Abstract
Background:
Consumption of arsenic contaminated water has been implicated in metalloid-induced carcinogenesis. Dietary intake of certain plant products with chemoprotective properties may protect against the onset of diseases and promote maintenance of health.
Objectives:
We investigated the outcome of black walnut Juglans nigra (JN) consumption on sodium arsenite (SA)-induced toxicity in rats.
Materials and Methods:
Wister albino rats were treated as follows: Control, SA only (positive control) (2.5 mg/kg body weight), JN only (100 mg/kg weight), and JN+SA coadministered. After 5 weeks animals were sacrificed whole blood, femur, liver and testis harvested were assessed for hepatic transaminases and clastogenicity. Histology of the liver, sperm morphology and quality were also assessed. Data were analyzed (ANOVA) and expressed as means ±SD.
Results:
SA treatment elevated hepatic transaminases level in serum (P < 0.05), induced histological changes in liver: fibroplasia and periportal hepatocytes infiltration by mononuclear cells. These changes were ameliorated by JN (P < 0.05) coadministration. SA induced micronuclei formation (P < 0.05). Again JN decreased (P < 0.05) micronuclei formation by 50%. Sperm count and motility decreased (P < 0.05) in all groups compared to control.
Conclusion:
JN showed no protection against arsenite effect on sperm quality. Hepatoprotective and anticlastogenic effects were apparent suggesting a chemopreventive potential active against arsenite genotoxicity and chromosomal instability which have implication for metalloid-induced carcinogenesis.
Keywords: Carcinogenesis, chemoprevention, Juglans nigra, micronuclei, sodium arsenite
INTRODUCTION
Arsenic poisoning in Bangladesh has been described as the largest mass scale poisoning in human history.[1] Till date arsenic -a well-known human carcinogen- is a ubiquitous environmental contaminant in aquifers, ground water supplying municipal water. Both trivalent and pentavalent arsenic are present in contaminated ground water.[2] Contamination above the permissible upper limits of 10 μg/L,[3] poses a public health concern in various countries.[4] Increase in cancer risk observed from epidemiological studies is associated with the presence of inorganic trivalent arsenic.[5,6]
Arsenicosis, heart disease, diabetes, and cancers are some of the complications arising from arsenic poisoning.[7] Arsenic has been reported to act with other risk factors synergistically to induce deoxyribonucleic acid (DNA) damage (mutations) and cancer development.[8,9] Although several hypotheses have been proposed, the mechanisms responsible for arsenic carcinogenesis has not been established. One possible mechanism is the generation of reactive oxygen species (ROS) which has a direct damaging impact on cellular macromolecules including DNA[10] resulting from arsenic metabolism. Arsenic intoxication in experimental animals has also been associated with hepatic tumors,[11] the inhibition of testicular steroidogenic function and spermatogenesis.[12] Medical intervention has not been helpful in dealing with long-term implications of arsenic exposure and subsequent poisoning, underscoring the need for preventive mechanisms to mitigate these public health concern.
This underscores a potential role of antioxidants in the prevention and or management of arsenic toxicity.[13,14] Recent studies have suggested chemoprevention -the use of antioxidants and antioxidant-rich foods- for the management of arsenicosis[15] especially in the natural forms present in plant. Consumption of healthy diet provides antioxidants and in addition to nutrients. Nuts are rich in polyphenolic and flavonoids compounds which are known to exhibit strong antioxidant properties, hence, may confer health benefits. Black walnuts -Juglans nigra- (JN) contains a plethora of chemical compounds[16] including antioxidants omega-3 fatty acids and phytosterols. Black walnut has been reported to exhibit antiparasitic and cholesterol-lowering effects.[17] JN has also been claimed to have antioxidant properties, scientific data in support of some of these claims are scanty. The present study was undertaken to evaluate the chemoprotective effect of aqueous extract of JN on sodium arsenite (SA)-induced toxicity in male Wistar albino rat.
MATERIALS AND METHODS
Chemicals
SA (BDH Chemicals Ltd., Poole, England) 2.5 mg/kg body weight (corresponding to 1/10th of the oral LD50 of SA) was administered to the experimental animals.[18,19] All other reagents and chemicals were of analytical grade.
Preparation of aqueous extract of Juglans nigra
The nuts of JN were removed from its kernels, chopped into small pieces and dried at ambient room temperature. The dried nuts were finely milled and extracted with distilled water (1:2) respectively for 72 hours. The aqueous extract was concentrated in a rotary evaporator, lyophilized, and preserved for further use.
Phytochemical screening
JN extract was subjected to the phytochemical test using Trease and Evans,[20] and Harbourne,[21] methods for flavonoids, alkaloids, tannins, anthraquinones, saponins, and cardenolides.
Experimental animals
Twelve week's male albino Wistar rats (200-250 g) purchased from Covenant Farms (Ibadan, Nigeria) were housed in the Department of Biochemistry, University of Ibadan, Nigeria Primate colony. Rats were fed with rat pellet from Ladokun Livestock Feeds (Ibadan, Nigeria) and water ad libitum.
Experimental protocol
Animals were randomly distributed into four groups of five rats each. Group I served as the control. Groups II, III, and IV were treated with SA (2.5 mg/kg body weight), JN (100 mg/kg body weight) and SA and JN (2.5 mg/kg and 100 mg/kg body weight), respectively. SA and JN was administered weekly and every third day respectively by gavage for 5 weeks. The animals were injected (i.p.) with 0.04 % colchicine (1 mL/100 g body weight) 2 hours before sacrifice. Twenty-four hours after the last treatment the animals were bled by retro-orbital puncture and sacrificed by cervical dislocation and the liver, femur, and epididymis harvested.
Micronucleus assay
Clastogenicity was evaluated in the rat bone marrow using the micronucleus assay as described by Heddle and Salmone, modified by Heddle et al.[22] Bone marrow cells were extruded with fetal calf serum from both femurs and smeared on clean glass slides. The slides were fixed in methanol, air-dried, and treated first with May-Grunewald solution then stained with Giemsa solution. The slides were scored for the presence of micronucleated polychromatic erythrocytes (mPCEs) using Nikon Epi-fluorescent microscope model E200.
Sperm motility assay
The caudal epididymis was minced in pre-warmed normal saline (37°C). One drop of sperm suspension was placed on a clean glass slide to analyse 200 motile sperm in four different fields with a Nikon eclipse 200 microscope at 200X magnification as described by Zemjanis.[23] The motility of epididymal sperm was evaluated microscopically within 2-4 min of their isolation from the epididymis and motility were expressed as percentages of total number of sperm counted.
Sperm count
Epididymal sperm was obtained by mincing the epididymis in normal saline, and filtering through a nylon mesh (80 pt. pore size). The sperm were counted using a hemocytometer; the number of sperm in five squares were counted and averaged following the methodology of Freund and Arol.[24,25]
Morphological abnormalities
A portion of the sperm suspension was placed on a clean glass slide, smeared out with another slide, fixed in 95% ethanol and stained with eosin. A total of about 400 sperm from each rat were examined for abnormalities in different regions of spermatozoa, according to the method described by Wyrobek and Bruce.[26]
Data analysis
All results were expressed as mean ± Standard deviation (±S.D). Differences between the groups were analyzed by one-way analysis of variance (ANOVA) with the aid of Statistical Package for Social Sciences (SPSS) software, SPSS Inc., Chicago, Standard version 10.0.1. P-values <0.05 were considered statistically significant.
RESULTS
Phytochemical profile of JN indicated the presence of saponins, anthraquinones, alkaloids, and cardenolides [Table 1]. There was no significant increase in body weight throughout the study. However, there was a significant decrease in liver and relative liver weights of group II animals compared with other treatment groups [Table 2]. Increases in serum hepatic transaminases were significant [Table 3] in SA-only-treated group compared with the control and JN-only groups. Groups treated with JN, serum transaminases were similar to control group.
Table 1.
Phytochemical result showing the components present in Juglans nigra fruit

Table 2.
Influence of Juglans nigra treatment on body weight, change in body and relative liver weight of the rats treated with sodium arsenite
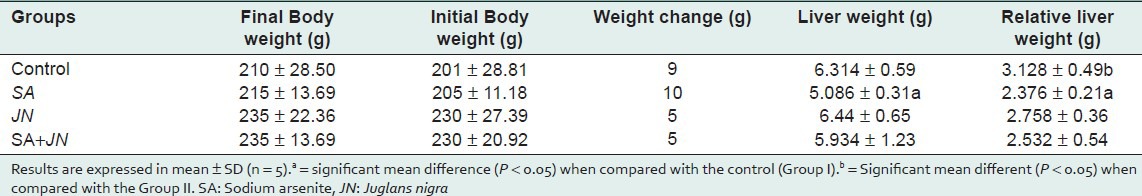
Table 3.
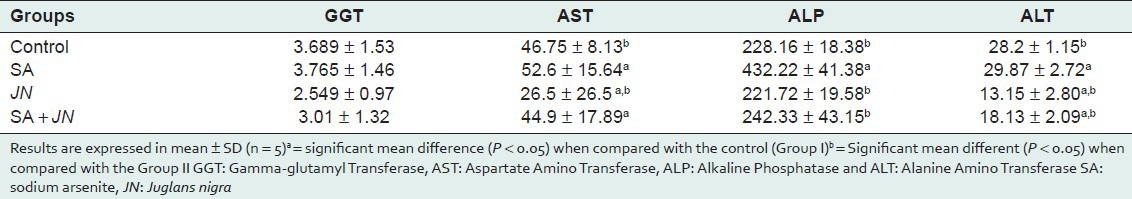
Influence of Juglans nigra administration on serum level of hepatic transaminases (GGT, AST ALT, ALP, and ALT) in rats treated with sodium arsenite
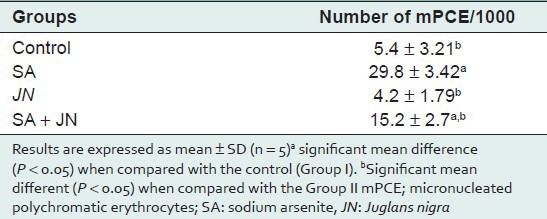
Histopathology of liver from control group showed no visible lesions. JN-treated group was comparable to the control. SA treatment resulted in diffuse hydropic degeneration of hepatocytes, portal congestion, fibrotic changes, and periportal cellular infiltration by mononuclear cells. JN and SA group exhibited mild vacuolar degeneration of hepatocytes [Figure 1]. Compared with control group, SA significantly induced micronuclei formation in the rat polychromatic erythrocytes [Table 4]. The observed SA-induced mPCEs was, however, lowered significantly by JN co-treatment, but higher than control and JN-only groups.
Figure 1.
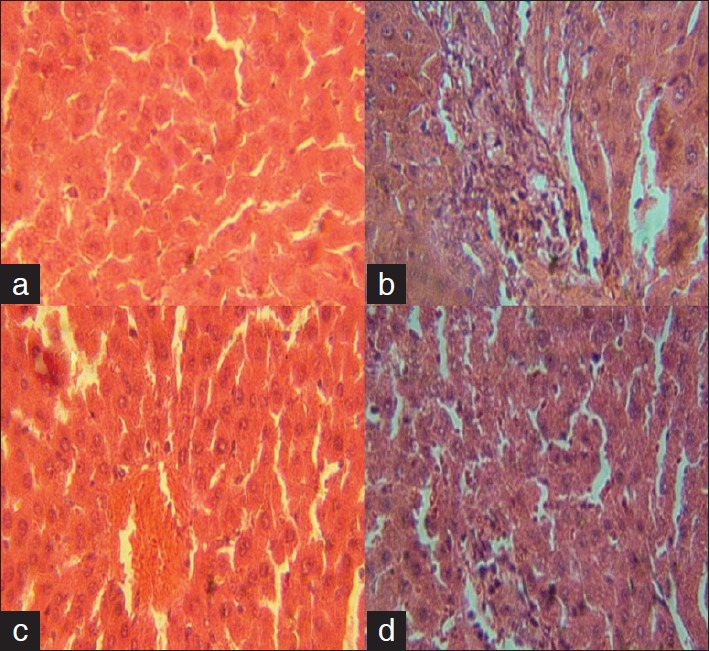
Photomicrograph of liver sections rats treated with Juglans nigra (JN) and sodium arsenite for 5 weeks. (a) control showing no visible lesions, (b) NaAsO2 (2.5 mg/kg body weight) showing diffused degeneration of hepatocytes, portal congestion, fibrotic changes and periportal cellular infiltration by mononuclear cells, (c) JN only (100 mg/kg body weight), showing no visible lesions and (d) JN + SA (100 mg/kg and 2.5 mg/kg and 100 mg/kg body weight) showing very mild vacuolar degeneration of hepatocytes
Table 4.
Effect of Juglans nigra administration on mPCE formation in rats bone marrow treated with sodium arsenite
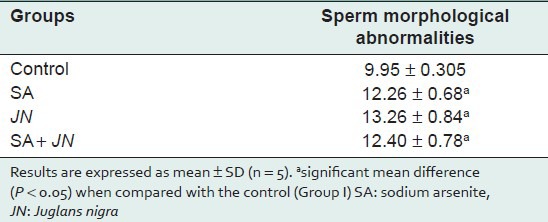
Sperm quality assessment [Table 5] indicated a significant decrease in sperm count and motility in treated groups compared with the control. Morphologically, spermatozoa's were not significantly affected [Table 6].
Table 5.
Effect of Juglans nigra treatment on sperm quality (count, motility and viability) in rats treated with sodium arsenite
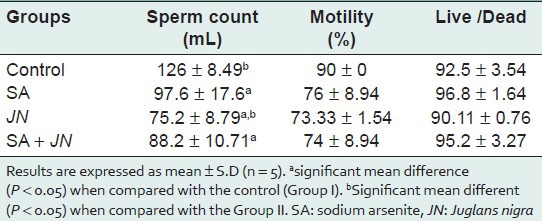
Table 6.
Effect of Juglans nigra treatment on sperm morphological abnormalities rats treated with sodium arsenite
DISCUSSION
Arsenic is a naturally occurring metalloid and can contaminate water sources when found in ground water supplying human population both in the rural and urban settings common in some regions of the world. Recently, in Bangladesh, there was arsenic contamination that resulted in mass poisoning of whole populations (WHO, 2000). While certain amount of arsenic is permitted in municipal water (>10 μg/L) beyond this threshold, arsenic can be a cause of concern constituting health hazard. In recent years, environmental exposure to arsenic has been implicated in the occurrence of various cancers and numerous health problems.[7] Certain cancers have been linked to the inadvertent consumption of arsenic contaminated water. Studies have related the presence of inorganic trivalent arsenic as playing a role in arsenic-induced carcinogenesis.
Given the challenges that exist in treating cancer effectively,[27] prevention strategies represent an essential part in cancer control.[28] It has been reported that effective prevention initiatives can decrease cancer incidence and mortality by ≥ 50%.[29] Consumption of food products rich in antioxidants have been suggested and shown to hold promise in mitigating disease onset and chemical-induced carcinogenesis. One target of arsenic induced toxicity appears to be the liver, central to metabolism of xenobiotic. Radicals generated from normal cellular activities can cause oxidative stress when cellular clearance mechanisms are overwhelmed. Macromolecules necessary for optimal cell function can be damaged as a result e.g., DNA. This may cause mutations and chromosomal instability. DNA damaging events have been implicated in chemical carcinogenesis and by extension a possible mechanism metalloid-induced carcinogenesis. These study reports the effect of the aqueous extract of Juglans nigra on SA-induced toxicity on albino Wistar rats.
JN extract contains saponins, alkanoids, tannins, saponins, and cardenolides [Table 1], which may be beneficial or toxic. Alkaloids and cardenolides are known to possess antioxidant properties that can mitigate cellular macromolecule damage from oxidative radical species.[30] Such as DNA strand damage, resulting in genomic instability implicated in carcinogenesis. Saponins have been reported to have hemolytic properties, although they are not toxic when administered orally. Polyphenolic tannins have been reported to exhibit metal ions chelating properties that can help in mopping up trivalent arsenite upon exposure.[31] However, tannins can be poisonous by depriving the body of essential metal ions needed for proper functioning.
There was no significant increase in body weight throughout the course of the study [Table 2]. Compared with control, there was significant decrease in liver weight of SA-only-treated group. This resulted from arsenic toxicity in the liver and increased hepatic metabolism to eliminate it. These observations are consistent with reports that SA toxicity can compromise the integrity of the liver in mouse, rat, and goat.[32,33] Liver weights and relative liver weights of groups treated with SA and JN (IV) and JN (III) were similar to those of the control, indicative of JN exhibiting a potent protective effect on hepatocytes on the one hand and in response to SA-induced toxicity thereby ameliorating SA-induced toxicity on hepatocytes.
Exposure to SA had been shown to increase activity of liver transaminases in the blood,[34] this is an index of hepatotoxicity which might have resulted from oxidative stress-related damages to hepatocytes membrane and leakage of hepatic transaminases into extracellular spaces ultimately finding their way into the blood from the liver. From the results presented in Table 4 there is a significant increase in the activities of AST, ALP, and ALT (P < 0.05) in the serum of SA-treated rats when compared with the control. However, there is a significant decrease (P < 0.05) in serum activities of AST and ALP in the SA+JN-treated group compared with control. This is suggestive of hepatocytes protection from JN co-treatment against SA-induced damages. This can be attributed to the antioxidant present in JN. This is in accordance with some recent studies that recommend the use of antioxidants and antioxidant-rich foods and herbal medicinal plant for the management of arsenicosis.[15] The activities of all the liver enzymes (GGT, AST, ALT, and ALP) were significantly low in the JN-only-treated group confirming protective property of walnut. Our finding from liver histopathology suggests SA-induced toxicity and ameliorative potentials of JN on SA induced hepatocytes damage. Histological examinations of liver sections of treated animals showed that SA was potentially hepatotoxic as reflected by portal congestion, fibrotic changes, and periportal cellular infiltration by mononuclear cells. Liver sections from SA and JN-treated group exhibited very mild hepatic degeneration, while those of the JN-treated group showed no visible lesions confirming a modulatory effect of JN on SA-induced hepatocytes damage.
SA significantly (P < 0.05) induced the formation of micronuclei in the polychromatic erythrocytes (mPCEs) of the rat bone marrow cells [Table 5]. This observation is consistent with our earlier observation on the clastogenic potentials of SA in the bone marrow.[35,36,37] There was a significant (P < 0.05) increase in the number of mPCEs in the SA-treated group when compared with the control [Table 5]. This may be due to the fact that arsenite generates free radicals that can attack DNA leading to chromosomal breakage. There is, however, a significant decrease (P < 0.05) in mPCEs formation in the SA and JN-treated group when compared to the SA-only-treated group. This reduction can be attributed to both the antioxidant-scavenging properties provided by the walnut extract.[38] The number of mPCEs in the JN-only-treated group was similar to that of the control, indicative that JN on its own may not be genotoxic or result in any clastogenic activity leading to chromosomal instability and disease development. Sperm morphological abnormality, the results obtained in this study with regards sperm to quality [Table 6] there is a significant (P < 0.05) decrease in sperm count and motility in the SA-treated group compared with the control. There was a decrease (P < 0.05) in sperm count and motility in the SA+JN and the JN-only treatment groups, indicating that JN did not seem to offer any protection against SA-induced reproductive toxicity.
Overall the results suggest that JN protects cells against oxidative DNA damages from ROS generated from arsenic metabolism. Damaged DNA may adversely impact genomic stability with deleterious effect on normal cellular function and disease development.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Smith AH, Lingas EO, Rahman M. Contamination of drinking water by arsenic in Bangladesh: A public health emergency. Bull World Health Organ. 2000;78:1093–103. [PMC free article] [PubMed] [Google Scholar]
- 2.Schwerdtle T, Walter I, Mackiw I, Hartwig A. Induction of oxidative DNA damage by arsenite and its trivalent and pentavalent methylated metabolites in cultured human cells and isolated DNA. Carcinogenesis. 2003;24:967–74. doi: 10.1093/carcin/bgg018. [DOI] [PubMed] [Google Scholar]
- 3.U.S. EPA (U.S. Environmental Protection Agency) National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring; final rule. Fed Reg. 2001;66:6976–7066. [Google Scholar]
- 4.International Agency for Research on Cancer (IARC) working group on the evaluation of carcinogenic risks to humans. Some drinking water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risks Hum. 2004;84:1–477. [PMC free article] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer (IARC) Some metals and metallic compounds. IARC Monogr Eval Carcinog Risks Hum. 1980;23:39–142. [PMC free article] [PubMed] [Google Scholar]
- 6.Tinwell H, Stephens SC, Ashby J. Arsenite as the probable active species in the human carcinogenicity of arsenic: Mouse micronucleus assays on Na and K arsenite, orpiment, and Fowler's solution. Environ Health Perspect. 1991;95:205–10. doi: 10.1289/ehp.9195205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida T, Yamauchi H, Fan Sun G. Chronic health effects in people exposed to arsenic via the drinking water: Dose-response relationships in review. Toxicol Appl Pharmacol. 2004;198:243–52. doi: 10.1016/j.taap.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Evans CD, LaDow K, Schumann BL, Savage RE, Jr, Caruso J, Vonderheide A, et al. Effect of arsenic on benzo[a]pyrene DNA adduct levels in mouse skin and lung. Carcinogenesis. 2004;25:493–7. doi: 10.1093/carcin/bgg199. [DOI] [PubMed] [Google Scholar]
- 9.Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite cocarcinogenesis: An animal model derived from genetic toxicology studies. Environ Health Perspect. 2002;110(Suppl 5):749–52. doi: 10.1289/ehp.02110s5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchet JP, Lauwerys R, Roels H. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsenate, or dimethylarsenate in man. Int Arch Occup Environ Health. 1980;48:71–9. doi: 10.1007/BF00405933. [DOI] [PubMed] [Google Scholar]
- 11.Waalkes MP, Ward JM, Diwan BA. Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: Promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis. 2004;25:133–41. doi: 10.1093/carcin/bgg181. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar M, Chaudhuri GR, Chattopadhyay A, Biswas NM. Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats. Asian J Androl. 2003;5:27–31. [PubMed] [Google Scholar]
- 13.Wang TS, Kuo CF, Jan KY, Huang H. Arsenite induces apoptosis in Chinese hamster ovary cells by generation of reactive oxygen species. J Cell Physiol. 1996;169:256–68. doi: 10.1002/(SICI)1097-4652(199611)169:2<256::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Flora SJ, Flora G, Saxena G, Mishra M. Arsenic and lead induced free radical generation and their reversibility following chelation. Cell Mol Biol (Noisy-le-grand) 2007;53:26–47. [PubMed] [Google Scholar]
- 15.Das NK, Sengupta SR. Arsenicosis: Diagnosis and treatment. Indian J Dermatol Venereol Leprol. 2008;74:571–81. doi: 10.4103/0378-6323.45098. [DOI] [PubMed] [Google Scholar]
- 16.Maguire LS, O’sullivan SM, Galvin K, O’Connor TP, O’Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts, and the macadamia nut. Int J Food Sci Nutr. 2004;55:171–8. doi: 10.1080/09637480410001725175. [DOI] [PubMed] [Google Scholar]
- 17.Kris-Etherton PM, Zhao G, Binkoski AE, Coval SM, Etherton TD. The effects of nuts on coronary heart disease risk. Nutr Rev. 2001;59:103–11. doi: 10.1111/j.1753-4887.2001.tb06996.x. [DOI] [PubMed] [Google Scholar]
- 18.Preston RJ, Dean BJ, Galloway S, Holden H, McFee AF, Shelby M. Mammalian in vivo cytogenetic assays. Analysis of chromosome aberrations in bone marrow cells. Mutat Res. 1987;189:157–165. doi: 10.1016/0165-1218(87)90021-8. [DOI] [PubMed] [Google Scholar]
- 19.Escribano A, Revilla M, Hernández ER, Seco C, González-Riola J, Villa LF, et al. Effect of lead on bone development and bone mass: A morphometric, densitometric, and histomorphometric study in growing rats. Calcif Tissue Int. 1997;60:200–3. doi: 10.1007/s002239900214. [DOI] [PubMed] [Google Scholar]
- 20.Trease GE, Evans WC. 14th ed. Ohio: Brown Publications; 1983. Pharmacognosy. [Google Scholar]
- 21.Harbourne JB. London: Chapman and Hall; 1983. Phytochemical Methods: A guide to modern technique of plants analysis; pp. 94–103. [Google Scholar]
- 22.Heddle JA, Salamone MF. The micronucleus assay I: In vitro. In: Stich H.F, San R.H.C, editors. Topics in environmental physiology and medicine. Short term tests for chemical carcinogens. New York: Springer Verlag; 1981. pp. 243–9. [Google Scholar]
- 23.Zemjanis R. Collection and evaluation of semen. 2nd ed. Baltimore: William and Wilkins Company; 1970. Diagnostic and therapeutic technique in animal reproduction; pp. 139–153. [Google Scholar]
- 24.Freund M, Carol B. Factors affecting haemocytometer counts of sperm concentration in human semen. J Reprod Fertil. 1964;8:149–55. doi: 10.1530/jrf.0.0080149. [DOI] [PubMed] [Google Scholar]
- 25.Moraes GES. Espermocitologia espermocitograma em criterio estrito. Educs. (2nd ed) 2007:61–93. ISBN 8570614209. [Google Scholar]
- 26.Wyrobek AJ, Bruce WR. The induction of sperm shape abnormalities in mice and human. In: Hollaender A, De Serres FJ, editors. Chemical mutagens. Vol. 5. New York: Plenum Press; 1980. pp. 257–85. [Google Scholar]
- 27.Bailar JC, 3rd, Gornik HL. Cancer undefeated. N Engl J Med. 1997;336:1569–74. doi: 10.1056/NEJM199705293362206. [DOI] [PubMed] [Google Scholar]
- 28.Kreiger N, Ashbury FD, Purdue MP, Marrett LD. Workshop report: Environmental exposures and cancer prevention. Environ Health Perspect. 2003;111:105–8. doi: 10.1289/ehp.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller AB. Canadian contributions to cancer control. Can J Oncol. 1994;4:238–42. [PubMed] [Google Scholar]
- 30.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: Evidence from laboratory investigations. Am J Clin Nutr. 2005;81(Suppl 1):284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 31.Okonkwo TJ, Okorie O, Okonta JM, Okonkwo CJ. Sub-chronic hepatotoxicity of Anacardium occidentale (Anacardiaceae) inner stem bark extract in rats. Indian J Pharm Sci. 2010;72:353–7. doi: 10.4103/0250-474X.70482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma A, Sharma MK, Kumar M. Modulatory role of Emblica officinalis fruit extract against arsenic induced oxidative stress in Swiss albino mice. Chem Biol Interact. 2009;180:20–30. doi: 10.1016/j.cbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Roy S, Roy M, Pandey PK, Tiwari SP. Effects of tissue trace minerals status and histopathological changes in chronic arsenicosis in goats. Vet World. 2009;2(1):8–9. [Google Scholar]
- 34.Chattopadhyay S, Ghosh S, Debnath J, Ghosh D. Protection of sodium arsenite-induced ovarian toxicity by coadministration of L-ascorbate (vitamin C) in mature wistar strain rat. Arch Environ Contam Toxicol. 2001;41:83–9. doi: 10.1007/s002440010223. [DOI] [PubMed] [Google Scholar]
- 35.Moore LE, Smith AH, Hopenhayn-Rich C, Biggs ML, Kalman DA, Smith MT. Micronuclei in exfoliated bladder cells among individuals chronically exposed to arsenic in drinking water. Cancer Epidemiol Biomarkers Prev. 1997;6:31–6. [PubMed] [Google Scholar]
- 36.Odunola OA. Comparative effects of some local food condiments on sodium arsenite-induced clastogenicity. Afr J Med Med Sci. 2003;32:75–80. [PubMed] [Google Scholar]
- 37.Celik A, Ogenler O, Cömelekoglu U. The evaluation of micronucleus frequency by acridine orange fluorescent staining in peripheral blood of rats treated with lead acetate. Mutagenesis. 2005;20:411–5. doi: 10.1093/mutage/gei055. [DOI] [PubMed] [Google Scholar]
- 38.Vattem DA, Ghaedian R, Shetty K. Enhancing health benefits of berries through phenolic antioxidant enrichment: Focus on cranberry. Asia Pac J Clin Nutr. 2005;14:120–30. [PubMed] [Google Scholar]


