Abstract
Background:
Helicobacter pylori infection is a basic risk factor for chronic gastritis, and gastric carcinoma. Based on some studies, the reason is binding of H. pylori to H and Leb antigens in gastric mucosa. However, some other findings have not determined any association between the infection and these antigens. Because of this controversy and the fact that H. pylori infection and gastric adenocarcinoma are common diseases in Iran, the assessment of the association of H. pylori infection with these blood groups could be valuable.
Materials and Methods:
In a cross sectional study on 135 adult dyspeptic patients in Mashhad, Iran, from 2009 to 2010, H. pylori infection was evaluated by using the Heliprobe 14C-urea breath test and the ABO and Lewis blood group antigens were determined by the tube method. Association between the Lewis and ABO phenotypes with H. pylori infection were analysed by Fisher's exact test. A P ≤ 0.05 was considered to be significant.
Results:
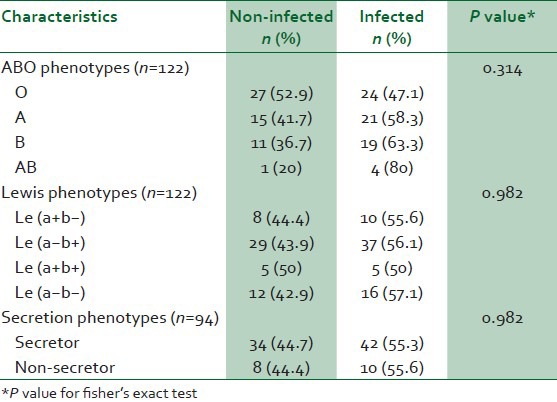
68 (50.4%) patients were positive for H. pylori infection. The frequencies of the ABO, Lewis and secretion phenotypes were not significant in the infected and non-infected patients. We also did not find a significant association between Lea and Leb antigens and this infection.
Conclusion:
We could not establish a significant association between the Lewis, ABO and secretion phenotypes with H. pylori infection. Diversity of sequences of blood group antigen b-binding adhesion (babA gene) of H. pylori may be a reason why our findings are different from other studies in other geographic areas.
Keywords: ABO blood groups, gastritis, Helicobacter pylori, Lewis blood group, secretor blood group
INTRODUCTION
Helicobacter pylori (H. Pylori) infection is high prevalence particularly in underdeveloped and developing countries.1,2,3,4 It affects about 90% of adult persons in some countries.5 In Iran, prevalence of the infection in adults is estimated to be about 70-80%.6,7 The infection is a basic risk factor for chronic gastritis, peptic ulcer and gastric adenocarcinoma,8,9,10,11,12 which is the most common malignancy in north and northwest Iran.7
H. pylori has several lipopolysaccharides such as O antigen on its outer membrane expressing Lea and Leb antigens. The Lewis antigen expression on the membrane of H. pylori for antigenic mimicry may create persistent colonisation and surviving of bacteria in the stomach mucosa. In addition, expression of Leb antigens in gastric mucosa may play as a receptor for bacterial adhesion. It seems blood group antigen b-binding adhesion (babA) on the outer membrane of H. pylori has a major role in persistent colonisation of the bacteria with attachment to Leb antigens of gastric mucosa.13,14 Binding of H. pylori to H and Leb antigens in gastric mucosa probably describes higher incidence of chronic gastritis and gastric adenocarcinoma in O blood group phenotype and secretors (expressing Leb antigen).15,16
Some other reports, however, have not determined any association between the infection and the Lewis5 and ABO blood groups.17 Some heterogeneity has been characterised in expression of the outer membrane protein, especially babA, that describes various capacities of different H. pylori for adhesion to Leb antigen on the gastric mucosa, a factor determining some differences in clinical outcomes of the infection.18,19 Based on controversial associations between H. pylori infection and these blood groups and the fact that H. pylori infection and gastric adenocarcinoma are the frequent diseases in developing countries, the assessment of the association of the infection with the blood groups would be valuable.
MATERIALS AND METHODS
The study was ethically approved by Vice President for research and the local ethical committee in Mashhad University of Medical Sciences, Iran. In a cross-sectional study from 2009 to 2010, we evaluated adult dyspeptic patients referred to the nuclear medicine laboratory of Imam Reza hospital (a major teaching hospital located in Mashhad, northeast Iran) from various gastrointestinal clinics for 14C-urea breath test (UBT). Based on medical history, patients who had received H2 receptor antagonists, proton-pump inhibitors and antibiotics within the last 4 weeks of conducting the study, were excluded to avoid false negative results. We also excluded pregnant or breast feeding women from the study. After exclusions, first a UBT was performed and then the blood ABO, Rh (D) and Lewis phenotypes of the remaining 135 patients were determined.
Current infection with H. pylori was detected by using the Heliprobe system 14C-UBT (Kibion-sweden™). After 10-20 minutes of ingestion of a capsule containing 14C-urea, the patients exhaled into the Breath Card until a pH-sensitive indicator altered color from orange to yellow because of CO2 saturation of the pads. Then, the BreathCards were put into a Heliprobe analyser and the radioactivities of the samples were measured. Finally, the results were mentioned in a numerical order: 0 as not infected (radioactivity as count per minute (CPM) <25, 1 as borderline result (25-50 CPM) and 2 as infected (>50 CPM). Borderline results were disregarded in comparative analysis.
After getting written informed consent, 2 mL blood for blood group typing was taken. ABO, Rh (D) and Lewis phenotypes were typed by commercial antibodies using direct tube agglutination test according to manufacturer's instructions (Biotest™, Germany). Based on expression or non-expression of Leb antigen, subjects were divided to secretor and non-secretor groups, respectively. Secretion status cannot be inferred from Le (a−b−) phenotype (n = 33); therefore, it was ignored in the evaluation of secretion phenotype.15
Statistical analysis
Descriptive analysis of the demographic features, the frequencies of ABO, Lea and Leb antigens, the Lewis phenotypes and H. pylori infection were performed. Association of the Lewis antigens and the Lewis and ABO phenotypes with H. pylori infection was analysed by Fisher's exact test, and means of continuous variables were compared with independent sample t-test. A P ≤ 0.05 was considered as a significant level. All data were analysed by Statistical Package for the Social Sciences (SPSS) software (version 11.5).
RESULTS
We studied 135 dyspeptic patients including 59 (43.7%) males and 76 (56.3%) females with an age range of 18-78 years and a mean (±SD) of 40 (±13) years. Out of all cases, 68 (50.4%) patients were positive for H. pylori infection, 54 (40%) patients were negative and 13 (9.6%) patients had borderline results. We didn't find a significant association between age and H. pylori infection (P = 0.666); however, the infection was more common in females than males (P = 0.024). The most common phenotype in the ABO groups was O (41.5%) followed by A (28.9%), B (25.2%) and AB (4.4%) and the most common phenotype in the Lewis groups was Le (a−b+) (53.4%) followed by Le (a−b−) (24.4%), Le (a+b−) (14.1%) and Le (a+b+) (8.1%).
As shown in Table 1, the frequencies of the ABO, Lewis and secretion phenotypes did not show a significant difference between infected and non-infected patients. We also did not find a significant difference between Lea and Leb antigens with the infection (P = 0.830).
Table 1.
Distribution of ABO, Lewis and secretion phenotypes between two groups (infected and non-infected by H. pylori)
DISCUSSION
There are many examples showing importance of blood groups in pathogenesis of some diseases. The role of blood groups in pathogenesis of norovirus, cholera and malaria infection is only a few examples.20 For the expression of ABH antigens, the first step is the synthesis of the H or group O antigen and then glycosyltransferases synthesise A and B antigens. Synthesis of the Lewis antigens is biochemically related to ABO antigens. The secretor gene (Se) express a fucosyltransferase II adding fucose to the terminal galactose of the type 1 precursor chain and forms type 1 H chain. Fucosyltransferase type III, the product of the Lewis gene, adds fucose to type 1 precursor chains and forms the Lea antigen or type 1 H chain and forms the Leb antigen. As a result, subjects lacking the Se gene, non-secretors, can't form type 1H chain and antigens derived from it (Leb).11,12,17 Hence, non-secretors can only form the Lea antigen.20,21
Diagnosis of H. pylori can be made by various invasive methods such as biopsy for a rapid urease test, culture and polymerase chain reaction and non-invasive methods such as serum H. pylori IgG antibody titer and UBT. The 14C-UBT (Heliprobe system) is an easy and rapid way with sensitivity of 94% and specificity of 100% for diagnosis of H. pylori infection in patients with dyspeptic complaints.22,23
Many studies have shown adherence of H. pylori to H and Leb antigens (secretors) in gastric mucosa and babA on the outer membrane of H. pylori mediates adherence of H. pylori to Leb antigens expressed on the mucosa.13,24,25 This may cause high incidence of chronic gastritis and gastric adenocarcinoma in the O and Le (a−b+) phenotypes.21 However, in our research, we did not determine any association between ABO and Lewis phenotypes with this infection. de Mattos et al., studied the association between the ABO and Lewis blood group and secretor status in 120 infected and non-infected patients by H. pylori by using breath and urease tests. They showed H. pylori is more frequent in the O blood group but not in the Lewis nor in the secretors.5 In another study, Heneghan et al., determined the Lewis and ABO phenotypes on 207 patients during gastric endoscopy and culture for H. pylori infection; like our findings, they did not characterise any relationship between these phenotypes or secretor status with H. pylori infection.26 In contrast to many other findings, Rothenbacher et al., demonstrated higher frequency of H. pylori infection in Le (a+b−) phenotype compared with Le (a−b+).13 Perhaps part of the differences in the results across studies is due to the number of patients included, especially the difference with Rothenbacher study may be due to different criteria. Rothenbacher study included only immediate postpartum period and breast feeding women, while in our study, this population was excluded.
These data show that the association of ABO and Lewis antigens and, secretor status with H. pylori infection is controversial. Recent studies have tried to resolve this controversy. Based on some findings, strains of H. pylori from various areas of the world differed about 1,500-fold in binding affinity to O antigen, because of diversity in babA gene sequences.18,20,27 Accordingly, all strains are not so specific for O and Leb. In a study in Sweden, Aspholm-Hurtig et al., considered that more than 95% of adherent strains bind to A, B, and also O antigens whereas only 60% of adherent strains in South American Amerindian adhere better to O antigen. Many strains from outside South America have binding capabilities for A and Leb in addition to O and Leb.20,27 Con et al., characterised a higher frequency of babA2 in Japan (96.8%) than in Costa Rica (73.7%) by genotyping of 95 Costa Ricans and 95 Japanese H. pylori isolates; however, babA2/B was higher in Costa Rican (11.6%) than in the Japanese (1.1%). They suggested that babA2 and babA2/B had geographic diversities.28
SabA, a sialic acid-binding adhesion, is another virulence factor in H. pylori determined lately. It also has geographic diversity and is more frequent in European than Japanese H. pylori isolates.29,30 The babA distinguishes both H-type 1 and Lewis B blood-group antigens on gastric mucosa of secretor persons, initiating the first steps of the infection. After that, persistent infection results in inflammatory reaction with concomitantly expression of sialylated antigens. Furthermore, the SabA causes H. pylori binding to gastric mucosa by sialyl-Lewisa and sialyl-Lewis x antigens.25 A limitation of the study was lack of genotype determination of H. pylori. We suggest the determination of virulence factors of H. pylori such as babA and SabA in other study in addition to ABO and Lewis antigens.
CONCLUSION
We did not establish a significant association between the Lewis, ABO or secretion phenotypes with H. pylori infection. Diversity in babA gene sequences of H. pylori can probably describe why our results were different from others in other geographic areas.
ACKNOWLEDGMENTS
This study was the results of a medical student thesis supported financially by the Vice President for research, Mashhad University of Medical Sciences. We also thank Dr. Ismaili for statistical advice and Miss Zamanipour for performing laboratory tests.
Footnotes
Source of Support: Supported financially by the Vice President for research, Mashhad University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Keramati MR, Siadat Z, Mahmoudi M. The correlation between H. pylori infection with serum ferritin concentration and iron deficiency anemia. Int J Hematol Oncol. 2007;17:16–20. [Google Scholar]
- 2.Magalhães Queiroz DM, Luzza F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2006;11:1–5. doi: 10.1111/j.1478-405X.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell H, Megraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2002;7:8–16. doi: 10.1046/j.1523-5378.7.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 4.Metanat M, Sharifi-Mood B, Izadi S. Prevalence of Helicobacter pylori infection in healthcare workers. Turk J Med Sci. 2010;40:965–9. [Google Scholar]
- 5.de Mattos LC, Rodrigues CJ, Sanches FE, Alves da Silva Rde C, Ruiz MA, Moreira HW. ABO, Lewis, secretor and non-secretor phenotypes in patients infected or uninfected by the Helicobacter pylori bacillus. Sao Paulo Med J. 2002;120:55–8. doi: 10.1590/S1516-31802002000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babamahmoodi F, Ajemi A, Kalhor M, Shfiei GR, Khalilian AR. A seroepidemiological study of Helicobacter pylori infection in Sari in 2001-02. J Mazandaran Univ Med Sci. 2004;43:39–48. [Google Scholar]
- 7.Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: Epidemiology and risk factors. Arch Iran Med. 2009;12:576–83. [PubMed] [Google Scholar]
- 8.Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci. 2010;14:302–8. [PubMed] [Google Scholar]
- 9.Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. 2010;25:479–86. doi: 10.1111/j.1440-1746.2009.06188.x. [DOI] [PubMed] [Google Scholar]
- 10.Ramírez Ramos A, Sánchez Sánchez R. Helicobacter pylori and gastric cancer. Rev Gastroenterol Peru. 2008;28:258–66. [PubMed] [Google Scholar]
- 11.Ruggiero P. Helicobacter pylori infection: What's new. Curr Opin Infect Dis. 2012;25:337–44. doi: 10.1097/QCO.0b013e3283531f7c. [DOI] [PubMed] [Google Scholar]
- 12.Dovhanj J, Kljaic K, Vcev A, Ilakovac V. Helicobacter pylori and trace elements. Clin Lab. 2010;56:137–42. [PubMed] [Google Scholar]
- 13.Rothenbacher D, Weyermann M, Bode G, Kulaksiz M, Stahl B, Brenner H. Role of Lewis A and Lewis B blood group antigens in Helicobacter pylori infection. Helicobacter. 2004;9:324–9. doi: 10.1111/j.1083-4389.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 14.Montecucco C, Rappuoli R. Living dangerously: How Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol. 2001;2:457–66. doi: 10.1038/35073084. [DOI] [PubMed] [Google Scholar]
- 15.Schmaier AH, Thornburg CD, Pipe SW. Coagulation and fibrinolysis. In: Mcpherson RA, Pincus MR, editors. Henry's Clinical Diagnosis and Management by Laboratory Methods. 21st ed. Philadelphia: Saunders Elsevier; 2007. pp. 729–43. [Google Scholar]
- 16.Beadling WV, Cooling L. Immunohematology. In: Mcpherson RA, Pincus MR, editors. Henry›s Clinical diagnosis and Management by Laboratory Methods. 21st ed. Philadelphia: Saunders Elsevier; 2007. pp. 636–7. [Google Scholar]
- 17.Keller R, Dinkel KC, Christl SU, Fischbach W. Interrelation between ABH blood group 0, Lewis (B) blood group antigen, Helicobacter pylori infection, and occurrence of peptic ulcer. Z Gastroenterol. 2002;40:273–6. doi: 10.1055/s-2002-30115. [DOI] [PubMed] [Google Scholar]
- 18.Hennig EE, Mernaugh R, Edl J, Cao P, Cover TL. Heterogeneity among Helicobacter pylori strains in expression of the outer membrane protein BabA. Infect Immun. 2004;72:3429–35. doi: 10.1128/IAI.72.6.3429-3435.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishijima N, Suzuki M, Ashida H, Ichikawa Y, Kanegae Y, Saito I, et al. BabA-mediated adherence is a potentiator of the Helicobacter pylori type IV secretion system activity. J Biol Chem. 2011;286:25256–64. doi: 10.1074/jbc.M111.233601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anstee DJ. The relationship between blood groups and disease. Blood. 2010;115:4635–43. doi: 10.1182/blood-2010-01-261859. [DOI] [PubMed] [Google Scholar]
- 21.Cooling L, Downs T. Immunohematology. In: Mcpherson RA, Pincus MR, editors. Henry's Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia: Elsevier Saunders; 2011. pp. 696–7. [Google Scholar]
- 22.Mansour-Ghanaei F, Sanaei O, Joukar F. Clinical validation of an office-based C-UBT (Heliprobe) for H. pylori diagnosis in iranian dyspeptic patients. Gastroenterol Res Pract 2011. 2011 doi: 10.1155/2011/930941. 930941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozdemir E, Karabacak NI, Degertekin B, Cirak M, Dursun A, Engin D, et al. Could the simplified (14) C urea breath test be a new standard in noninvasive diagnosis of Helicobacter pylori infection? Ann Nucl Med. 2008;22:611–6. doi: 10.1007/s12149-008-0168-6. [DOI] [PubMed] [Google Scholar]
- 24.Bäckström A, Lundberg C, Kersulyte D, Berg DE, Borén T, Arnqvist A. Metastability of Helicobacter pylori bab adhesin genes and dynamics in Lewis b antigen binding. Proc Natl Acad Sci U S A. 2004;101:16923–8. doi: 10.1073/pnas.0404817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magalhaes A, Reis CA. Helicobacter pylori adhesion to gastric epithelial cells is mediated by glycan receptors. Braz J Med Biol Res. 2010;43:611–8. doi: 10.1590/s0100-879x2010007500049. [DOI] [PubMed] [Google Scholar]
- 26.Heneghan MA, Moran AP, Feeley KM, Egan EL, Goulding J, Connolly CE, et al. Effect of host Lewis and ABO blood group antigen expression on Helicobacter pylori colonisation density and the consequent inflammatory response. FEMS Immunol Med Microbiol. 1998;2:257–66. doi: 10.1111/j.1574-695X.1998.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 27.Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, Roche N, et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–22. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 28.Con SA, Takeuchi H, Nishioka M, Morimoto N, Sugiura T, Yasuda N, et al. Clinical relevance of Helicobacter pylori babA2 and babA2/B in Costa Rica and Japan. World J Gastroenterol. 2010;16:474–8. doi: 10.3748/wjg.v16.i4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molnar B, Galamb O, Sipos F, Leiszter K, Tulassay Z. Molecular pathogenesis of Helicobacter pylori infection: The role of bacterial virulence factors. Dig Dis. 2010;28:604–8. doi: 10.1159/000320060. [DOI] [PubMed] [Google Scholar]
- 30.Shao L, Takeda H, Fukui T, Mabe K, Han J, Kawata S, et al. Genetic diversity of the Helicobacter pylori sialic acid-binding adhesin (sabA) gene. Biosci Trends. 2010;4:249–53. [PubMed] [Google Scholar]


