Abstract
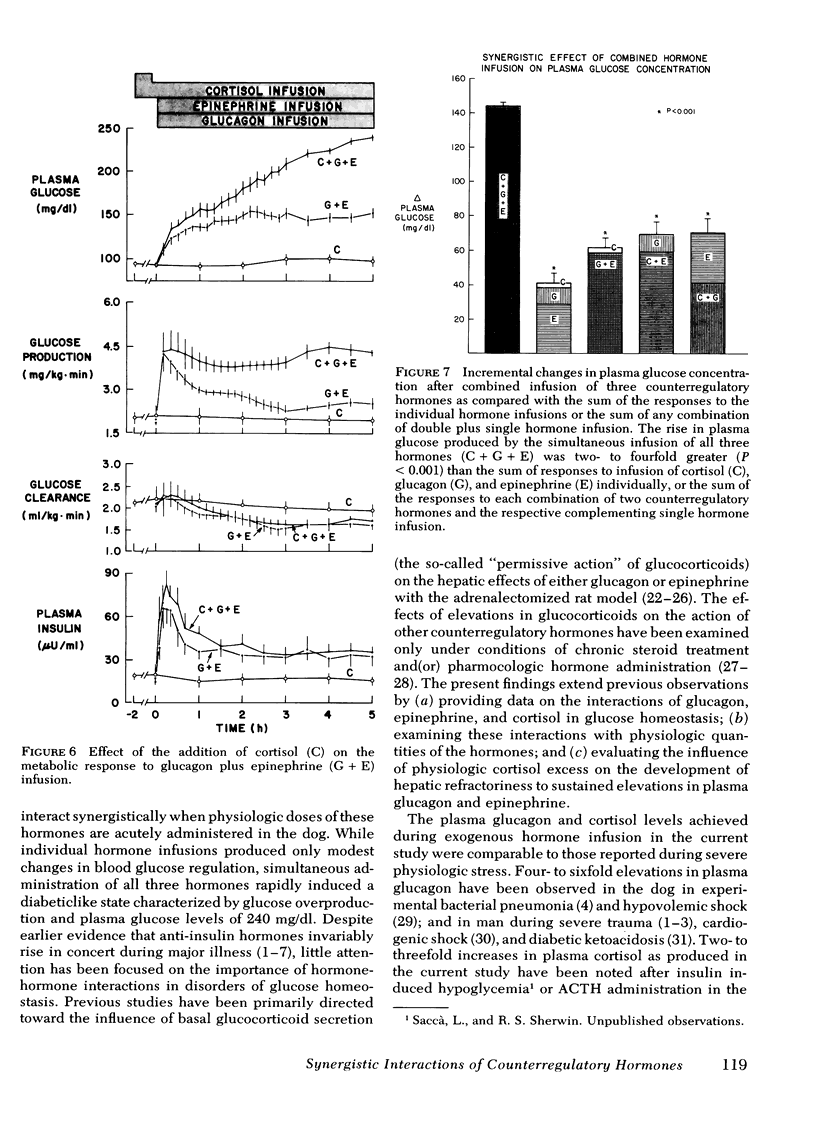
To evaluate the role of anti-insulin hormone actions and interactions in the pathogenesis of stress-induced hyperglycemia, the counterregulatory hormones, glucagon, epinephrine, and cortisol were infused alone as well as in double and triple combinations into normal conscious dogs in doses that were designed to simulate changes observed in severe stress. Infusion of glucagon, epinephrine, or cortisol alone produced only mild or insignificant elevations in plasma glucose concentration. In contrast, the rise in plasma glucose produced by combined infusion of any two counterregulatory hormones was 50-215% greater (P < 0.005-0.001) than the sum of the respective individual infusions. Furthermore, when all three hormones were infused simultaneously, the increment in plasma glucose concentration (144±2 mg/dl) was two- to fourfold greater than the sum of the responses to the individual hormone infusions or the sum of any combination of double plus single hormone infusion (P < 0.001).
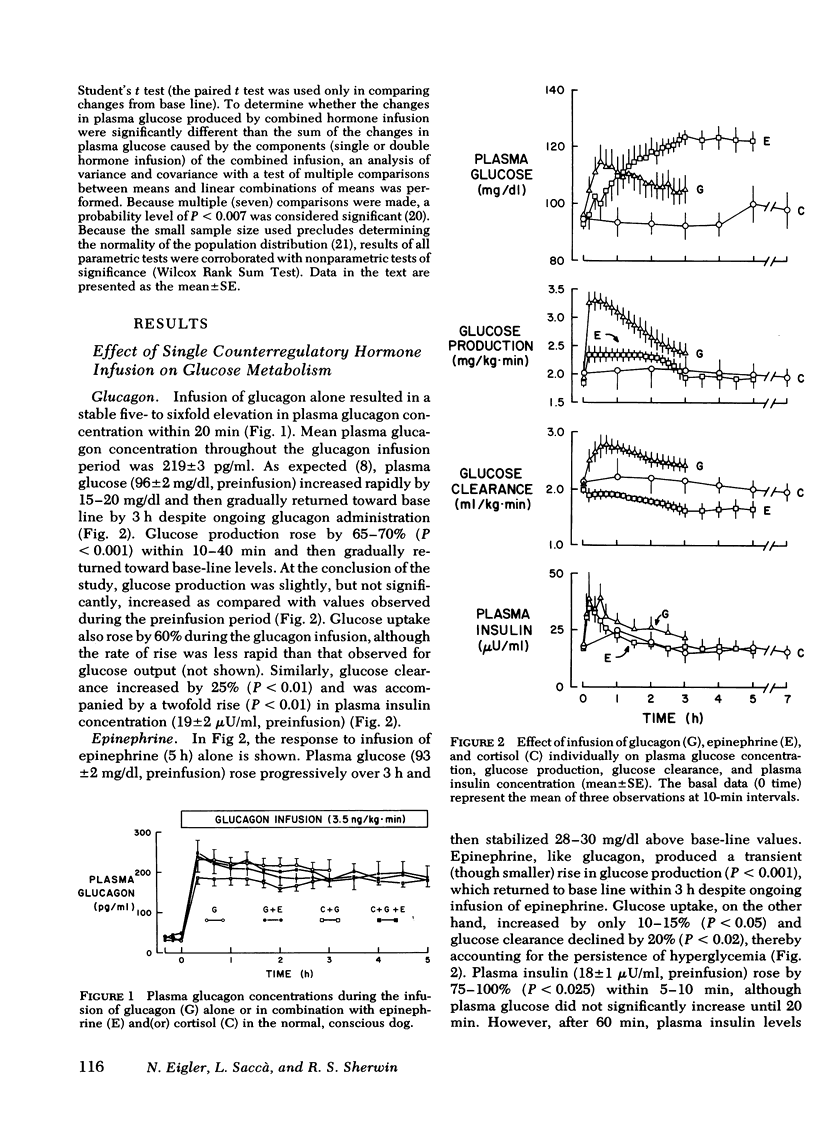
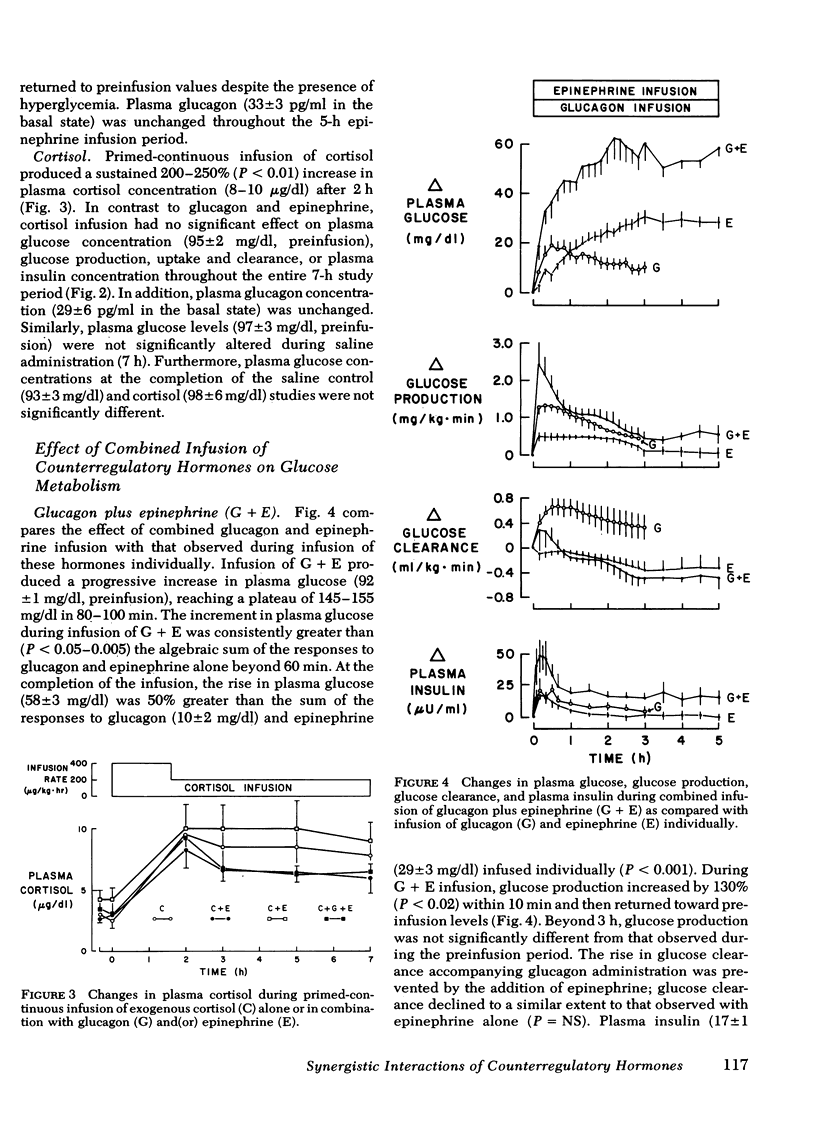
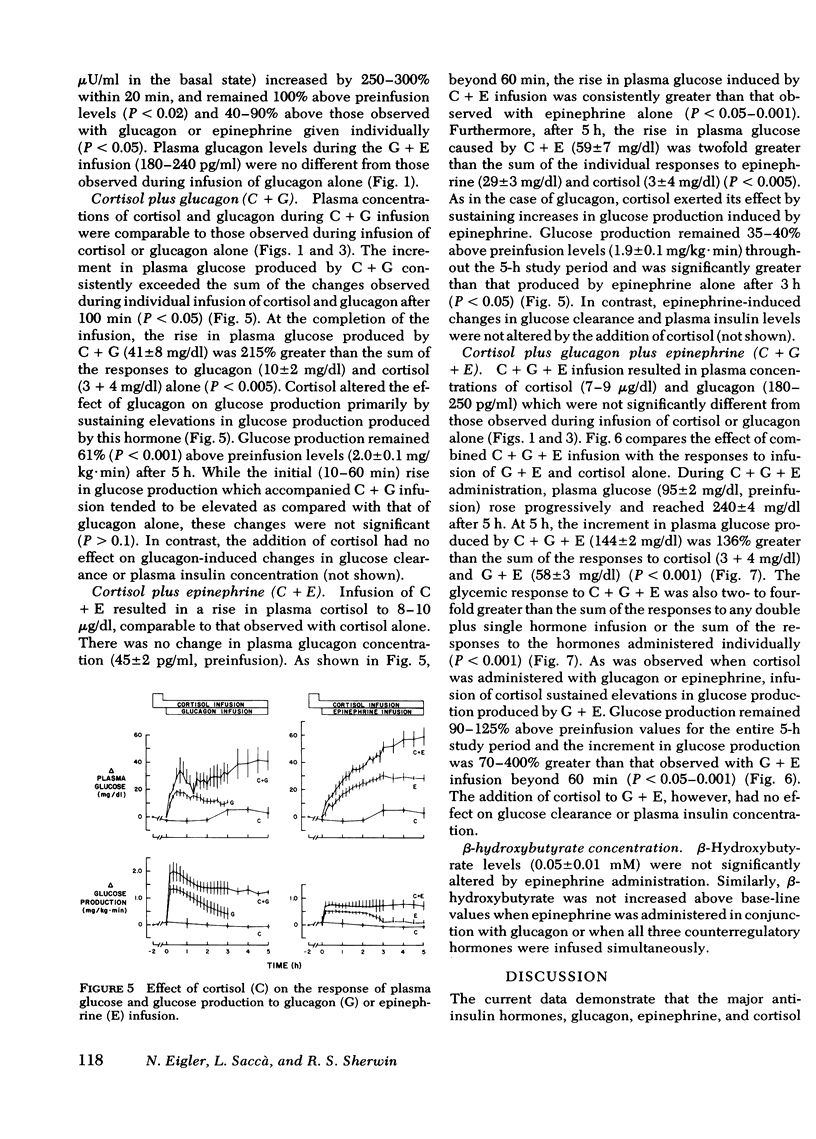
Infusion of glucagon or epinephrine alone resulted in a transient rise in glucose production (as measured by [3-3H]glucose). While glucagon infusion was accompanied by a rise in glucose clearance, with epinephrine there was a sustained, 20% fall in glucose clearance. When epinephrine was infused together with glucagon, the rise in glucose production was additive, albeit transient. However, the inhibitory effect of epinephrine on glucose clearance predominated, thereby accounting for the exaggerated glycemic response to combined infusion of glucagon and epinephrine. Although infusion of cortisol alone had no effect on glucose production, the addition of cortisol markedly accentuated hyperglycemia produced by glucagon and(or) epinephrine primarily by sustaining the increases in glucose production produced by these hormones. The combined hormonal infusions had no effect on β-hydroxybutyrate concentration.
It is concluded that (a) physiologic increments in glucagon, epinephrine, and cortisol interact synergistically in the normal dog so as to rapidly produce marked fasting hyperglycemia; (b) in this interaction, epinephrine enhances glucagon-stimulated glucose output and interferes with glucose uptake while cortisol sustains elevations in glucose production produced by epinephrine and glucagon; and (c) these data indicate that changes in glucose metabolism in circumstances in which several counterregulatory hormones are elevated (e.g., “stress hyperglycemia”) are a consequence of synergistic interactions among these hormones.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Steele R., Rathgeb I., De Bodo R. C. Glucose metabolism and plasma insulin level during epinephrine infusion in the dog. Am J Physiol. 1967 Mar;212(3):677–682. doi: 10.1152/ajplegacy.1967.212.3.677. [DOI] [PubMed] [Google Scholar]
- Becker M. J., Helland D., Becker D. N. Serum cortisol (hydrocortisone) values in normal dogs as determined by radioimmunoassay. Am J Vet Res. 1976 Sep;37(9):1101–1102. [PubMed] [Google Scholar]
- Bomboy J. D., Jr, Lewis S. B., Lacy W. W., Sinclair-Smith B. C., Liljenquist J. E. Transient stimulatory effect of sustained hyperglucagonemia on splanchnic glucose production in normal and diabetic man. Diabetes. 1977 Mar;26(3):177–174. doi: 10.2337/diab.26.3.177. [DOI] [PubMed] [Google Scholar]
- CONN J. W., FAJANS S. S. Influence of adrenal cortical steroids on carbohydrate metabolism in man. Metabolism. 1956 Mar;5(2):114–127. [PubMed] [Google Scholar]
- Cherrington A. D., Williams P. E., Harris M. S. Relationship between the plasma glucose level and glucose uptake in the conscious dog. Metabolism. 1978 Jul;27(7):787–791. doi: 10.1016/0026-0495(78)90213-5. [DOI] [PubMed] [Google Scholar]
- Christensen N. J. Plasma norepinephrine and epinephrine in untreated diabetics, during fasting and after insulin administration. Diabetes. 1974 Jan;23(1):1–8. doi: 10.2337/diab.23.1.1. [DOI] [PubMed] [Google Scholar]
- Christensen N. J., Videbaek J. Plasma catecholamines and carbohydrate metabolism in patients with acute myocardial infarction. J Clin Invest. 1974 Aug;54(2):278–286. doi: 10.1172/JCI107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- DE MOOR P., STEENO O., RASKIN M., HENDRIKX A. Fluorimetric determination of free plasma 11-hydroxycorticosteroids in man. Acta Endocrinol (Copenh) 1960 Feb;33:297–307. doi: 10.1530/acta.0.xxxiii0297. [DOI] [PubMed] [Google Scholar]
- Eisenstein A. B., Strack I. Effects of glucagon on carbohydrate synthesis and enzyme activity in rat liver. Endocrinology. 1968 Dec;83(6):1337–1348. doi: 10.1210/endo-83-6-1337. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Gluconeogenesis. Metabolism. 1972 Oct;21(10):945–990. doi: 10.1016/0026-0495(72)90028-5. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Jefferson L. S., Jr, Butcher R. W., Park C. R. Gluconeogenesis in the perfused liver. The effects of fasting, alloxan diabetes, glucagon, epinephrine, adenosine 3',5'-monophosphate and insulin. Am J Med. 1966 May;40(5):709–715. doi: 10.1016/0002-9343(66)90151-3. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Mallette L. E., Jefferson L. S., Wong E. H., Friedmann N., Miller T. B., Jr, Park C. R. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- FRANKSSON C., GEMZELL C. A., VON EULER U. S. Cortical and medullary adrenal activity in surgical and allied conditions. J Clin Endocrinol Metab. 1954 Jun;14(6):608–621. doi: 10.1210/jcem-14-6-608. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of physiologic hyperglucagonemia on basal and insulin-inhibited splanchnic glucose output in normal man. J Clin Invest. 1976 Sep;58(3):761–765. doi: 10.1172/JCI108523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J., Sherwin R., Hendler R. Insulin, glucagon, and somatostatin in normal physiology and diabetes mellitus. Diabetes. 1976 Dec;25(12):1091–1099. doi: 10.2337/diab.25.12.1091. [DOI] [PubMed] [Google Scholar]
- Forbath N., Hall J. D., Hetenyi G., Jr The effect of methyl-prednisolone on the turnover of lactate and the conversion of lactate to glucose in dogs. Horm Metab Res. 1969 Jul;1(4):178–182. doi: 10.1055/s-0028-1095151. [DOI] [PubMed] [Google Scholar]
- Friedmann N., Exton J. H., Park C. R. Interaction of adrenal steroids and glucagon on gluconeogenesis in perfused rat liver. Biochem Biophys Res Commun. 1967 Oct 11;29(1):113–119. doi: 10.1016/0006-291x(67)90550-5. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Cryer P. E., Santiago J. V., Haymond M. W., Pagliara A. S., Kipnis D. M. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest. 1976 Jul;58(1):7–15. doi: 10.1172/JCI108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Karam J. H., Forsham P. H. Stimulation of glucagon secretion by epinephrine in man. J Clin Endocrinol Metab. 1973 Sep;37(3):479–481. doi: 10.1210/jcem-37-3-479. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Borkow I. Effect of catecholamines and dibutyryl-cyclic-AMP on glucose turnover, plasma free fatty acids, and insulin in dogs treated with methylprednisolone. Can J Physiol Pharmacol. 1972 Oct;50(10):999–1006. doi: 10.1139/y72-144. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Borkow I. Effect of glucagon and glucose load on glucose kinetics, plasma FFA, and insulin in dogs treated with methylprednisolone. Metabolism. 1973 Jan;22(1):39–49. doi: 10.1016/0026-0495(73)90027-9. [DOI] [PubMed] [Google Scholar]
- KINSELL L. W., MARGEN S., MICHAELS G. D., REISS R., FRANTZ R., CARBONE J., LANGE J., LIEBERT G. Studies in fat metabolism. III. The effect of ACTH, of cortisone, and of other steroid compounds upon fasting-induced hyperketonemia and ketonuria. J Clin Invest. 1951 Dec;30(122):1491–1502. doi: 10.1172/JCI102559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey A., Santeusanio F., Braaten J., Faloona G. R., Unger R. H. Pancreatic alpha-cell function in trauma. JAMA. 1974 Feb 18;227(7):757–761. [PubMed] [Google Scholar]
- Lindsey C. A., Faloona G. R., Unger R. H. PLasma glucagon levels during rapid exsanguination with and without adrenergic blockade. Diabetes. 1975 Apr;24(4):313–316. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Hormonal control of ketogenesis. Biochemical considerations. Arch Intern Med. 1977 Apr;137(4):495–501. [PubMed] [Google Scholar]
- Muller W. A., Aoki T. T., Egdahl R. H., Cahill G. F., Jr Effects of exogenous glucagon and epinephrine in physiological amounts on the blood levels of free fatty acids and glycerol in dogs. Diabetologia. 1977 Jan;13(1):55–58. doi: 10.1007/BF00996328. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med. 1973 Jan;54(1):52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- PLAGER J. E., KNOPP R., SLAUNWHITE W. R., Jr, SANDBERG A. A. CORTISOL BINDING BY DOG PLASMA. Endocrinology. 1963 Sep;73:353–358. doi: 10.1210/endo-73-3-353. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Measurement and validation of nonsteady turnover rates with applications to the inulin and glucose systems. Fed Proc. 1974 Jul;33(7):1855–1864. [PubMed] [Google Scholar]
- Robertson R. P., Porte D., Jr Adrenergic modulation of basal insulin secretion in man. Diabetes. 1973 Jan;22(1):1–8. doi: 10.2337/diab.22.1.1. [DOI] [PubMed] [Google Scholar]
- Rocha D. M., Santeusanio F., Faloona G. R., Unger R. H. Abnormal pancreatic alpha-cell function in bacterial infections. N Engl J Med. 1973 Apr 5;288(14):700–703. doi: 10.1056/NEJM197304052881402. [DOI] [PubMed] [Google Scholar]
- Ross H., Johnston I. D., Welborn T. A., Wright A. D. Effect of abdominal operation on glucose tolerance and serum levels of insulin, growth hormone, and hydrocortisone. Lancet. 1966 Sep 10;2(7463):563–566. doi: 10.1016/s0140-6736(66)93036-4. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Saccà L., Sherwin R., Felig P. Effect of sequential infusions of glucagon and epinephrine on glucose turnover in the dog. Am J Physiol. 1978 Sep;235(3):E287–E290. doi: 10.1152/ajpendo.1978.235.3.E287. [DOI] [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P. Modulation of fatty acid metabolism by glucagon in man. IV. Effects of a physiologic hormone infusion in normal man. Diabetes. 1976 Oct;25(10):978–983. doi: 10.2337/diab.25.10.978. [DOI] [PubMed] [Google Scholar]
- Schaeffer L. D., Chenoweth M., Dunn A. Adrenal corticosteroid involvement in the control of liver glycogen phosphorylase activity. Biochim Biophys Acta. 1969 Nov 18;192(2):292–303. doi: 10.1016/0304-4165(69)90368-7. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Fisher M., Hendler R., Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. N Engl J Med. 1976 Feb 26;294(9):455–461. doi: 10.1056/NEJM197602262940901. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R. G., Felig P. Effect of ketone infusions on amino acid and nitrogen metabolism in man. J Clin Invest. 1975 Jun;55(6):1382–1390. doi: 10.1172/JCI108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]
- Weber G., Singhal R. L., Stamm N. B., Fisher E. A., Mentendiek M. A. Regulation of enzymes involved in gluconeogenesis. Adv Enzyme Regul. 1964;2:1–38. doi: 10.1016/s0065-2571(64)80003-0. [DOI] [PubMed] [Google Scholar]
- Willerson J. T., Hutcheson D. R., Leshin S. J., Faloona G. R., Unger R. H. Serum glucagon and insulin levels and their relationship to blood glucose values in patients with acute myocardial infarction and acute coronary insufficiency. Am J Med. 1974 Nov;57(5):747–752. doi: 10.1016/0002-9343(74)90848-1. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W. Carbohydrate metabolism in trauma. Clin Endocrinol Metab. 1976 Nov;5(3):731–745. doi: 10.1016/s0300-595x(76)80048-5. [DOI] [PubMed] [Google Scholar]
- Wise J. K., Hendler R., Felig P. Influence of glucocorticoids on glucagon secretion and plasma amino acid concentrations in man. J Clin Invest. 1973 Nov;52(11):2774–2782. doi: 10.1172/JCI107473. [DOI] [PMC free article] [PubMed] [Google Scholar]



