Abstract
Background:
Diabetic mellitus and hypothyroidism lead to serum lipoproteins disorders. This study aims to investigate the potential effect of fresh ginger extracts Zingiber officinale roscoe (Family: Zingebiraceae) on serum lipid profile and on blood glucose in alloxan-induced diabetes and propylthiouracil-induced hypothyroidism in rats. Rats were divided into 11 groups: The normal G1, diabetic control rats G2, ginger 500 mg/kg treated diabetic rats G3, 10 mg/day atorvastatine-treated diabetic rats G4, [5 mg/day atorvastatine combined with 500 mg/kg ginger] treated diabetic rats G5, glibenclamid-treated diabetic rats G6, hypothyoidism control rats G7, 300 mg/kg ginger-treated hypothyroidism rats G8, 500 mg/kg ginger-treated hypothyroidism rats G9, 10 mg/day atorvastatine-treated hypothyroidism rats G10, [atorvastatine combined with 500 mg/kg ginger]treated hypothyroidism rats G11. Thirty days after treatment, samples were collected, to compare treated groups with normal and control groups, using Mann-Whitney U test P < 0.01.
Results:
It revealed a decrease in the levels of total cholesterol (TC), and low density lipoprotein (LDL) in the serum of rats that were treated by ginger extracts, compared with the control groups. Previous extracts were also able to cause reduction in LDL to similar levels compared to normal group and that was the same effect of atorvastatin 10 mg/day. Combined effect was clear between the act of ginger at a dose of 500 mg/kg and atorvastatin; that levels of both TC and LDL in animals which received [atorvastatin 5 mg/day combined with ginger extract] was almost equal to levels in animals that received atorvastatin 10mg/day. Clear reduce in triglyceride, and clear increase in high density liopprotein were also recorded in the ginger-treated groups. Ginger was more active in hypothyroidism rats than in diabetic rats in reducing LDL and TC. Glucose levels were substantially reduced in ginger- treated diabetic groups.
Keywords: Alloxan-induced diabetes, Atorvastatin, ginger, glibenclamide, lipoproteins
INTRODUCTION
Diabetes mellitus is a common disorder, characterized by chronic hyperglycemia,[1] also hypothyroidism is the most common disorder of thyroid function and it can be divided into primary hypothyroidism and central hypothyroidism,[2] while ginger Zingiber officinal roscoe is a perennial herb that belongs to the Zingiberaceae family,[3] its rhizome has a great medical value and wide common use for gastrointestinal disorders,[4] and for its anti-inflammatory effect.[5] A lot of research were done to clarify ginger's effect on lipid profile [total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL), triglycerides (TG)].[6] Ginger's effect on glucose and lipid metabolism in both liver and blood was investigated,[4] effects of ethanolic ginger extract on rabbits with hyperlipidemia[7] was also studied, ginger extract showed an ability of lowering liver cholesterol and cholesterol oxidation in E0 rats.[8] In 2003 ZT{[E]-8b,7-epoxylabd-21-ene-15,16-dial} was isolated from ginger, ZT can inhibit the HMG-Co-A reductase which determines the rate of cholesterol biosynthesis,[9] in 2006 Al-Amin declared that ginger decreased glucose and lipids in STZ-induced diabetic rats.[10] Finally, ginger effect on lipid profile in human is studied in 2008.[11] Moreover, ginger seems to interfere with the thyroid function,[12] but only a little research study ginger's effect on thyroid function, also in our knowledge no research was conducted about ginger's effect lipid profile in hypothyroidism cases in vivo nor study ginger's combined effect with atorvastatine.
This study aims to : investigate the action of fresh ginger extracts in two doses 300 mg/kg, 500 mg/kg orally on the lipid profile and glucose blood levels in rats with hypercholesterolemia due to two causes: Alloxan-induced diabetes mellitus and [6-n-propylthiouracil] propylthiouracil (PTU)-induced hypothyroidism and investigate the combined effect between ginger and atorvastatin then compare between ginger effectiveness on both rat models and study the dose-dependent action in two doses of 300,500 mg/kg in hypothyroidism rats.
MATERIALS AND METHODS
Materials
Methanol (Merck®) [Germany], alloxan monohydrate (Fluka®) [Argentine], glibenclamid (Thamico Drugs Industry®) [Syria], atorvastatin (Al-Fares Drugs Dawliya Drugs Industry®) [Jordan], kits of HDL, LDL, TG, TC, purchased from Medison. Ltd®. [Syria], kits of glucose from Roche® [Germany], standardized ginger extract for High-performance thin layer chromatography (HPTLC) from Universal Medicament Pvt. Ltd.® Nagpur, [India].
Apparatus
Rotary vapor (Push RE 111®) [Germany], electric grinder, silk filtration, CAMAG HPTLC Scanner III® [Duetchland], centrifugator Eppendrof S810R® [Germany], automatic biochemical analyzer Olympus AU400® [USA], Accu-check active® blood glucose meter device with its stripes [Germany].
Plant extracts
Ginger was obtained from local market then was identified by the herbarium staff of Botany department, Damascus University, Syria.
It was peeled, crushed and extracted by cold percolation in methanol for 24 hours. The extract was recovered and methanol was further added to the plant material and the extract was continued, the process was repeated three times, the three extracts were pooled together and concentrated under reduced pressure (22-26 mmHg) at (45°) C using rotary vapor until the oleoresin was obtained[13] the golden brawny viscous oleoresin was maintained in dark glass-container, at (–4°) C until use.
Animals
A total of 88 male Wistar rats at the age of 8 weeks and weight of 200-250 g from Leen. Ltd. Company [Damascus], were housed in standard conditions in air-conditioned room at (22°) C, 55% humidity, and 12 hours day-light circle. Rats were fed with normal diet and water ad libitium in the [experimental animals house] in Faculty of Pharmacy in University of Damascus.
PROCEDURES
Experimental protocol
In the first step, eight rats were separated to be normal group which were called group (1) then rats were divided into two groups to induce hypercholesterolemia by the following two ways:
-
(a)
Inducing diabetes mellitus: Diabetes was induced in (12) hours fasted rats by single dose of intraperitoneal injection of 150 mg/kg body weight of alloxan monohydrate, after the injection, animals had free access of food and water, also after 6 hours of injection rats were given solution of 5% glucose to drink over night to avoid hypoglycemia shock. The diabetic state was assessed by measuring non-fasting plasma glucose levels after 72 hours of alloxan injection, rats with plasma glucose levels above 250 mg/dL were accepted for experiment and considered as diabetics.[14]
-
(b)
Inducing hypothyroidism: This was induced by adding PTU 3% (w/v) to the drinking water for 21 days[15] then serum LDL levels were measured and animals with LDL levels above 22 mg/dL were approved.
Diabetic rats were divided into five groups of eight rats: Group (2): Control diabetic rats, group (3): Ginger 500 mg/kg treated rats, group (4): Atorvastatin treated rats [equivalent to 10mg/day therapeutic dose in human (0.18mg)],[16] group (5): [Atorvastatin in half of previous dose combined with ginger extract 500mg/kg] treated rats, and group (6): Glibenclamid [equal to 10mg/day in human] treated rats.
Hypothyroidism group was also divided into four groups: Group (7): Control hypothyroidism rats, group (8): 300mg/kg ginger-treated rats, group (9): 500mg/kg ginger treated rats, group (10): Atorvastatin treated rats, group (11): [atorvastatin at half of therapeutic dose combined with 500 mg/kg ginger extract] treated rats.
All groups also received free diet and water ad libitium. The treatment lasted for 4 weeks.
Blood samples
At the end of the 4th week, blood samples were collected by direct puncher in heart under light anesthetic, samples were collected in clean EDTA tubes, plasma was separated by centrifugation, at (4°C) then animals were sacrificed under anesthetic.[17]
Biochemical analysis
By automatic analyzer Olympus AU400, using [LDL, HDL, TC, TG] kits which were purchased from Medision®, Syria, while glucose kits were purchased from Rouche.
Extracts standardization
By HPTLC[18] comparing to standard plant obtained from Universal Medicament Pvt. Ltd. using HPTLC scanner to determine the presence and quantities of main Constituents. Figure 1 shows the peaks in the studied extract (left) compared to the standard one (Right). Peaks in both chromatograms have the same retention times.
Figure 1.
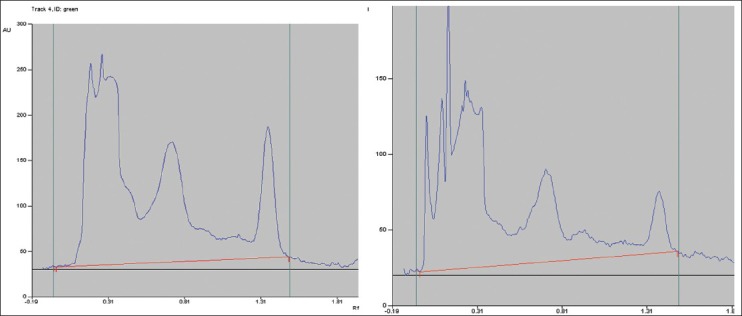
HPTLC High-performance thin layer chromatography chromatogram
Statistical analysis
Mann-Whitney U test was used to analyze data by using SPSS program; it showed differences between treated groups and each of normal and control groups, it also clarified the effect size of each material that was added to the treated groups.[19]
RESULTS
The results are divided into diabetic rats in Table 1 and hypothyroidism rats in Table 2. Every table includes the M and the SD of each group.
Table 1.
Diabetic rats values
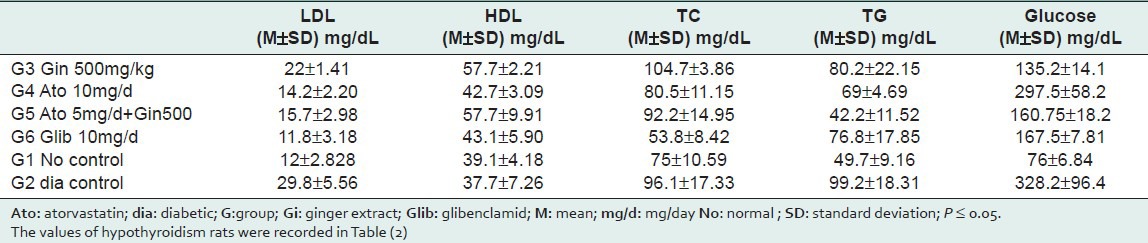
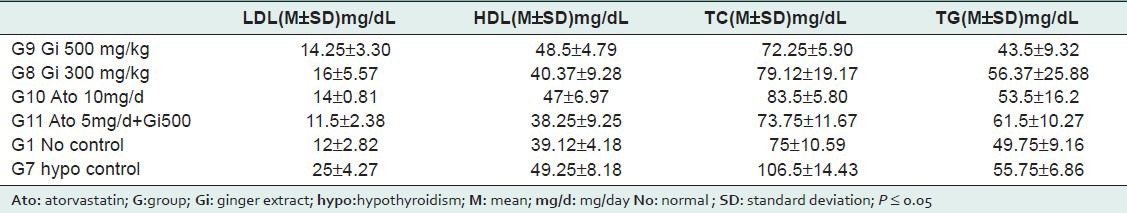
Table 2.
Hypothyroidism rats values
Lipid profile
Diabetic rats: As shown in Table 1, alloxan produced significant hypercholesterolemic action that a significant increase in levels of plasma TC and TG were recorded when compared to normal group. HDL levels were not statistically changed when compared to the normal group. Treatment with ginger produced significant reduction in LDL when compared to the control diabetic group. Also ginger caused significant increase in HDL compared to normal and diabetic control groups. Combined effect was clear between atorvastatin and ginger; that TC levels in [atorvastatin 5 mg/day combined with 500 mg/kg ginger extract] group, and [10mg/day atorvastatin] group were nearly equal.
Hypothyroidism rats: As shown in Table (2), PTU produced hypercholesterolemia that remarkable increase in TC and LDL plasma levels were recorded compared to the normal group, HDL levels was also increased. Treatment with ginger in two doses produced significant reduction in LDL and TC compared to control hypothyroidism group and this action was equal to atorvastatin action, that groups (8), (9), and (10) became similar to normal group. Combined effect was also clear; that LDL and TC levels in [10 mg/day atorvastatin] group and [atorvastatine 5mg/day combined with ginger] group were similar.
Ginger was more active in lowering cholesterol in Hypothyroidism rats.
Glucose
Administration of ginger to diabetic rats reduced plasma glucose levels compared to diabetic control group, this action exceeded glibenclamid action, but it was not enough to reach normal group as shown in Table (1). In addition, no dose-dependent action for 300,500 mg/kg doses was noticed, but the effect size was more in the higher dose.
DISCUSSION
The present work was carried out to study the effect of ginger extract as an antihypercholesterolemic agent in both diabetic and hypothyroidism male Wistar rats (in vivo). While ginger 500 mg/kg has similar effectiveness to atorvastatin 10 mg/day in hypothyroidism rats, it does not have the same effectiveness in diabetic rats, but there was a combined effect between ginger and atorvastatin in both rat models. The efficacy of ginger may be due to the presence of (ZT) compound that was isolated from ginger, which lowered plasma cholesterol levels in rats and mice by cholesterol biosynthesis blockage,[9] these results are compatible with the results of previous research which applied ginger orally on high cholesterol fed rabbits to cause reduction in atherogenesis and lipid levels, by disruption of cholesterol absorption from gastrointestinal tract.[7] Ginger's effect may also be due to the pharmacological action of ginger which elevates the activity of hepatic cholesterol-7α-hydroxylase which is the rate-limiting enzyme in the biosynthesis of bile acids and stimulates the conversion of cholesterol to bile acids.[20] Moreover, ginger antihypercholesterolemic effect may be due to the inhibition of cellular cholesterol synthesis,[21] this may be due to the presence of niacin in ginger,[3] niacin causes increased clearance of VLDL, lower TG levels, increase hepatic uptake of LDL, and inhibition of cholesterogenesis.[22,23] Aqueous ginger infusion 5% yielded nearly the same antioxidant activity toward lipid peroxidation as did the synthetic antioxidant butylhydroxyanizole, this may be due to the essential oil content.[24] Also this antioxidant activity may be due to the high polyphenols content[25] and the presence of polyphenolic flavonoids prevents coronary artery disease by reducing plasma cholesterol levels or by inhibition LDL oxidation.[26] The main antioxidant active principles in ginger are the polyphenolic compounds which called gingerols and some related phenolic ketone derivatives.[3] The effect of ginger could also be due to the inhibition or scavenging radicals of rat body in different degrees,[27] or by increasing the antioxidative defense mechanisms of liver cells.[28] When ginger was added to an animal diet, a considerable increase in the pancreatic and intestine lipase occurred; lipase is the other key factor that plays a vital role in fat digestion,[29] the previous activity of ginger may be responsible for the TG reduction effect. The increase in HDL may occur because niacin causes reduction in the catabolic rate of HDL,[30] research reported that consumption of 500 mg of curcumin (a constituent of ginger) by volunteers for 10 days induced an increase in HDL-c by 29%.[31] Observed ginger hypoglycemic action may be due to the inorganic part of ginger which contains mainly mineral elements,[32] a number of main minerals (Ca, Zn, Mn, and Cr) are known to be associated with the mechanisms of insulin release and its activity in different animals and in human beings,[33] the potential antidiabetic activity is possibly involving 5-HT receptor.[28] On the other hand, ginger's effect on hypothyroidism rats may be due to ginger stimulating action on thyroid gland that ginger must be avoided in women with subacute thyroiditis,[11] but this stimulating action may exceed ginger's ameliorative action on diabetes mellitus so ginger's effects on hypothyroidism rats was more. Actually, this point needs more research to be clear. In summary, results of the present work demonstrate that the use of ginger extract at dose of 300,500 mg/kg has hypoglycemic effect and antihyperchoesterolemic effect, which has equal efficacy to atorvastatin in hypothyroidism rats. Also clear combined effect between ginger and atorvastatin was noticed, these findings suggest that ginger may have therapeutic potential as antihypercholesterolaemic agent in itself or as support with atorvastatin in half of its dose, which may enable to reduce atorvastatin's dose 50%. Eventually ginger is available and has hypoglycemic effect. This may be a novel finding, because it shows ginger's effect on lipid profile in hypothyroidism rats which have not been studied yet, in our knowledge, and compare between ginger action on two models of rats: Diabetic and hypothyroidism. Finally, it shows the combined effect between ginger and atorvastatin in reducing cholesterol.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Elshater A, Salman M, Moussa M. Effect of ginger extract consumption on levels of blood glucose, lipid profile and kidney function in Alloxan induced-diabetic rats. Egypt Acad J Biolog Sci. 2009;2:153–62. [Google Scholar]
- 2.Brunton L, Goodman LS, Donald B, Buxton L. Thyroid and antithyroid drugs. In: Laurence LB, Lazo JS, Parder KL, editors. Goodman and Gilmans’ Manual of Pharmacology and Therapeutics. New York: McGraw-Hill; 2007. p. 986. [Google Scholar]
- 3.Zachariah TJ. Ginger. In: Parthasarathy VA, Champakam B, Zachariah TG, editors. Chemistry of Spices. CABI. 2008. pp. 70–100. [Google Scholar]
- 4.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological, and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol. 2008;46:409–20. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 5.Shen CL, Hong KJ, Kim SW. Effects of ginger (Zingiber officinale Rosc.) on decreasing the production of inflammatory mediators in sow osteoarthritic cartilage explants. J Med Food. 2003;6:323–8. doi: 10.1089/109662003772519877. [DOI] [PubMed] [Google Scholar]
- 6.Mascolo N, Jain R, Jain SC, Capasso F. Ethnopharmacologic investigation of ginger (Zingiber officinale) J Ethnopharmacol. 1989;27:129–40. doi: 10.1016/0378-8741(89)90085-8. [DOI] [PubMed] [Google Scholar]
- 7.Bhandari U, Sharma JN, Zafar R. The protective action of ethanolic ginger extract in cholesterol-fed rabbits. J Ethnopharmacol. 1998;61:167–71. doi: 10.1016/s0378-8741(98)00026-9. [DOI] [PubMed] [Google Scholar]
- 8.Fuhrman B, Rosenblant M, Hayek T, Coleman R, Aviram M. Ginger extract consumption reduces plasma cholesterol, inhibits LDL oxidation and attenuates development of atherosclerosis in atherosclerotic lipoprotein E-deficient mice. J Nutr. 2000;130:1124–31. doi: 10.1093/jn/130.5.1124. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe M, Chen YD, Saito K, Kano Y. Cholesterol biosynthes inhibitory component from Zingiber officinale Roscoe. Chem Pharm Bull (Tokyo) 1993;41:710–3. doi: 10.1248/cpb.41.710. [DOI] [PubMed] [Google Scholar]
- 10.Al-Amin ZM, Thomson M, Al-Qattan KK, Peltonen-Shalaby R, Ali M. Anti- diabetic and hypolipidaemic properties of ginger in streptozotocin-induced diabetic rats. Br J Nutr. 2006;96:660–6. doi: 10.1079/bjn20061849. [DOI] [PubMed] [Google Scholar]
- 11.Sanavi S, Afshar R. Subacute thyroiditis following ginger (Zingiber officinale) consumption. Int J Ayurveda Res. 2010;1:47–8. doi: 10.4103/0974-7788.59944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alizadeh-Navaei R, Roozbeh F, Saravi M, Pouramir M, Jalali F, Moghadamnia AA. Investigation of the effect of ginger on the lipid levels. A double blind controlled clinical trial. Saudi Med J. 2008;29:1280–4. [PubMed] [Google Scholar]
- 13.Bhandari U, Kanojia R, Pillai KK. Effect of etanolic extract of Zingiber officinale on dyslipidemia in diabetic rats. J Ethnopharmacol. 2005;97:227–30. doi: 10.1016/j.jep.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Huang Y, Hou T, Wang Y. Hypoglycaemic effect of Artemisia sphaerocephala Krasch seed polysaccharide in alloxan-induced diabetic rats. Swiss Med Wkly. 2006;136:529–32. doi: 10.4414/smw.2006.11348. [DOI] [PubMed] [Google Scholar]
- 15.Laezza C, Mazziotti G, Fiorentino L, Gazzero P, Portella G, Gerbasio D, et al. HMG-CoA reductase inhibitor inhibit rat proyithiouracil- induced goiter by modulating the ras-MAPK pathway. J Mol Med (Berl) 2006;84:967–73. doi: 10.1007/s00109-006-0079-8. [DOI] [PubMed] [Google Scholar]
- 16.Paget GF, Barnes JM. Toxicity test. In: Laurence DR, Bacharach AL, editors. Evaluation of Drug Activities: Pharmacometries. London: Academic Press; 1964. pp. 135–66. [Google Scholar]
- 17.NIH Guide for the Care and Use of Laboratory Animals. (a) DHEW Publication No. (NIH) 8-23, revised 1978 and (b) NIH Publication No. 85-23, revised 1985. US Department of Health, Education, and Welfare [Google Scholar]
- 18.Melianita F, Cholifah S, Sumarlik E, Kartinasari WF. Simultaneous densitometric determination of 6-gingerol and 6- shogaol in some commercial gingers. J Liquid Chrom Relat Tech. 2007;30:2941–51. [Google Scholar]
- 19.Darweesh RM. Mann-Whitny U test in Statistical tests in psychology Damascus University Publication. 1997:38–45. [Google Scholar]
- 20.Srinivasan K, Sambaiah K. The effect spices on cholesterol 7 alpha-hydroxylase activity on serum and on hepatic cholesterol levels in rat. Int J Vitam Nutr Res. 1991;61:364–9. [PubMed] [Google Scholar]
- 21.Ness GC, Zhao Z, Lopez D. Inhibitor of cholesterol biosynthesis increase hepatic low-density lipoprotein receptor protein degradation. Arch Biochem Biophys. 1991;325:242–8. doi: 10.1006/abbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 22.Durrington P. Dyslipidaemia. Lancet. 2003;362:717–31. doi: 10.1016/S0140-6736(03)14234-1. [DOI] [PubMed] [Google Scholar]
- 23.Cardia G, Grisorio D, Impedovo G, Lillo A, Regina G. Plasma lipid as a risk factor in peripheral vascular disease. Angiology. 1990;41:19–22. doi: 10.1177/000331979004100103. [DOI] [PubMed] [Google Scholar]
- 24.Murcia MA, Egea I, Romojaro F, Parras P, Jiménez AM, Martínez-Tomé M. Antioxidant evaluation in dessert spices compared with common food additives. Influence of irradiation procedure. J Agric Food Chem. 2004;52:1872–81. doi: 10.1021/jf0303114. [DOI] [PubMed] [Google Scholar]
- 25.Yen GC, Chong Y, Su S. Antioxidant activity and active compounds of rice koji fermented with Aspergillus candidus. Food Chem Toxicol. 2003;83:49–52. [Google Scholar]
- 26.Belinky PA, Aviram M, Fuhrman B, Rosenblat M, Vaya J. The antioxidant effects of isoflavon glibridin on endogenous constituents of LDL during its oxidation. Atherosclerosis. 1998;137:49–61. doi: 10.1016/s0021-9150(97)00251-7. [DOI] [PubMed] [Google Scholar]
- 27.Liu N, Huo G, Zhang L, Zhang X. Effect of Zingiber officinale roscoe on lipid per oxidation in hyperlipidemia rats. Wei Sheng Yan Jiu. 2003;32:22–3. [PubMed] [Google Scholar]
- 28.Akhany SP, Vishwakarma SL, Goyal RK. Anti-diabetic activity of i officinale in streptozotocin-induced type 1 diabetic rats. J Pharm Pharmacol. 2004;56:101–5. doi: 10.1211/0022357022403. [DOI] [PubMed] [Google Scholar]
- 29.Platel K, Srinivasan K. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Nahrung. 2000;1:42–6. doi: 10.1002/(SICI)1521-3803(20000101)44:1<42::AID-FOOD42>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 30.Mary JM, John PK. Agents used in hyperlipidemia. In: Katzung BG, editor. Basic and clinical pharmacology. 8th ed. New York: McGraw-Hill; 2000. pp. 581–95. [Google Scholar]
- 31.Soni KB, Rajan A, Kuttan R. Reversal of aflatoxin induced liver damage by turmeric and curcumin. Cancer Lett. 1992;66:115–21. doi: 10.1016/0304-3835(92)90223-i. [DOI] [PubMed] [Google Scholar]
- 32.Kar A, Choudhary BK, Bandyopadhyay NG. Preliminary studies on the inorganic constituents of some indigenous hypoglycemic herbs on oral glucose tolerance test. J Ethnopharmacol. 1991;64:179–84. doi: 10.1016/s0378-8741(98)00118-4. [DOI] [PubMed] [Google Scholar]
- 33.Castro VR. Chromium in series of Portuguese plants used in the herbal treatment of diabetes. Biol Trace Elem Res. 1998;62:101–6. doi: 10.1007/BF02820025. [DOI] [PubMed] [Google Scholar]


