy). Intra-assay coefficient of variation was 2.9% and inter-assay coefficient of variation was 5.1%. Samples for vitamin D were frozen at −20°C, and after adequate sample size was obtained, vitamin D concentration was measured by direct competitive chemiluminescent immunoassay (IDS, Boldons, UK). This assay has 100% specificity for 25-hydroxyvitamin D3 and 75% specificity for 25-hydroxyvitamin D2. The intra-assay coefficient of variation was 5.6% for 67.1 nmol/l and inter-assay coefficient of variation was 6.4% for 72 nmol/l.
Pre-diabetic patients from the previous project had been randomized by using a random number table into three treated groups: Vitamin D (injection) alone, vitamin D (injection) and calcium intake, and calcium intake alone.[15] In our study, those patients who were eligible and still pre-diabetic according to baseline measurements were merged into two groups randomly. The first group was treated with a single intramuscular injection of 300,000 IU vitamin D3[17] (Iran hormone, Tehran, Iran), and the second and the third groups were treated with 50,000 IU oral vitamin D (Zahravi, Tabriz, Iran) weekly or every other week,[18] if serum vitamin D concentration was less or more than 75 nmol/l, respectively. A new group of pre-diabetic patients was selected as control on the basis of the exclusion criteria given above. Calcium carbonate 500 mg (Ramopharmin, Tehran, Iran) daily was given to all groups.[19,20] After 12 weeks, anthropometric and lab measurements were repeated and oral glucose test was performed.
Insulin sensitivity and resistance were calculated from Matsuda index and homeostasis model assessment of IR (HOMA-IR), respectively. The Matsuda index is defined as 10,000/square root of (fasting glucose × fasting insulin) × [mean glucose × mean insulin during oral glucose tolerance test (OGTT)].[21] HOMA-IR is defined as: (fasting glucose × fasting insulin)/22.5.[22] β-cell function was measured from HOMA-B, which is defined as 20 × fasting insulin/(fasting plasma glucose −3.5).[22] Area under the curve (AUC) glucose and AUCinsulin were calculated using the trapezoidal rule. Delta percent of changes from baseline were also analyzed.
Results were analyzed using SPSS software version 15. Changes in results between baseline and after intervention were tested with paired t-test and Wilcoxon signed ranks test for normally distributed or non-normally distributed continuous variables, respectively. For comparison between more than two groups, we used analysis of variance (ANOVA) test and Kruskal–Wallis test for normally or non-normally distributed variables, respectively. Correlation analysis was performed using Pearson's technique. P values less than 0.05 were considered statistically significant.
RESULTS
Forty-eight patients were enrolled, 31 patients from the previous project and 17 new pre-diabetic patients. Three of the patients did not participate in the follow-up. One in the control group, because of journey, and two refused to do OGTT (one in the control group and one in oral vitamin D treated group). However, 21 patients in oral vitamin D and calcium treated group (group A), 9 patients in vitamin D injection and calcium group (group B), and 15 patients in calcium treated group (group C) completed the study. Mean (SD) age was 47.4 ± 6.6 (range 33-61) years, and mean (SD) BMI and mean waist circumference were 29.4 ± 3.6 kg/m2 and 91.8 ± 8.4 cm, respectively. The mean of weight, BMI, and waist of patients before and after the intervention and their comparison among the three groups was not statistically significant [Table 1]. The serum concentration of vitamin D increased significantly after oral therapy with vitamin D (from 77.5 ± 39.2 to 118.8 ± 56.3 nmol/l, P = 0.009); however, the changes after intramuscular vitamin D or in the control group were not significant. Insulin sensitivity that was calculated by Matsuda Index decreased significantly after treatment with oral vitamin D (from 11.4 ± 3 to 9.9 ± 3.2, P = 0.046) and non-significantly after intramuscular vitamin D (from 11.1 ± 1.9 to 10.5 ± 2.4, P = 0.1) treatment. Change in control group was not significant (10.7 ± 3 to 10.8 ± 2.1, P = 0.7). Comparison of these changes among the three groups was not significant. Decrease in insulin resistance that was assessed by HOMA-IR was not significant in each group. The β-cell function (HOMA-B) increased non-significantly after oral and intramuscular vitamin D (P = 0.3 and P = 0.2, respectively). For patients in group A who had vitamin D less than 75 nmol/l (n = 15), the vitamin D level increased from 54 ± 13.1 to 114 ± 65.5 nmol/l (P = 0.006). In this subgroup, Matsuda index increased non-significantly (P = 0.1) (not shown in the table). Decrement in AUCglucose and AUCinsulin was not significant in each group. Changes in HbA1c, calcium, and phosphate were not significant in each group. Results of comparison between groups are shown in Table 2.
Table 1.
Demographic and anthropometric characteristics of pre-diabetic people (mean±SD)
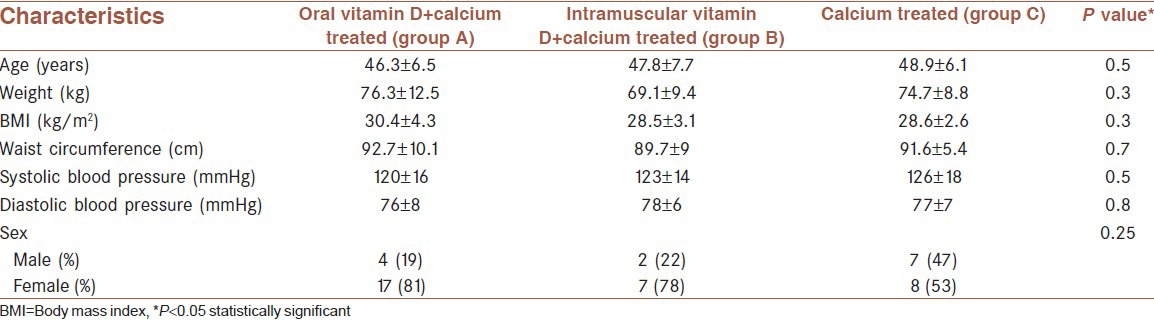
Table 2.
Different variables on admission and after 12 weeks follow-up in pre-diabetic people (mean±SD)
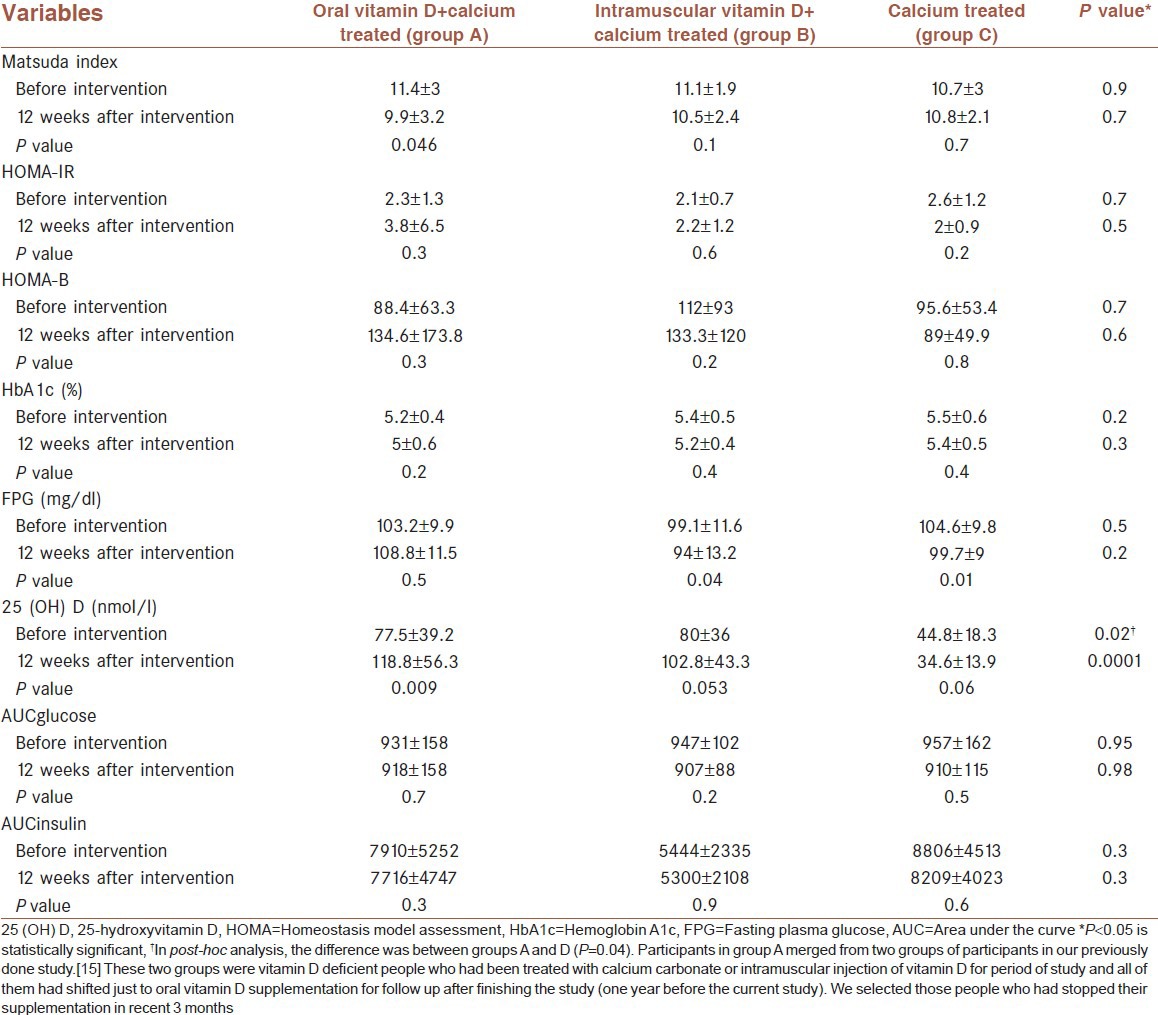
BMI was significantly higher (31.6 ± 4.6 vs. 28.4 ± 2.5 kg/m2, P = 0.049) in patients with baseline vitamin D < 50 nmol/l in comparison with subjects with vitamin D 50–75 nmol/l. However, correlation analysis did not show any relation between BMI and vitamin D concentration at baseline. BMI had direct relationship with HOMA-IR (r = 0.42 P = 0.002), and was inversely related to Matsuda index (r = −0.37, P = 0.009) and had correlation with AUCinsulin (r = 0.34, P = 0.021). These correlations persisted after treatment with vitamin D in all patients. There was no correlation between vitamin D and insulin sensitivity or insulin resistance indexes. Delta percent of changes from baseline were not statistically significant. There was no difference between men and women in our findings.
DISCUSSION
In our study, although insulin sensitivity was reduced after oral intake of vitamin D, in comparison with other groups, this reduction was not statistically significant. This finding is similar to the results of our previous study that was conducted in our center on pre-diabetic patients.[15] In that study, insulin sensitivity was reduced 2 months after the intramuscular injection of 300,000 IU vitamin D. However, in comparison with controls, this change was not significant. The present study did not show any difference between oral and intramuscular vitamin D on insulin resistance. It shows that the route of administration does not have a key role on the effect of vitamin D on insulin sensitivity.
Insulin sensitivity is measured from standard tests such as euglycemic clamp test or intravenous glucose tolerance test (IVGTT). Such techniques are difficult to do and time consuming. Thus, insulin sensitivity is calculated from fasting plasma glucose and insulin, or OGTT-based formulas standardized with euglycemic clamp test or IVGTT are routinely used. Studies that used euglycemic clamp test or IVGTT to measure insulin sensitivity after vitamin D treatment are few in number. Ljunghall et al.[23] investigated 65 vitamin D sufficient Caucasian men with impaired glucose tolerance using IVGTT. They prescribed oral, 0.75 μg/d of alphacalcidiol or placebo for 3 months. Insulin sensitivity did not change after treatment. Results of similar studies were consistent.[24,25]
Studies that measure fasting plasma glucose (HOMA-IR) primarily measure hepatic insulin resistance, and OGTT-derived indexes such as Matsuda measure total body insulin sensitivity. Studies with OGTT for calculating insulin sensitivity are also rare. Insulin sensitivity did not change in most of these studies. In Nagpal et al.'s[5] study, in 100 centrally obese Indian men, after 3 doses of oral 120,000 IU cholecalciferol fortnightly versus placebo, on 3-h oral glucose, the insulin sensitivity index (Mari's formula) was improved, whereas other oral glucose insulin sensitivity indexes remained unaffected. In comparison, subjects in our study were pre-diabetic and the first-degree relatives of T2DM patients and more obese (mean BMI 29.4 vs. 26.7 kg/m2). Correlation analysis showed persistence of relation of BMI with insulin resistance after vitamin D treatment. Risk factors for diabetes and insulin resistance in our study were more potent than in the Nagpal et al.'s study. Furthermore, insulin resistance calculated by HOMA-IR in our study and Nagpal et al.'s study showed this difference (2.3 ± 1.3 and 1.47 ± 1.16, respectively).
In our study, insulin resistance as assessed by HOMA-IR increased non-significantly. There are several studies that showed the administration of vitamin D does not improve HOMA-IR.[26,27] In von Hurst et al.'s[9] study, after 4000 IU vitamin D administration, serum 25(OH) D concentrations increased significantly from 21 nmol/l at baseline to 93 nmol/l at 3 months, and then declined to 80 nmol/l at 6 months. After 3 months, the insulin resistance improved non-significantly, but significant improvement was observed after 6 months of treatment. Improvement was seen in patients in whom vitamin D concentration was 80 nmol/l or more. This study showed sufficient dose and sufficient length of time are needed to enhance insulin sensitivity. In our study, we followed patients for only 3 months. In vitamin D deficient subgroup, insulin sensitivity was not improved with vitamin D treatment. Small sample size or short time of follow-up may be the cause of inconclusive results.
This study has some possible limitations. The small sample size may be a limiting factor, particularly in group B. However, in this study, we wanted to examine the effect of oral vitamin D on insulin sensitivity or resistance. We chose this group to make sure if the effect of intramuscular injection of vitamin D which had been observed in the previous study was seen again or not. Another limitation is that 12-week follow-up may be too short to determine the effect of vitamin D on insulin sensitivity or resistance. However, vitamin D reaches its equilibrium in this period.[9]
CONCLUSION
Oral vitamin D had no effect on insulin sensitivity or resistance in pre-diabetes patients who were the first-degree relatives of T2DM patients. A randomized double-blind study with a longer duration of treatment is suggested.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Majid Abyar for his technical help with the computer, and all the individuals who accepted our invitation and participated in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia. 1995;38:1239–45. doi: 10.1007/BF00422375. [DOI] [PubMed] [Google Scholar]
- 2.Orwoll E, Riddle M, Prince M. Effects of vitamin D on insulin and glucagon secretion in non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1994;59:1083–7. doi: 10.1093/ajcn/59.5.1083. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, Davies M, Zakaria Y, Mawer EB, Gordon C, Olukoga AO, et al. Improvement in glucose tolerance and beta-cell function in a patient with vitamin D deficiency during treatment with vitamin D. Postgrad Med J. 1994;70:440–3. doi: 10.1136/pgmj.70.824.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak RH. 1,25-Dihydroxyvitamin D3 corrects insulin and lipid abnormalities in uremia. Kidney Int. 1998;53:1353–7. doi: 10.1046/j.1523-1755.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 5.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 6.Pinelli NR, Jaber LA, Brown MB, Herman WH. Serum 25-hydroxy vitamin D and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care. 2010;33:1373–5. doi: 10.2337/dc09-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract. 1995;27:181–8. doi: 10.1016/0168-8227(95)01040-k. [DOI] [PubMed] [Google Scholar]
- 8.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–8. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 9.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient-a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–55. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 10.Pittas AG, Dawson-Hughes B, Li T, van Dam RM, Willett WC, Manson JE, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–6. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 11.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective Study 1990-2000. Diabetes. 2008;57:2619–25. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knekt P, Laaksonen M, Mattila C, Härkänen T, Marniemi J, Heliövaara M, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666–71. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 13.Parekh D, Sarathi V, Shivane VK, Bandgar TR, Menon PS, Shah NS. Pilot study to evaluate the effect of short-term improvement in vitamin D status on glucose tolerance in patients with type 2 diabetes mellitus. Endocr Pract. 2010;16:600–8. doi: 10.4158/EP09300.OR. [DOI] [PubMed] [Google Scholar]
- 14.Taylor AV, Wise PH. Vitamin D replacement in Asians with diabetes may increase insulin resistance. Postgrad Med J. 1998;74:365–6. doi: 10.1136/pgmj.74.872.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iraj B, Aminorroaya A, Amini M. Does the intramuscular injection of vitamin D increase insulin resistance? J Res Pharm Pract. doi: 10.4103/2279-042X.108372. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care. 2011;34:S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heikinheimo RJ, Inkovaara JA, Harju EJ, Haavisto MV, Kaarela RH, Kataja JM, et al. Annual injection of vitamin D and fractures of aged bones. Calcif Tissue Int. 1992;51:105–10. doi: 10.1007/BF00298497. [DOI] [PubMed] [Google Scholar]
- 18.Pietras SM, Obayan BK, Cai MH, Holick MF. Vitamin D2 treatment for vitamin D deficiency and insufficiency for up to 6 years. Arch Intern Med. 2009;169:1806–8. doi: 10.1001/archinternmed.2009.361. [DOI] [PubMed] [Google Scholar]
- 19.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–6. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 20.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–6. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Ljunghall S, Lind L, Lithell H, Skarfors E, Selinus I, Sørensen OH, et al. Treatment with one-alpha-hydroxycholecalciferol in middle-aged men with impaired glucose tolerance-a prospective randomized double-blind study. Acta Med Scand. 1987;222:361–7. doi: 10.1111/j.0954-6820.1987.tb10684.x. [DOI] [PubMed] [Google Scholar]
- 24.Fliser D, Stefanski A, Franek E, Fode P, Gudarzi A, Ritz E. No effect of calcitriol on insulin-mediated glucose uptake in healthy subjects. Eur J Clin Invest. 1997;27:629–33. doi: 10.1046/j.1365-2362.1997.1520699.x. [DOI] [PubMed] [Google Scholar]
- 25.Lind L, Pollare T, Hvarfner A, Lithell H, Sørensen OH, Ljunghall S. Long-term treatment with active vitamin D (alphacalcidol) in middle-aged men with impaired glucose tolerance. Effects on insulin secretion and sensitivity, glucose tolerance and blood pressure. Diabetes Res. 1989;11:141–7. [PubMed] [Google Scholar]
- 26.Kaviani M, Abdollahian M, Almasi V, Amini M, Yamini AA. Effects of vitamin D on insulin resistance in nursing home residents: An interventional study. Endokrynol Pol. 2012;63:191–5. [PubMed] [Google Scholar]
- 27.Tai K, Need AG, Horowitz M, Chapman IM. Glucose tolerance and vitamin D: Effects of treating vitamin D deficiency. Nutrition. 2008;24:950–6. doi: 10.1016/j.nut.2008.04.009. [DOI] [PubMed] [Google Scholar]


