Abstract
Background:
Nyctanthes arbortristis has been used in traditional medicine for the treatment of asthma and cough.
Aim:
In this study, bronchodilatory effect of ethanolic extract of the N. arbortristis was investigated under in vitro conditions. The concentration–response curve of the tracheal smooth muscle (TSM) to histamine was recorded in presence or absence of ethanolic extract and Nω-nitro-l-arginine methyl ester (L-NAME). Dose–response effect of ethanolic extract on pre-constricted tissues was investigated. The ethanolic extract inhibited the histamine-induced maximum contractile responses of TSM (P < 0.001). Ethanolic extract also cause dose-dependent relaxation of TSM. These effects were reversed by L-NAME. Phytochemical analysis showed the presence of typical plant constituents. These results suggest the possible use of extract of the leaves of N. arbortristis as a bronchodilator in therapeutic treatment of asthma.
Keywords: Asthma, ethanolic extract Nyctanthes arbortristis, tracheal smooth muscle
INTRODUCTION
Among several non-infectious respiratory disorders in humans, allergic asthma is the most common disease that affects breathing. Allergic asthma is a chronic inflammatory disease of the airways, characterized by bronchial obstructive reactions, airway inflammation, and airway hyper-reactivity to variety of stimuli such as allergens, histamine, methacholine, etc.[1] Bronchoconstriction is the main component of the early asthmatic reaction on exposure to allergen.[2] It was confirmed that bronchial obstructive reaction and airway hyper-sensitivity under asthmatic conditions were associated with the deficiency of nitric oxide (NO) production.[3] NO is a relaxant molecule that plays a major role in maintenance of airway balance. It is produced by a family of NO synthase (NOS) that utilize the amino acid l-arginine.[4]
Current therapy in the management of asthma includes bronchodilators such as β2-agonists, muscarinic receptor antagonists, etc.[5] Despite the beneficiary effects in relief of symptoms of asthma, the use of these bronchodilators is limited because of their adverse effect profile.[5] Therefore, there is a need to find alternative therapies that are more effective and safe to treat asthmatic conditions.[6] Based on the fact that many anti-asthmatic drugs are of plant origin, herbal medicine is a promising approach for current efforts of finding improved anti-asthmatic drugs.
Nyctanthes arbortristis L. (Family: Oleaceae) commonly known as “Night Jasmine” is generally distributed in tropical and sub-tropical regions.[7] The plant is a large shrub that grows up to 10 m high. The leaves are opposite, simple, with an entire margin. Traditionally, it is used in several ailments including asthma, cough, rheumatism, high blood pressure, etc.[7,8] Pharmacological studies have shown that the leaves of N. arbortristis have anti-asthmatic and anti-allergic properties possibly due to the presence of β-sitosterol.[9] Also a recent study showed that the leaves of this plant possess anti-spasmodic activity.[10] The ethanolic leaf extract inhibited acetylcholine-induced contractions of the guinea pig ileum preparations.[10] Although some pharmacological studies were undertaken to explain the dilatatory effect of different parts of N. arbortristis, the effect of the leaves of this plant on airway smooth muscle is unclear. Therefore, the aim of this study was to test the relaxant effect of ethanolic extract of the leaves of N. arbortristis on tracheal smooth muscle (TSM) and to explore the possible mechanisms of this effect.
MATERIALS AND METHODS
Plant material
Fresh leaves of N. arbortristis L. were collected from the surrounding of Dhaka, Bangladesh and its aerial part was identified taxonomically by the experts of Bangladesh National Herbarium, Mirpur, Dhaka. The voucher specimen of the plant has been deposited (No. DACB-37905) in the herbarium for further reference.
Collected leaves were gently washed to remove the dust. Leaves were shade dried at room temperature for 20 days. About 1 kg of dried powdered leaves of the plant was extracted by cold maceration with ethanol (80% v/v) for 48 h, with intermittent agitation. The extract was then filtered using the filter paper (Whatman No. 3). The filtrate obtained was concentrated in a Rotavapour (Buchii Rota-vapour R-205, BUCHI Labortechnik AG, Flawil, Switzerland) under reduced pressure and completely dried over a regulated water bath maintained at 50°C. The percentage yield was calculated to be approximately 26.5%. This ethanolic extract was kept at 4°C until the experiments for pharmacological screening were done.
Phytochemical analysis
The extract was subjected to phytochemical analysis for identification of constituents using standard procedures.[11]
Studies on the guinea pig trachea
Guinea pigs were housed in the laboratory animal facility. All experiments were conducted in accordance with the Accepted International Laboratory Animals Care, and guidelines and rules for animal use in experiments. Guinea pigs of either sex (350-550 g) were used for these experiments. Animals were sacrificed by a blow on the head and exsanguinations. The isolated trachea was removed immediately and under dissecting microscope was prepared with the intact epithelium, free of serosal connective tissue, in an ice-cold oxygenated Krebs–Henseleit (KH) solution (concentration in mM: 118.2 NaCl, 25 NaHCO3, 4.6 KCl, 1.2 KH2 PO4, 1.2 MgSO4, 2.5 CaCl2, and 10 dextrose; pH = 7.4; all chemicals were purchased from Sigma-Aldrich Chemie Gmbh, Munich, Germany). The trachea was then cut transversely between cartilaginous rings, and a cotton thread was tied to the cartilages to form tracheal chain containing four rings. The chain was suspended in a 20-ml thermo-regulated organ bath (Radnotti Glass Technology, Monrovia, CA, USA) filled with 15 ml KH solution, maintained at 37°C and continuously oxygenated with a gas mixture, 95% O2 and 5% CO2. These tracheal preparations were anchored between a stainless hook at the bottom of the organ bath and a force displacement transducer on recording device. The responses of TSM were recorded with Grass 7E Polygraph 5 × D.C. Amplifier. An initial load (1.0 g) was applied and then tissues were allowed to equilibrate for 60 min in the organ baths containing KH solution. The solution was changed every 20 min. After equilibration, a concentration–response curve to histamine (10–6-10–3.5 M; Sigma-Aldrich, Chemie Gmbh, Munich, Germany) was recorded. Effect of ethanolic extract on histamine-induced contraction was evaluated by pre-incubating tracheal preparations on ethanolic extract (400 μg/ml) for 10 min and then treated with various concentrations of histamine. In order to study the involvement of (NO) on the effects of ethanolic extract, an NO synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (L-NAME; 10–4 M; Sigma-Aldrich, Germany) was used 30 min prior to the addition of ethanolic extract.
To study whether ethanol extract induces direct relaxant responses on TSM, the preparations were pre-constricted with histamine (3 × 10–4.5 M, the concentration elicited 75% of maximal constriction) and then treated with different concentrations of ethanolic extract (100-1600 μg/ml) in absence or presence of L-NAME. The relaxation was expressed as a percentage of the total pre-constricted state for each tracheal preparation.
Statistical analysis
The data of the experiments were expressed as mean ± SEM. The results obtained were analyzed using one way ANOVA. In all cases, P < 0.05 was considered statistically significant.
RESULTS
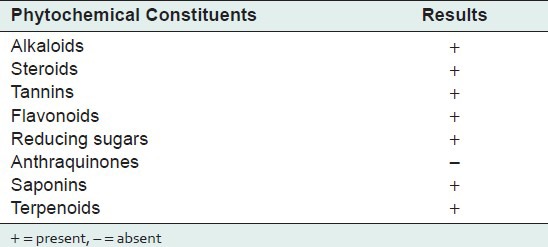
Phytochemical analysis of ethanolic extract showed the presence of typical plant constituents such as alkaloids, steroids, tannins, flavonoids, reducing sugars, saponins, and terpenoids [Table 1].
Table 1.
Phytochemical constituents of the leaves extract of Nyctanthes arbortristis
Evaluation of the effect of ethanolic extract to histamine-induced dose-dependent contraction of TSM, indicated that ethanolic extract (400 μg/ml) exhibited a significant inhibition (P < 0.001) of contractile responses produced by histamine, confirming a bronchodilatory activity of ethanolic extract. In order to find the mechanisms of this inhibition caused by the ethanolic extract of the leaves of N. arbortristis, prior the administration of ethanolic extract, tissues were pre-incubated with an NOS inhibitor – L-NAME (10–4 M). L-NAME reversed the inhibitory effect of ethanolic extract to histamine-induced contraction of TSM [Figure 1]. The differences were statistically significant (P < 0.05). The maximal responses of TSM to histamine in absence or presence of ethanolic extract and L-NAME are shown in Table 2.
Figure 1.
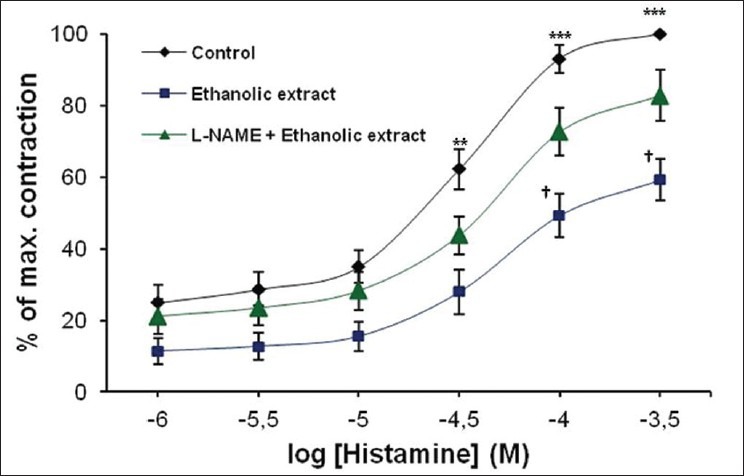
Effect of ethanolic extract of the leaves of Nyctanthes arbortristis on histamine-induced contraction of tracheal smooth muscle. Data are expressed as means ± SEM, n = 7; *Control vs. ethanolic extract, *P < 0.05, ***P < 0.001, †Ethanolic extract vs. Nω-nitro-l-arginine methyl ester + ethanolic extract, †P < 0.05
Table 2.
Maximal contractile responses to histamine and EC50 values
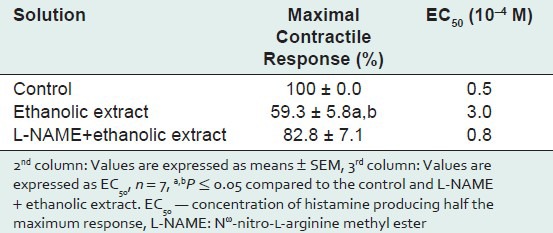
The effective concentration of histamine producing 50% of the maximum response (EC50) in presence of ethanolic extract significantly increased (P < 0.01), thereby decreasing the sensitivity of TSM to histamine. This concentration of histamine was six times higher as compared to control. – However, in the presence of L-NAME, this effect of ethanolic extract was reversed (P < 0.05) [Table 2].
Cumulative doses of ethanolic extract relaxed pre-constricted tracheal preparations in dose–response manner. The relaxant responses were significant as compared to the total pre-constricted state (P < 0.001). Relaxant effect of ethanolic extract was prevented by L-NAME, though not completely, but significantly reduced the relaxation of TSM produced by ethanolic extract [ P < 0.01; Figure 2], showing an important role of NO on bronchodilatory activity of the leaf extract of N. arbortristis.
Figure 2.
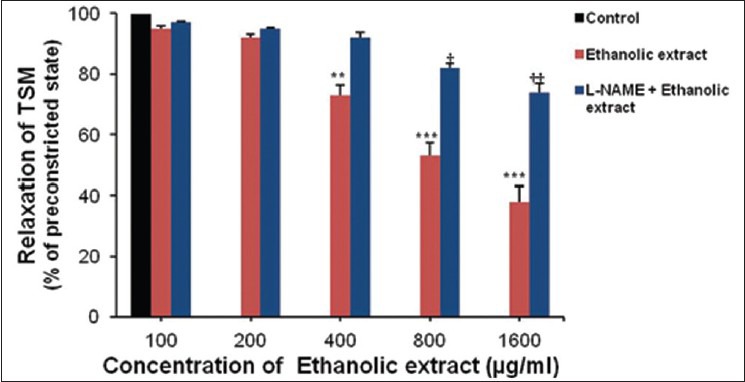
Relaxant effect of ethanolic extract of the leaves of Nyctanthes arbortristis on pre-constricted tracheal smooth muscle with histamine. Data are expressed as means ± SEM, n = 7, *Ethanolic extract vs. control, **P < 0.01, ***P < 0.001, †Nω-nitro-l-arginine methyl ester + ethanolic extract vs. ethanolic extract, †P < 0.05, ††P < 0.01
DISCUSSION
The pathophysiology of asthma is marked by early and late asthmatic reactions, characterized by changes in airway's function, inflammation, and airway remodeling. All these changes can be included by a cascade of inflammatory reactions involving mediators.[1,2] Histamine as a mediator implicated in the pathophysiology of asthma mimics several symptoms of disease, including bronchoconstriction. Therefore in this study, we tested the ability of ethanolic extract of the leaves of N. arbortristis to counteract the histamine-induced contractile responses of TSM. Histamine induced the concentration-dependent constriction of airways. Ethanolic extract of the leaves of N. arbortristis inhibited the histamine-induced contractile response of TSM, displaying a rightward shift of the concentration–response curve of histamine with reduced maximum response. In presence of ethanolic extract, the EC50 of histamine concentration was increased almost six folds. To our knowledge, our data confirm for the first time the bronchodilatory effect of ethanolic extract of the leaves of N. arbortristis on airway smooth muscle. The ability of ethanolic extract to inhibit the effects of bronchoconstrictors such as histamine suggests for possible use in asthma treatment. Our data are in agreement with other studies showed relaxant effect of petroleum ether extract of the barks of N. arbortristis on ileum preparations of guinea pigs.[12] Pre-incubation of tissues with NOS inhibitor reduced the inhibitory effect of ethanolic extract to histamine, shifting the concentration–response curve toward the control level. This indicates that acting mechanism of ethanolic extract is through the increase of NO production. NO can initiate the relaxation cascade inside the smooth muscle cells via cyclic guanosine monophosphate (cGMP) signaling to balance the contractile responses induced by bronchoconstrictors. On other side in prior studies, it was demonstrated that NO can also inhibit the release of mast cell mediators such as histamine, preventing degranulation of mast cells, thus decreasing the airway smooth muscle tone as a consequence. These proposed mechanisms are consistent with prior findings revealed that increase in NO production attenuates/inhibits allergen-induced mast cell activation.[13,14] Nirmal et al. also found that petroleum ether extract has mast cell stabilizing property using a mouse model.[12]
Furthermore, the results indicated that ethanolic extract of the leaves of N. arbortristis induced dose-dependent relaxation of pre-constricted TSM. Inhibition of NO production reduced the relaxant effect of ethanolic extract, complementing the above results obtained from contraction experiment. These results are in agreement with other previous studies showing a relaxant effect of petroleum ether extract of the barks of N. arbortristis.[12]
Phytochemical analysis showed the presence of typical plant constituents such as alkaloids, steroids, tannins, flavonoids, reducing sugars, saponins, and terpenoids. Previous study showed that leaves of N. arbortristis supposed to have anti-asthmatic activity due to the presence of β-sitosterol.[9] Other chemical constituents isolated in the leaves of this plant are iridoid glucosides; main alkaloid nyctanthin; arbortristosides A, B, C; nyctanthic acid; tannic acid; d-mannitol; methyl salicylate; volatile oil; carotene; terpenoid; and cardiac glucosides.[15] These activities of the leaves of N. arbortristis connect with the traditional use of this plant in treatment of diseases marked with bronchoconstriction.
In conclusion, the extract of leaves has a direct relaxant effect on airways via increasing the NO production. Further investigations on the efficacy and the isolation of the specific bioactive molecule situated in the ethanolic extract are needed in order to lead to the development of the leaves extract into a new drug for treatment of asthma.
Footnotes
Source of Support: DGHS-011 and Erasmus Mundus mobility program
Conflict of Interest: None declared
REFERENCES
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–45. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 2.Rang HP, Dale MM, Ritter JM, Flower RJ. Rang and Dale's Pharmacology. 6th ed. London: Churchill Livingstone; 2007. Respiratory system; pp. 356–367. [Google Scholar]
- 3.Samb A, Pretolani M, Dinh-Xuan AT, Ouksel H, Callebert J, Lisdero C, et al. Decreased pulmonary and tracheal smooth muscle expression and activity of type 1 nitric oxide synthase (nNOS) after ovalbumin immunization and multiple aerosol challenge in guinea pigs. Am J Respir Crit Care Med. 2001;164:149–54. doi: 10.1164/ajrccm.164.1.2004030. [DOI] [PubMed] [Google Scholar]
- 4.Moncada S, Palmer RM, Higgs EA. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989;38:1709–15. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- 5.Undem BJ. Pharmacotherapy of asthma. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill; 2006. pp. 717–36. [Google Scholar]
- 6.Barnes PJ. Current therapies for asthma. Promise and limitations. Chest. 1997;111:17S–26. doi: 10.1378/chest.111.2_supplement.17s. [DOI] [PubMed] [Google Scholar]
- 7.Sandhar HK, Kaur M, Kumar B, Prasher S. An update on Nyctanthes arbortristis Linn. Internationale Pharmaceutica Sciencia. 2011;1:77–86. [Google Scholar]
- 8.Nawaz AH, Hossain M, Karim M, Khan M, Jahan R, Rahmatullah M. An ethnobotanicals survey of Jessore district in Khulna division. Am-Eurasian J Sustain Agric. 2009;3:238–43. [Google Scholar]
- 9.Nirmal SA, Pal SC, Mandal SC. Antiasthmatic activity of Nyctanthes arbortristis leaves. Lat Am J Pharm. 2011;30:654–60. [Google Scholar]
- 10.Das S, Sasmal D, Basu SP. Antispasmodic and anthelmintic activity of Nyctanthes arbortristis Linn. Int J Pharm Sci Res. 2010;1:51–5. [Google Scholar]
- 11.Treas GE, Evans WC. Pharamcognosy. 12th ed. United Kingdom: Balliere Tindal; 1983. Drugs of biological origin; pp. 309–540. [Google Scholar]
- 12.Nirmal SA, Pal SC, Mandal SC. Mast cell stabilizing and bronchodilatory activity of Nyctanthes arbortristis bark. Phytopharmacology. 2012;2:234–42. [Google Scholar]
- 13.Sopi RB, Mirjam Simoons, Michael J. Sanderson, Herman Meurs, Harm Maarsingh. Allergen-induced airway constriction in guinea pig lung slices is attenuated by arginase inhibition via increased nitric oxide production. FASEB J. 2012;26:1061.2. [Google Scholar]
- 14.Coleman JW. Nitric oxide: A regulator of mast cell activation and mast cell-mediated inflammation. Clin Exp Immunol. 2002;129:4–10. doi: 10.1046/j.1365-2249.2002.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathore B, Paul B, Chaudhury BP, Saxena AK, Sahu AP, Gupta YK. Comparative studies of different organs of Nyctanthes arbortristis in modulation of cytokines in murine model of arthritis. Biomed Environ Sci. 2007;20:154–9. [PubMed] [Google Scholar]


