Abstract
Background:
Highly active antiretroviral therapy (HAART) has become more accessible to Human immunodeficiency virus infection/Acquired Immunodeficiency Syndrome (HIV/AIDS) patients worldwide. There is growing concern that the metabolic complications associated with HIV and HAART may increase cardiovascular risk and lead to cardiovascular diseases. We, therefore, set out to describe the cardiovascular risk profile of HIV/AIDS patients receiving HAART at a health facility in northern part of Nigeria.
Materials and Methods:
This cross-sectional study was conducted at the Aminu Kano Teaching Hospital, Kano, Nigeria. Consenting patients, who had been receiving HAART, were compared with age and sex matched HAART-naive subjects. Questionnaire interview, electrocardiography, anthropometric and blood pressure measurements were conducted under standard conditions. Blood samples were obtained for the determination of plasma glucose, uric acid and lipid levels.
Results:
Two hundred subjects were studied, 100 were on HAART (group 1) and the other 100 (group 2) were HAART-naive. Subjects’ mean age for all the participants was 32.5 (7.6) years. The prevalence of hypertension was 17% in group 1 and 2% in group 2 (P < 0.001). Similarly, 11% and 21% of group 1 subjects were obese or had metabolic syndrome compared with 2% and 9% of group 2 patients (P < 0.05 for both).
Conclusion:
HAART treatment was associated with significantly higher prevalences of hypertension, obesity and metabolic syndrome.
Keywords: Cardiovascular risk factors, highly active antiretroviral therapy, human immunodeficiency virus infection/Acquired Immunodeficiency Syndrome, metabolic syndrome, Nigeria
INTRODUCTION
Worldwide, the estimated number of persons living with the human immunodeficiency virus (HIV) in 2007 was 33.2 million.1 Sub-Saharan Africa remains the most affected by the global acquired immunodeficiency syndrome (AIDS) pandemic; more than two-thirds (68%) of all people living with HIV are in this region. Also, more than three-quarters of AIDS deaths in 2007 occurred in sub-Saharan Africa. The HIV epidemic in Nigeria is believed to have started in the 1980s. The first AIDS case in Nigeria was reported in 1986. At the end of 2006, the prevalence HIV/AIDS in Nigeria was estimated to be 4.4%.2 Untreated HIV infections usually progresses to AIDS. Recent advances in HIV therapy have enabled patients to live longer. Thus, individuals affected by HIV are more likely to develop long term complications of HIV/AIDS including cardiovascular (CV) complications. The introduction of highly active antiretroviral therapy (HAART) in the mid to late 1990s dramatically reduced HIV-associated morbidity and mortality in treated patients, so that they no longer inevitably succumb to opportunistic infections.3,4 The increasing accessibility of antiretroviral drugs in resource poor countries has led to a significant reduction in morbidity and mortality.
Side effects and toxicities are associated with these highly effective therapies. There is growing concern that the metabolic complications associated with HIV and antiretroviral therapy may lead to an increased risk for CV diseases.5,6 Following the introduction of HAART, studies reporting treatment related side-effects such as hyperlipidaemia, fat redistribution, insulin resistance and high blood pressure began to appear in the literature.
Some of these complications are also components of metabolic syndrome, which constitute a CV disease risk in the general population. Studies have reported higher prevalence of metabolic syndrome among HIV positive patients on HAART than in HIV negatives.7,8
Several studies on CV risk factors among HIV/AIDS patients on HAART have been reported in the western literatures but there is paucity of information on this subject in sub-Saharan Africa, which has the greatest HIV/AIDS burden and increasing access to HAART. Identification of these risk factors and appropriate intervention where necessary would allow assessment of impact of HAART on CV risk.
This study aimed to evaluate the CV risks profile of HIV/AIDS patients at a health facility in Kano, Nigeria. Our objectives were to compare CV risk factors burden among HAART-treated and HAART-naïve subjects.
MATERIALS AND METHODS
This was a cross-sectional study carried out at the HIV specialty clinic of Aminu Kano Teaching Hospital (AKTH), between May and August 2009. The study involved two groups of patients. Group 1 included HIV positive patients attending HIV specialty clinic who had been receiving HAART for at least 6 months. The Control group (group 2) comprised age and sex matched HIV positive patients who had indication for HAART but had not commenced, either because they are on a waiting list or they are undergoing investigations before commencing HAART. We excluded patients less than 18-years-old, those on HAART for less than 6 months, pregnant and lactating women, those with documented hypertension, diabetes and dyslipidaemia before commencing HAART, terminally ill patients and those who declined consent. Patients, who met the inclusion criteria, were recruited consecutively.
Group 1 patients were recruited during their routine follow up while group 2 patients were recruited during their initial visit. HAART was defined as a combination of at least three classes of antiretroviral drugs, namely protease inhibitors (PIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs) and nucleoside reverse transcriptase inhibitors (NRTIs), one of which was a PI or an NNRTI or a triple combination of (NRTIs).
Informed consent was obtained from each patient and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the AKTH Ethics committee. Data were collected using a pre-tested interviewer-administered questionnaire. History was obtained and thorough physical examination performed including measurement of blood pressure, palpation of pulses and anthropometry. Height was measured with a stadiometer with the patients standing erect on a flat surface without shoes or headgear. Weight was recorded in kilograms using a standard weighing scale on a firm horizontal surface with patients wearing light clothing.
Twelve lead surface electrocardiogram (ECG) will recorded for all patients in the ECG laboratory of AKTH using Schiller ECG machine in the standard fashion. Body mass index (BMI) and waist-hip ratio were calculated using the standard formulae. Venous blood was taken after a 10 hour overnight fast for the determination of fasting blood glucose (FBG), lipid profile and uric acid. Electrocardiography, liver function tests, CD4 cell counts and urinalysis were also conducted.
Hypertension was identified when the average of two blood pressure (BP) measurements was equal to or greater than 140/90 mmHg. Diabetes Mellitus (DM) and impaired fasting glycaemia (IFG) were defined using the American Diabetes Association (ADA)9 guidelines as follows: DM-Fasting plasma glucose ≥126 mmg/dl (7 mmol/l) or Random plasma glucose of ≥200 mg/dl (11.1 mmol/l) and IFG-Fasting plasma glucose (6.0-6.9 mmol/l). Dyslipidaemia and metabolic syndrome were defined using the National Cholesterol Education Programme (NCEP) adult treatment panel (ATP) III guidelines.10 Obesity was defined with the BMI using the World Health Organisation (WHO) classification.11 Patients were said to have increased waist circumference (WC) according to the NCEP ATP III guidelines: ≥102 cm for males and ≥88 cm for females, and waist hip ratio (WHR) was said to be abnormal if >0.9 for men and >0.85 for females. left ventricular hypertrophy (LVH) was assessed using the Lyon-Sokolow criteria.12
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 16.0. Analysis of continuous variables was carried out using the procedures of descriptive statistics and later, to identify any differences we used Student's “t” test. Continuous variables were analysed with descriptive statistics and compared using Student's t-tests. Categorical variables were analysed using contingency tables involving Chi-square (χ2) tests to identify statistical differences between the groups. The relationship between HAART and CV risk profile was determined using logistic regression analysis. For all statistical test P < 0.05 was considered significant.
RESULTS
Two hundred subjects were studied; 100 were on HAART (group 1) and the other 100 (group 2) were HAART-naive. Participants’ age ranged from 20 to 50 years while their mean age was 32.5 ± 7.55 years. The corresponding age range for group 1 and group 2 were 20 to 50 years and 20 to 48 years while the mean ages were 32.81 ± 7.63 years and 32.36 ± 7.50 years, respectively (t = 0.421, P = 0.675). Sixty-four percent of the respondents (n = 128) were aged between 20 and 34 years. Only 21.5% (43) of the respondents were above the age of 40 years. The proportions of females were 54% and 52% in group 1 and group 2, respectively. The duration of HIV diagnosis ranged from <1 year to 12 years for group 1 and about 99% of them were diagnosed more than 1 year ago. For those in group 2, duration of HIV diagnosis was from a month to 6 years; about 76% were diagnosed less than a year ago.
Figure 1 show the various HAART regimens. All the subjects on treatment (group 1) were on a backbone of either lamivudine (3TC) 78% or emtricitavine (FTC) 22% which are essentially similar drugs. Fifty-four percent were on zidovudine based (AZT), while 23% each were on stavudine (D4T) or tenoforvir-based regimens (TDF). Ninety percent of them were on nevirapine (NVP) as the third agent in the HAART, 9% on effaviranz (EFV), only a single participant was on lopinavir (LPVr). The duration of treatment ranged from 6-84 months.
Figure 1.
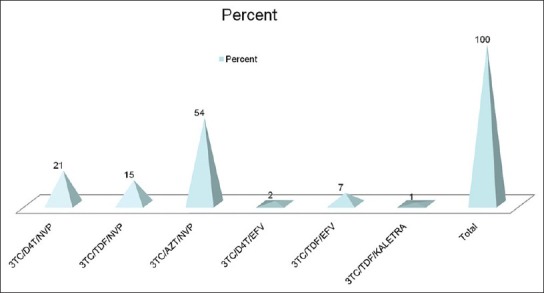
Types of HAART regimen among group 1 respondents 3TC – lamivudine; FTC – emtricitavine; AZT – zidovudine; D4T – stavudine; TDF – tenoforvir; NVP – nevirapine; EFV – effaviranz; LPVr – ritonovir boosted lopinavir
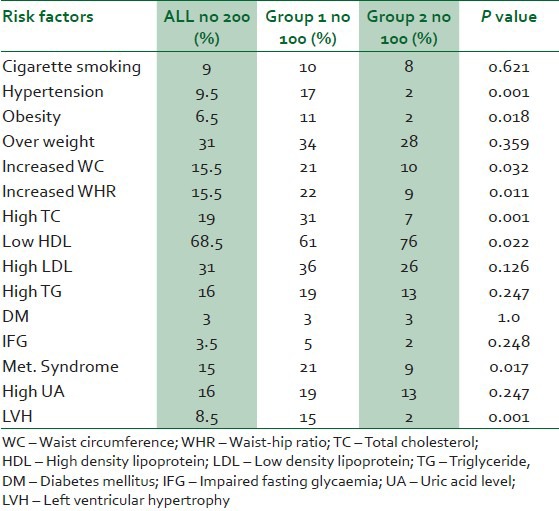
Table 1 shows the baseline clinical and laboratory parameters of the study subjects. The proportions of CV risk factors in the 2 groups are shown in Table 2. Hypertension was documented among 17% and 2% among groups 1 and 2 subjects respectively (P < 0.001). Among the hypertensives in group 1, seven were on stavudine based, six on zidovudine based and four on tenoforvir-based regimens. Hypertension was predicted by increased BMI (OR 3.69, 95% CI 5.75-280.68) and longer the duration on HAART (OR 1.37, 95% CI: 1.12-5.57). Hypercholesterolaemia was found among 31% of the subjects in group 1 and 7% among subjects in group 2 (P < 0.001). The prevalence of metabolic syndrome among group 1 and 2 were 21% and 9%, respectively (P = 0.017). Of the 21% of group 1 with metabolic syndrome 9%, 7% and 5% were on zidovudine, satavudine and tenoforvir-based regimens. Eleven percent of group 1 and 2% of group 2 were obese (P = 0.018). Thirty-four percent and 28%, respectively, were overweight (P = 0.359). There was statistically significant increase in BMI with increasing age (OR-0.964, 95% CI: 1.016-6.217), but no significant association was seen between increase in BMI and type or duration of HAART.
Table 1.
Clinical and laboratory characteristics of the subjects
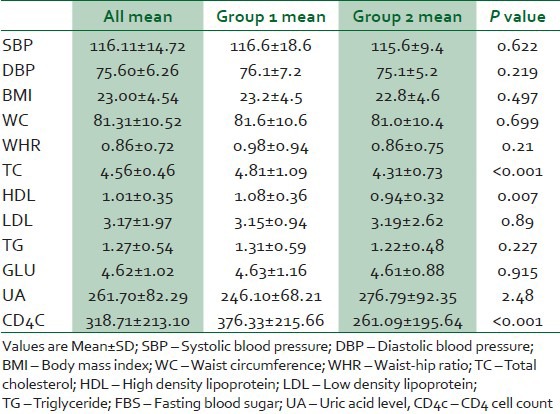
Table 2.
Proportion of cardiovascular disease risk factors and metabolic syndrome in the two groups
DISCUSSION
This study found a significantly higher burden of some CV disease risk factors (viz: Hypertension, hypercholesterolaemia, obesity and metabolic syndrome) among HAART-treated HIV patients than their HAART-naïve counterparts. Trends in published research findings on this subject have shown a mixture of association and lack of association between HAART use and CV risks.
Gazzaruso et al., compared the prevalence of hypertension among 287 HIV positive patients receiving HAART to 287 age- and gender-matched controls in relation to the metabolic syndrome, they found an increased prevalence of hypertension (34.2% vs 11.9%) and metabolic syndrome (33.1% vs 2.4%), in the patients on HAART.13 In contrast, Bergensen et al.,14 compared the prevalence of hypertension in HIV-infected patients’ naive to HAART, those receiving HAART and HIV-negative controls. They found no statistical difference across three groups. Similarly, a Kenyan study found no difference in the prevalence of hypertension between HIV positive subjects on HAART and those who were HAART naive.15 The finding of a significant difference in the prevalence of hypertension in our study might be as a result of muscle wasting and dehydration in the untreated patients. Some workers have observed that in untreated patients, systolic BP (SBP) is reduced progressively in HIV infection.16 Additionally, it has been postulated that untreated HIV disease may tend to lower BP, while normalisation of immune status and suppression of HIV replication with potent antiretroviral combinations elevate BP.17 Whether continued treatment with HAART results in an increased incidence of hypertension or a normalisation of BP from untreated disease is unknown.
In our study SBP correlated with waist-to-hip ratio, which is a marker metabolic syndrome and is associated with HAART use. The chances of having hypertension in this study were predicted by increasing BMI and longer duration of HAART. Increasing BMI has been shown to be a predictor of hypertension among patients on HAART by other investigators.18,19 Also, Seaberg et al., found the duration of HAART to be a predictor of hypertension. In their study, the prevalences of systolic and diastolic hypertension were found to be non-significantly different between patients who had received HAART for less than 2 years and HIV-negative controls.20 However, our study suggests that HAART predisposes to development of high blood pressure in patients who had treatment for 6 months or longer. It is important to note that the fairly high prevalence of hypertension (25-35% among adults) among the general population in the study area implies that exposure to HAART may be unmasking a pre-existing predisposition and may not be a direct cause.
The finding of hypercholesterolaemia is similar to previous findings of Manuthu et al., who found prevalences of elevated total cholesterol (TC) to be 39.2% and 10% among HAART-treated and HAART-naïve subjects.15 In this study, the prevalence of hypertriglyceridaemia was not statistically different between the two groups. This is concordant with the findings of Manuthu et al.15 The high prevalence of low High density lipoprotein cholesterol (HDL-C) among HAART-naïve subjects may be as a result altered lipid metabolism which is known to occur in association with HIV infection. An earlier publication by Riddler et al., suggested that HIV seroconversion resulted in reductions in total cholesterol, low-density lipoprotein cholesterol (LDL-C), and HDL-C levels and that initiation of HAART increased both TC and LDL-C levels but not HDL-C.21 Some studies suggest that effavirenz usage is associated with the development of increased TC levels, largely due to beneficial increases in HDL levels.22 Ninety-nine percent of the subjects on HAART arm of this study were on non-nucleoside reverse transcriptase inhibitors (NNRTI). This class of anti-retroviral (ARVs) was shown to raise HDL-C level substantially as immunological status improves which may provide a potential long term beneficial effect on CV risk profile.22 This finding suggests that NNRTIs could have beneficial effects on lipids profile in addition to reducing viral replication and improving immunological status.
There was higher prevalence of obesity (BMI >30 Kg/m2) and increased WC among subjects that were on HAART. This contrast the findings from Data Collection on Adverse Events of Anti-HIV Drugs (D: A: D) study were HAART-naïve subjects tended to be obese compared with HAART-treated subjects.23 Lipodystrophy with lipoatrophy might have contributed to this finding in DAD study. However, we did not record any case of lipodystrophy in our study. Additionally, this disparity may be partially explained by malnutrition, opportunistic infections like tuberculosis and the mode of presentations of our patients where presentation is usually late with AIDS-defining illness such as the HIV wasting syndrome.
We found no statistically significant difference between the prevalence of DM in the two groups. This is in agreement with findings of previous studies. Our finding of a 3% prevalence of DM among HAART recipients is comparable with the 2.5% reported in the DAD study. While it is still unclear if HIV increases the risk of insulin resistance, factors attributed to this association include use of PIs, which have been found to induce insulin resistance over a short treatment course.24
The overall prevalence of metabolic syndrome in our study was 15%. This was similar to the 17% prevalence of metabolic syndrome among Spanish HIV positives subjects.7 Metabolic syndrome was higher among subjects who were on HAART compared to HAART naive subjects. Several studies have estimated the prevalence of metabolic syndrome among HIV-positive HAART recipients. Reported prevalences have ranged from 14% to 25%.25,26 While some workers reported metabolic syndrome prevalence was similar to that of control groups, others reported it to be higher among HAART recipients.26 Jacobson et al., noted specific anthropometric limitations to the metabolic syndrome definitions when applied to HIV-infected HAART recipients.27 WCs were lower in HIV-infected HAART recipients compared with the uninfected population despite similar prevalence rates for metabolic syndrome.28 As such metabolic syndrome definitions may not be sufficiently sensitive for HIV-infected HAART recipients.
Metabolic syndrome in HIV-infected HAART recipients likely represents the synergy of drug effects on glucose and lipid metabolism, HIV/drug-induced impairment of adipose tissue function, underlying genetic predisposition to metabolic disease and lifestyle factors.7
While most classes of ARV drugs used in HAART could increase CV risk, the effect is mostly seen with regimens containing PIs. The fact that only one patient in this study had a PI containing regimen may suggest that the effect of HAART on the risk factors could have been underestimated. The comparison done was mainly between stavudine, tenoforvir and zidovudine, which are all NRTIs. Despite this, the study result has shown that non PI-containing HAART regimens, which are mostly used in sub-Saharan Africa also significantly, affect the CV risk factors.
CONCLUSION
This study showed that exposure to HAART whilst improving the immune status of patients, predispose them to development of hypertension, dyslipidaemia and metabolic syndrome. Thus, there is need for CV risk factors assessment before initiation of HAART followed by periodic monitoring while on the treatment. This will ensure prompt detection and management of CV risk factors and the prevention of CV events.
ACKNOWLEDGMENT
We are grateful to the staff of SS Wali Center for Antiretroviral Treatment, Aminu Kano Teaching Hospital, Kano, for the assistance provided to the investigators during the study. We also thank the staff of the Department of Chemical Pathology who helped with the laboratory analysis. Finally, we thank all the patients who agreed to participate in the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.WHO AIDS epidemic Update 2007. [Last accessed on 2012 Sep 30]. Available from: http://www.who.int/hiv/pub/epidemiology/epiupdate2007/en .
- 2.National HIV/Syphilis Sero-prevalence sentinel survey among pregnant women attending antenatal clinics. Nigeria: Federal Ministry of Health; Technical Report 2005. [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–30. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 5.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia, and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Autran B, Gorin I, Leibowitch M, Laroche L, Escande JP, Hewitt J, et al. AIDS in a Haitian woman with cardiac Kaposi's sarcoma and Whipple's disease. Lancet. 1983;1:767–8. doi: 10.1016/s0140-6736(83)92058-5. [DOI] [PubMed] [Google Scholar]
- 7.Jerico C, Knobel H, Montero M, Ordoñez-Llanos J, Guelar A, Gimeno JL, et al. Metabolic syndrome among HIV-infected patients: Prevalence, characteristics, and related factors. Diabetes Care. 2005;28:132–7. doi: 10.2337/diacare.28.1.132. [DOI] [PubMed] [Google Scholar]
- 8.Hadigan C, Meigs JB, Corcoran C, Rietschel P, Piecuch S, Basgoz N, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystropy. Clin Infect Dis. 2001;32:130–9. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 9.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 10.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 11.Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 12.Sokolow M, Lyon TP. Criteria for the diagnosis of right ventricular hypertrophy using unipolar limb and precordial leads. Am J Med. 1947;3:125. doi: 10.1016/0002-9343(47)90136-8. [DOI] [PubMed] [Google Scholar]
- 13.Gazzaruso C, Bruno R, Garzaniti A, Giordanetti S, Fratino P, Sacchi P, et al. Hypertension among HIV patients: Prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2003;21:1377–82. doi: 10.1097/01.hjh.0000059071.43904.dc. [DOI] [PubMed] [Google Scholar]
- 14.Bergersen BM, Sandvik L, Dunlop O, Birkeland K, Bruun JN. Prevalence of hypertension in HIV-positive patients on highly active retroviral therapy (HAART) compared with HAART-naive and HIV-negative controls: Results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infec Dis. 2003;22:731–6. doi: 10.1007/s10096-003-1034-z. [DOI] [PubMed] [Google Scholar]
- 15.Manuthu EM, Joshi MD, Lule GN, Karari E. Prevalence of dyslipidemia and dysglycaemia in HIV infected patients. East Afr Med J. 2008;85:10–7. doi: 10.4314/eamj.v85i1.9600. [DOI] [PubMed] [Google Scholar]
- 16.Kelly P, Zulu I, Amadi B, Munkanta M, Banda J, Rodrigues LC, et al. Morbidity and nutritional impairment in relation to CD4 count in a Zambian population with high HIV prevalence. Acta Trop. 2002;83:151–8. doi: 10.1016/s0001-706x(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 17.Chow DC, Souza SA, Chen R, Richmond-Crum SM, Grandinetti A, Shikuma C. Elevated blood pressure in HIV-infected individuals receiving highly active antiretroviral therapy. HIV Clin Trials. 2003;4:411–6. doi: 10.1310/5E7Q-PGWB-16UE-J48U. [DOI] [PubMed] [Google Scholar]
- 18.Sattler FR, Qian D, Louie S, Johnson D, Briggs W, DeQuattro V, et al. Elevated blood pressure in subjects with lipodystrophy. AIDS. 2001;15:2001–10. doi: 10.1097/00002030-200110190-00013. [DOI] [PubMed] [Google Scholar]
- 19.Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS. 2006;20:1019–26. doi: 10.1097/01.aids.0000222074.45372.00. [DOI] [PubMed] [Google Scholar]
- 20.Seaberg EC, Munoz A, Lu M, Detels R, Margolick JB, Riddler SA, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–60. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 21.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–82. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 22.Negredo E, Cruz L, Ruiz L, Gel S, Johnson S, Fumaz CR, et al. Program and abstracts of the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy. Toronto: 2000. Impact of switching from PI to NVP or EFV in patients with viral suppression; pp. 17–20. [Google Scholar]
- 23.Friis-Moller N, Weber R, Reiss P, Thiebaut R, Kirk O, d’Arminio Monforte A, et al. Cardiovascular disease risk factors in HIV patients–association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 24.Dube MP, Sprecher D, Henry WK, Aberg JA, Torriani FJ, Hodis HN, et al. Preliminary guidelines for the evaluation and management of dyslipidaemia in adults infected with human immunodeficiency virus and receiving antiretroviral therapy: Recommendations of the Adult AIDS Clinical Trial Group Cardiovascular Disease Focus Group. Clin Infect Dis. 2000;31:1216–24. doi: 10.1086/317429. [DOI] [PubMed] [Google Scholar]
- 25.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: A cohort study. Lancet. 1999;353:2093–9. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 26.Wand H, Calmy A, Carey DL, Samaras K, Carr A, Law MG, et al. Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS. 2007;21:2445–53. doi: 10.1097/QAD.0b013e3282efad32. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson DL, Tang AM, Spiegelman D, Thomas AM, Skinner S, Gorbach SL, et al. Incidence of metabolic syndrome in a cohort of HIV-infected adults and prevalence relative to the US population (National Health and Nutrition Examination Survey) J Acquir Immune Defic Syndr. 2006;43:458–66. doi: 10.1097/01.qai.0000243093.34652.41. [DOI] [PubMed] [Google Scholar]
- 28.Bonfanti P, Giannattasio C, Ricci E, Facchetti R, Rosella E, Franzetti M, et al. HIV and metabolic syndrome: A comparison with the general population. J Acquir Immune Defic Syndr. 2007;45:426–31. doi: 10.1097/QAI.0b013e318074ef83. [DOI] [PubMed] [Google Scholar]


