Abstract
Background
Artesunate is commonly used in malaria therapy. Many antimalarial drugs have been associated with male reproductive dysfunction. The effect of artesunate on male reproductive activities was studied using in–vivo and in-vitro experimental models.
Methods
Adult male rats (n=6) were orally given artesunate (2.9 mg/kg body weight) on daily basis for five days. Artesunate (2.9 mg/kg body weight) was administered to another group of rats daily for six weeks, while there was a recovery group of rats too. The control animals received the vehicle only. At the end of the treatment, sperm characteristics, serum follicle stimulating hormone, luteinizing hormone and testosterone levels, testicular and epididymal histology and fertility were assessed. Cultured Sertoli cells were treated with 0.3 µM to 10 µM artesunate for five days after which Sertoli cell viability, double-stranded deoxyribonucleic acid (ds-DNA) integrity and genetic expression of Glial cell line-derived neurotrophic factor (GDNF) and transferrin were assessed. The data were analyzed using Graphpad Instat Statistical software. A probability value of p <0.05 was considered significant.
Results
Artesunate did not cause any significant effects in short-term administration but significantly reduced the aforesaid parameters in long-term administration. There were visible lesions in the testicular and epididymal histological studies, although fertility was not significantly reduced. These changes were restored in the recovery experiment. In-vitro studies showed dose and duration dependent changes in Sertoli cell viability and ds-DNA integrity. However, transferrin and GDNF gene expressions were normal.
Conclusion
The results suggest that long-term administration of artesunate could induce reversible infertility in rats which may act via distortion of blood–testis barrier formed by Sertoli cells.
Keywords: Artesunate, Epididymis, Rat, Sertoli cells, Sperm, Testis, Testosterone
Introduction
Malaria affects about 500 million people annually and it is a leading cause of mortality and morbidity in the developing word. It remains a major public health problem in endemic regions (1). Effective treatment of malaria had been a great challenge to medicine and this has had a great impact on man's health and economy (2). Treatment failures have been linked majorly to the development of resistance of the malaria parasite to standard antimalarial agents (3–5). This resistance created a need for new drugs. Discovery of artemisinin and its derivatives (artesunate, artemether, arteether, and dihydroartemisinin) have given renewed hope for combating resistant malaria (6, 7). Artesunate is a semi-synthetic derivative of artemisinin. It is an alkylating agent which generates free radicals, thus, alkylating the parasite's membrane (8). It is used in combination therapy and is effective in cases of uncomplicated P. falciparum. Serious concern has been raised about uncontrolled use of artemisinin derivatives in endemic areas of the disease, such as Nigeria. Moreover, self-medication is quite common and purchase of antimalarials in the open market is rampant (9). The possibility of overdose administration and misappropriation in the usage of antimalarial agents are very common, all of which could lead to toxic effects of the drugs (10–12).
Many antimalarial drugs have been associated with male reproductive dysfunction. Chloroquine has been reported to reduce sperm motility and hence fertility by a reduction in the average number of fetuses of cohabited female rats (13). Orisakwe et al. (14) reported that halofantrine adversely affected sperm parameters. Pyrimethamine was reported to cause spermatogenic arrest and male infertility in a dose-dependent manner (15). A study by Raji et al. (16) showed that an oral artemisinin derivative, artemether, caused a significant reduction in the progressive sperm motility, viability, sperm count and serum testosterone levels in a dose-dependent fashion during an acute administration of the drug in male rats.
It has been reported that high doses of artesunate could produce neurotoxicity such as selective damage to brainstem centers, gait disturbances (17–19) and loss of spinal cord and pain response mechanisms in mice and rats (20). Artesunate has also been shown to cause a decrease in sperm motility in guinea pigs (21).
The effects of artesunate on female reproductive system have also been reported. It was shown to significantly reduce serum progesterone concentration and degenerate the decidual cells and fetus of treated pregnant rats (22). Artesunate has also been reported to cause significant embryo-fetal toxicity causing embryo deaths and malformations (23, 24).
The global use of this antimalarial drug thus made its detailed investigation on male fertility imperative. The present study was therefore aimed at evaluating the reproductive activities of artesunate in male rats and its possible site(s) of action.
Methods
Animals
Male and female Wistar strain albino rats (10–12 weeks; 180–220 g) obtained from the Central Animal House, College of Medicine, University of Ibadan were used for the in-vivo study while Sprague-Dawley albino male rats (16–18 day old; 18–22 g) obtained from the Central Animal House, Central Drug Research Institute, in Lucknow, India were used for the in-vitro study. The rats were provided with feed and water ad libitum all through the period of the study. They were housed in wire mesh cages under a photo-period controlled environment (12L:12D cycles). The drug was administered to the rats orally by the use of orogastric tubes. All the animals were cared for in accordance with the ‘Guide for the Care and Use of Laboratory Animals’ (1996). The use of the animals was reviewed and approved by the ethics committee at the Research Institute where the experiment was carried out.
Drugs and Chemicals
Artesunate tablets used for this study were produced by GlaxoSmithKline Pharmaceutical Company, Nigeria. The pure form of this drug was produced by and obtained from Sigma-Aldrich Inc, St. Louis, USA. The chemicals used in the Sertoli cell culture preparation were obtained from Sigma-Aldrich Inc., St. Louis, USA.
In-vivo studies
The thirty male rats used for this study were divided equally into five groups. Group 1 contained the control rats that were administered distilled water orally for 5 days. Group 2 contained rats being administered 2.9 mg/kg B.W. of artesunate daily for 5 days. This experiment was to mimic the dose and duration of administration of the drug in humans. Rats in group 3 were given 2.9 mg/kg B.W. of artesunate daily for 6 weeks. This was to expose the gametes to the chronic effects of the drug (25, 26). Group 4, received the same dose of artesunate daily for 6 weeks and were allowed to recover in 6 more weeks. Rats in group 5 received distilled water for 6 weeks and they served as the controls for the long-term experiment.
Anesthetic protocol and autopsy
The rats were examined daily throughout the experiment period for signs of toxicity. At the end of the treatment and recovery periods they were killed by exsanguination under 25% urethane anesthesia (0.6 ml/ 100 g B.W.). Urethane has no known spermatogenic or antifertility effects on rat testis (27). Body weight, weight of testes, epididymis and seminal vesicles were harvested at the time of necrospy.
Hormone assay
Blood was collected from each rat via cardiac puncture from which the serum was separated. Serum testosterone, luteinizing hormone and follicle stimulating hormone levels were measured using the enzyme-immunoassay (E.I.A.) technique (28). The E.I.A. kits were produced by Immunometrics (London, UK) and obtained from Nzemat (Lagos, Nigeria). The optical density was read using a spectrophotometer (Jenway, 6300 Spectrophotometer, UK) that was sensitive at wavelengths between 492–550 nm.
Sperm characteristic analysis
The testes were carefully exposed and one of them was removed together with its epididymis. The epididymis was separated and the epididymal fluid was collected from the caudal part and the progressive sperm motility, sperm count, live/dead ratio (viability) and sperm morphology were determined as described earlier (28–30).
Progressive sperm motility evaluation was done immediately after semen collection. Two drops of semen were placed on a microscope slide and two drops of warm 2.9% sodium citrate were added. The slide was then covered with a cover slip and examined under the microscope using 40×objectives with reduced light. Sperm viability was done using the eosin/nigrosin stain. The dead sperm took up the stain. Sperm morphology was carried out by means of the Walls and Ewas stain. Sperm count was carried out using the new improved Neuber's hemocytometer counting chamber.
Daily Sperm Production
Daily sperm production (DSP) was determined using a previously described procedure (31–32). Briefly, the testis was removed, decapsulated, weighed and homogenyized in 50 ml of buffer solution containing Tris and Sucrose (pH=7.5) using a homogenizer at low speed. Elongated spermatid nuclei with a shape characteristic of step 17–19 spermatids (Stage IV–VIII) and resistant to homogenization were counted. Spermatids in ten squares of the Mackler chamber (Sefi-Medical Instruments, Haifa, Israel) were counted. This was done four times and an average value was determined. The result was appropriately adjusted for by dilution and the values were divided by 6.10 days. This time divisor was obtained from the fact that steps 17–19 spermatids make up 48% of one seminiferous epithelium cycle. The results obtained were expressed in the form of count/g testis.
Testicular and Epididymal histology
The testis and epididymis were prefixed in Bouin-Hollande solution prior to histological studies. They were then embedded in paraffin. Five-micron thick paraffin sections were stained with hematoxylin-eosin and examined by light microscopy (33–34).
Fertility Studies
Male rats which had been treated for 6 weeks were cohabited with untreated parous proestrus female albino rats at a ratio of 1:2. The presence of a vaginal plug was considered as an index of positive mating. Calculation of a single time point fertility test for each rat was carried out using the formula: percentage of fertility success is equal to the number of pregnant female rats divided by mated females multiplied by 100. The number of litters delivered and their morphology were also recorded.
In-vitro effects of artesunate on cultured Sertoli cells
Sertoli cells were obtained from the testis of 16 to 18 day old Sprague–Dawley rats (35) and cultured in DMEM/F-12 medium supplemented with insulin (5 mg/L), transferrin (5 mg/L), gentamicin sulfate (25 mg/L), vitamins A and E (200 ng/ml) and sodium bicarbonate (1.2 g/L) in a humidified atmosphere of 5% CO2/95% air at 34 °C at a density of 1×106 cells/25 mm 2 area. After 48 hours, cells were treated with hypotonic solution (20 mM Tris buffer, pH=7.6) for 3 minutes to remove contaminating germ cells. The Sertoli cells were finally plated in 48-well tissue culture plates with fresh DMEM/F-12 medium supplemented further with testosterone (10-6 M) and follicle stimulating hormone (FSH) (0.05 Units/ml).
The following procedures were then carried out on the cultured Sertoli cells:
Sertoli cell viability test (M.T.T. Assay)
The cultured Sertoli cells were treated with 10, 5, 2.5, 1.3, 0.6, and 0.3 µM of the pure form of artesunate. The treatment was continued daily for 5 days in-vitro at 34 °C in an atmosphere of 5% CO2/95% air in a CO2 incubator. After treatment for 24, 72 and 120 hours, 20 µL of M.T.T. [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] was added to each well of the 48-well tissue culture plates (36). The solution was further incubated for 4 hours. Then the whole medium was removed from each well and replaced with 200 µL of Dimethyl Sulfoxide (D.M.S.O.) to dissolve the formazan crystals at 37 °C for 30 minutes. The absorbance was then measured on an automated microplate reader (Microquant Bio-tech Instruments Inc.) at a wavelength of 540 nm.
Determination of the nuclear integrity of treated Sertoli cells
This procedure was used to determine the integrity of the double stranded deoxyribonucleic acid (dsDNA) of treated Sertoli cells (37, 38). The cells were isolated (35) and plated on sterile cover slips in 12-well tissue culture plates at a density of 2.5×106 cells/well and the cells were allowed to grow on these slips. The cells were then treated daily with 10, 5 and 2.5 µM of the pure forms of artesunate for five days. The positive control group contained Sertoli cells that were treated only with Nonoxynol–9 (N–9), a known inducer of DNA fragmentation (39). At the end of the treatment period, the medium in each well was carefully removed. 500 Ml of P.B.S. and 1 µL of 4'-6-Diamidino-2-phenylindole (DAPI) (from a stock solution of 1 µg/µL) were added to each well. This was allowed to incubate on a plate shaker for about one hour. The medium was then gently drained and the cover slips were then carefully removed from each well, mounted on slides and observed under the fluorescence microscope (Nikon Eclipse 80i, Japan) at an excitation wavelength of 350 nm. Apoptotic nuclei were identified by the condensed chromatin gathering at the periphery of the nuclear membrane or a total fragmented morphology of nuclear bodies.
Quantitative Reverse Transcriptase-Polymerase Chain Reaction (QRT-PCR)
Sertoli cells were isolated (35) and plated into 6-well tissue culture plates at a density of 5×106 cells/well. The cells were then treated daily with 5, 2.5 and 1.25 µM of the pure form of artesunate for five days. After the period of treatment, the medium was removed and RNA was extracted from the treated cells. 2.5 µg of each RNA sample was used to form complementary DNA using standard procedures (40). The integrity of the house keeping gene, β-actin was checked and the expression of transferrin and GDNF (Glial cell line-derived neurotrophic factor) genes were determined using the reverse transcriptase polymerase chain reaction technique and resolved using the electrophoresis technique on a horizontal agarose resolving gel assembly. The products were visualized by ethidium bromide (1 µL of EtBr/10 ml of agarose solution) staining in a gel duct (Boivis, Mumbai, India) under ultraviolet light. These genes are responsible for the proper growth of Sertoli cells and essential for mammalian spermatogonial stem cell self-renewal. The rat gene primers (Sigma Genoys, USA) used for the study had the following forward and reverse sequences:
β-actin gene
F=5' AGGCATCCTGACCCTGAAGTA 3'
R=3' TCTTCATGAGGTAGTCTGTCA 5'
Transferrin gene
F=5' GCTGTGGCCAGTTTCTTCTC 3'
R=3' CCACATCTCCACCTCCATCT 5'
GDNF (Glial cell line-derived neurotrophic factor) gene
F=5' CCAATATGCCCGAAGATTATC 3'
R=3' TTCGTAGCCCAAACCCAAG 5'
Statistical Analysis
The data were presented as mean±standard error of mean ( ±SEM) and the statistical significance between the controls and the treated groups was calculated by the analysis of variance (ANOVA) with Student-Newman-Keuls multiple comparison test (41) using the GraphPad InStat Statistical software package. A difference with a probability value of p <0.05 was considered significant.
Results
Effect of artesunate on body and relative organ weights
There were no significant changes in body and relative reproductive organ weights of rats administered artesunate (2.9 mg/kg B.W.) for five days when compared with their controls. However, there were significant reductions (p <0.01) in these parameters in the rats treated for six weeks when compared with their controls. There were gradual recovery of these parameters in rats in the recovery group as there were significant increases in the weights (p <0.05) when compared with the treated group (Table 1).
Table 1.
Effects of artesunate administration on the body and relative organ weight
| Group | Body Weight (g) | Testes (%) | Epididymis (%) | Seminal Vesicle (%) |
|---|---|---|---|---|
| Control (5 days) | 208.00±3.74 | 1.46±0.06 | 0.26±0.01 | 0.53±0.04 |
| Artesunate (5 days) | 209.00±5.83 | 1.47±0.08 | 0.26±0.02 | 0.50±0.03 |
| Control (6 days) | 286.00±9.80 | 1.49±0.03 | 0.29±0.01 | 0.57±0.03 |
| Artesunate (6 weeks) | 236.00±9.80** | 1.30±0.03** | 0.24±0.01* | 0.48±0.02 |
| Recovery | 268.00±8.60† | 1.39±0.02† | 0.25±0.01† | 0.55±0.03 |
p <0.05
p <0.01, (control vs. treated)
p <0.05(recovery vs. treated)
Effect of artesunate on sperm parameters
Administration of artesunate for five days significantly decreased (p <0.001) the mean progressive sperm motility and viability of the treated rats when compared with the controls. There were no significant changes in the mean of their sperm counts. All of these parameters significantly decreased (p <0.001) in the rats given daily artesunate for six weeks when compared with the controls. However, all the parameters gradually restored in the recovery experiment as significant increases (p <0.05) were observed when compared with the treated group (Table 2).
Table 2.
Effects of artesunate administration on sperm parameters
| Sperm parameters | |||
|---|---|---|---|
|
|
|||
| Group | Motility (%) | Viability (%) | Count (106/ml) |
| Control (5 days) | 74.60±1.30 | 79.60±1.40 | 42.99±1.82 |
| Artesunate (5 days) | 60.80±1.16*** | 63.00±1.89*** | 39.94±3.19 |
| Control (6 days) | 81.40±1.86 | 85.80±1.80 | 48.04±0.92 |
| Artesunate (6 weeks) | 57.20±1.56*** | 60.20±1.39*** | 35.77±0.86*** |
| Recovery | 79.00±1.18††† | 83.20±1.36††† | 46.31±1.38††† |
p <0.001(control vs. treated)
p <0.001(recovery vs. treated)
Daily administration of artesunate to rats for 5 days caused no significant changes in the number of abnormal sperm compared with the controls. There was a significant increase (p <0.01) in the number of abnormal sperm in rats treated for six weeks when compared with the controls. However there was a significant decrease (p <0.05) in the number of abnormal sperm in the recovery group when compared with the treated group (Table 3).
Table 3.
Effects of artesunate administration on sperm morphological parameters and daily sperm production
| Group | % Abnormal sperms | Daily Sperm Production (10 6/g tissue) |
|---|---|---|
| Control (5 days) | 7.56±0.89 | 20.68±1.62 |
| Artesunate (5 days) | 9.88±1.19 | 17.61±1.26 |
| Control (6 days) | 4.94±0.63 | 22.04±0.38 |
| Artesunate (6 weeks) | 10.12±1.27** | 15.63±0.56*** |
| Recovery | 6.67±0.72† | 19.16±1.10** |
p <0.01(control vs. treated)
p <0.05(recovery vs. treated)
p <0.001 (control vs. treated)
Effects of artesunate on daily sperm production
The daily sperm production of artesunate rats administered for five days showed no significant changes when compared with the controls. There was a significant decrease (p <0.001) in the daily sperm production of rats treated for six weeks when compared with their controls. The daily sperm production of rats in the recovery group significantly increased (p <0.01) in comparison with the treated rats (Table 3).
Effects of artesunate on some reproductive hormones
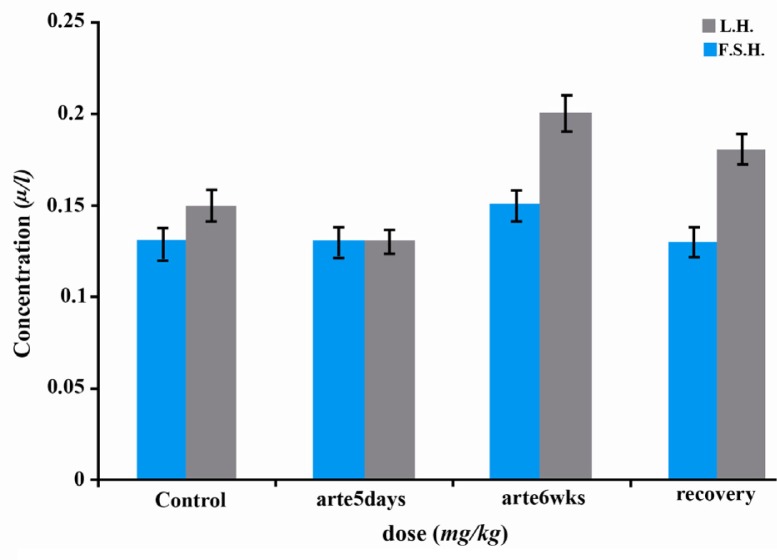
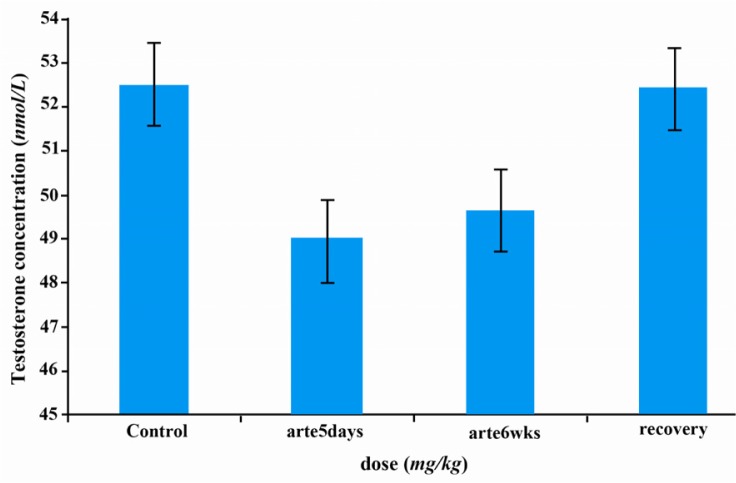
There were no significant changes in serum LH, FSH and testosterone concentrations of rats treated with artesunate daily for 5 days during 6 weeks when compared with the controls. This was also true in the recovery group (Figures 1–2).
Figure 1.
Effects of artesunate administration on serum LH and FSH concentrations. arte5days=artesunate administration for 5 days; arte6wks=artesunate administration for 6 weeks
Figure 2.
Effects of artesunate administration on serum testosterone concentration. arte5days=artesunate administration for 5 days; arte6wks=artesunate administration for 6 weeks
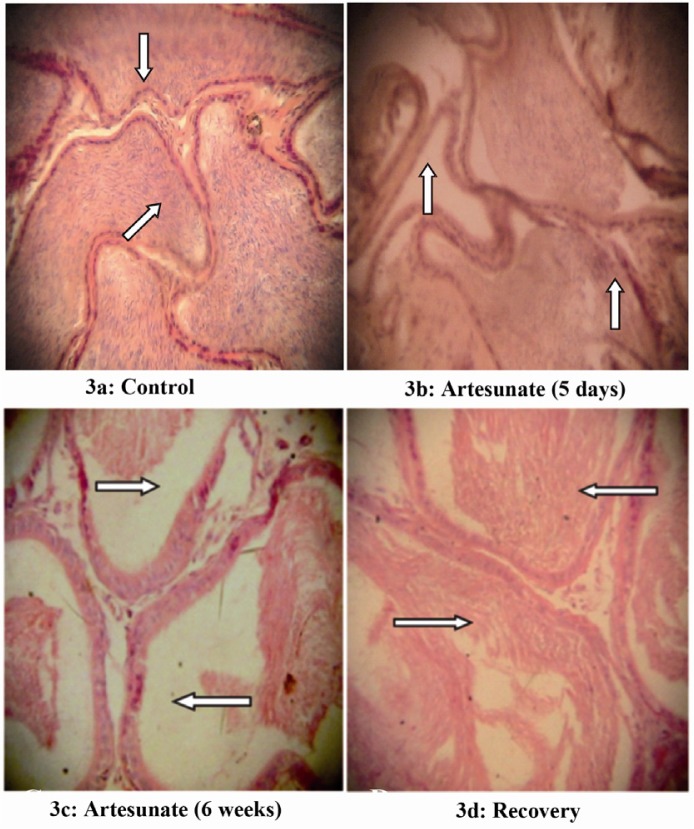
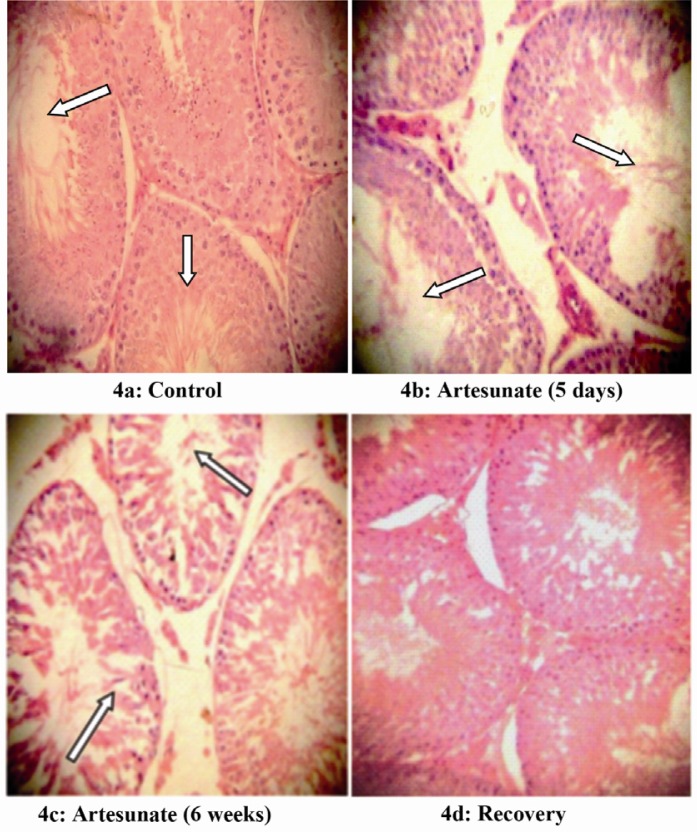
Effects of artesunate on the histology of rat epididymis and testis
The epididymal and testicular histology of treated rats showed duration dependent degenerative changes. Rats treated for five days showed mild while those treated for six weeks showed severe degenerative changes (Figures 3– 4). There was visible reduction in epididymal sperm content, while testicular histology showed disorganization of the seminiferous tubule architecture. There was degeneration of the plasmalemma in the basal portion of some of the seminiferous tubules with arrest of spermatogenesis. However, all these changes were gradually restored in the recovery group.
Figure 3.
Effects of artesunate administration on the histology of rat epididymis. (3a): Control, (3b): Artesunate (5 days), (3c) Artesunate (6 weeks) and (3d) Recovery. (Arrows showing stored sperm cells; Mag. x 100)
Figure 4.
Effects of artesunate administration on the histology of rat testis. (4a): Control, (4b): Artesunate (5 days), (4c): Artesunate (6 weeks) and (4d) Recovery. (Arrows showing somniferous tubules; Mag. x 100)
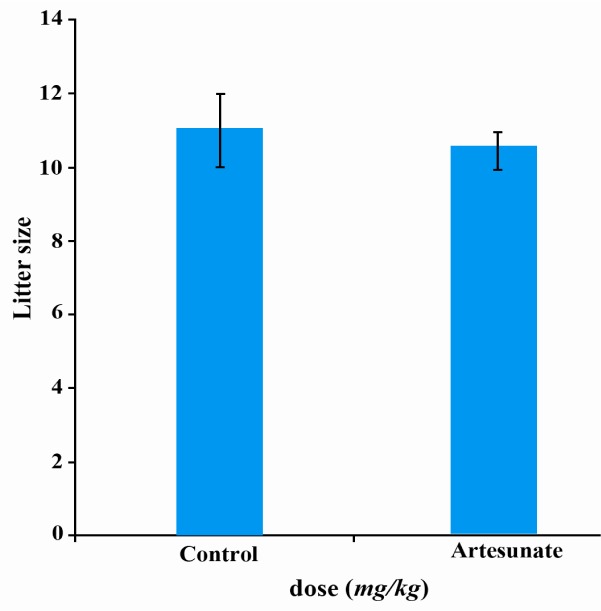
Effects of artesunate on litter size
There were no significant changes in the number of pups produced by the female rats cohabited with treated male rats when compared with the controls (Figure 5).
Figure 5.
Effects of artesunate administration on litter size
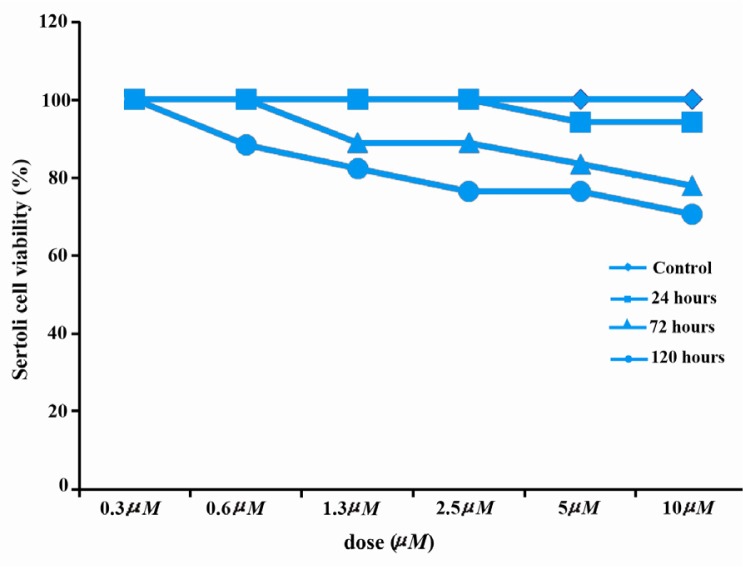
Effects of artesunate on viability
No reduction was observed in Sertoli cell viability when cultured with 0.3 µM, 0.6 µM, 1.3 µM and 2.5 µM of artesunate for 24 hours when compared with the controls. However, a reduction was observed when Sertoli cells were treated with 5 µM and 10 µM of artesunate. There was a duration dependent decrease in the viability of Sertoli cells cultured for 72 hours, and 120 hours, respectively (Figure 6).
Figure 6.
Effects of artesunate on Sertoli cell viability
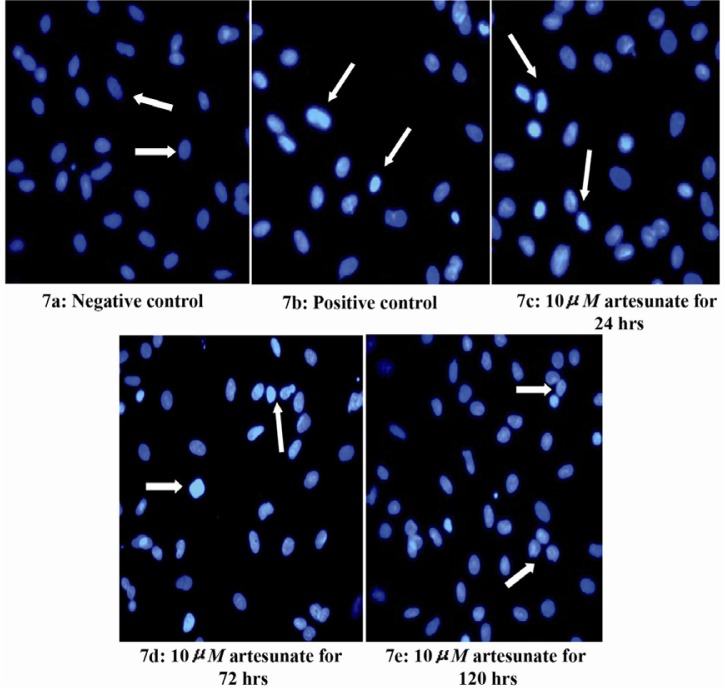
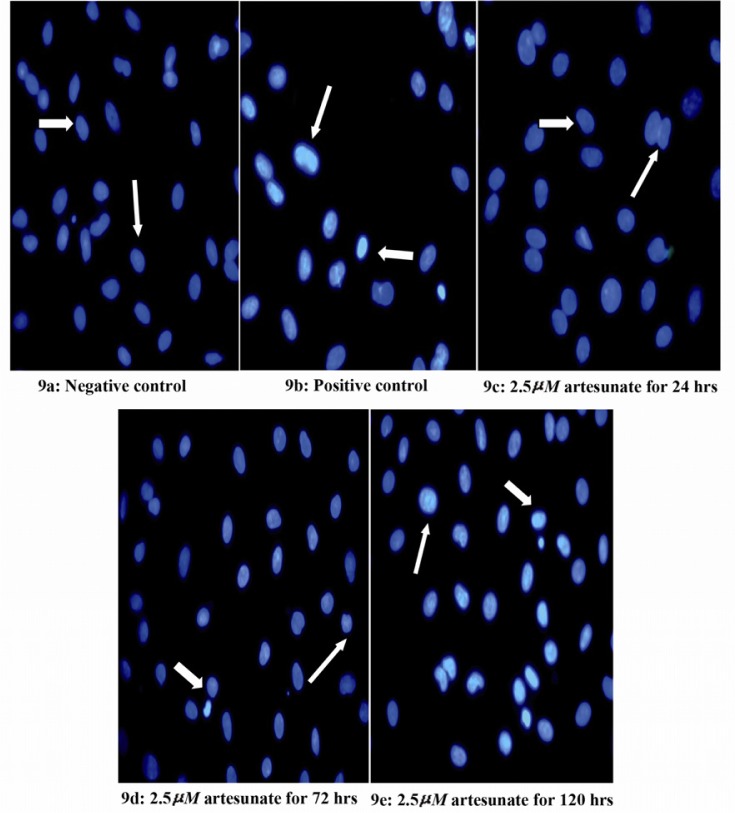
Effects of artesunate on DNA integrity
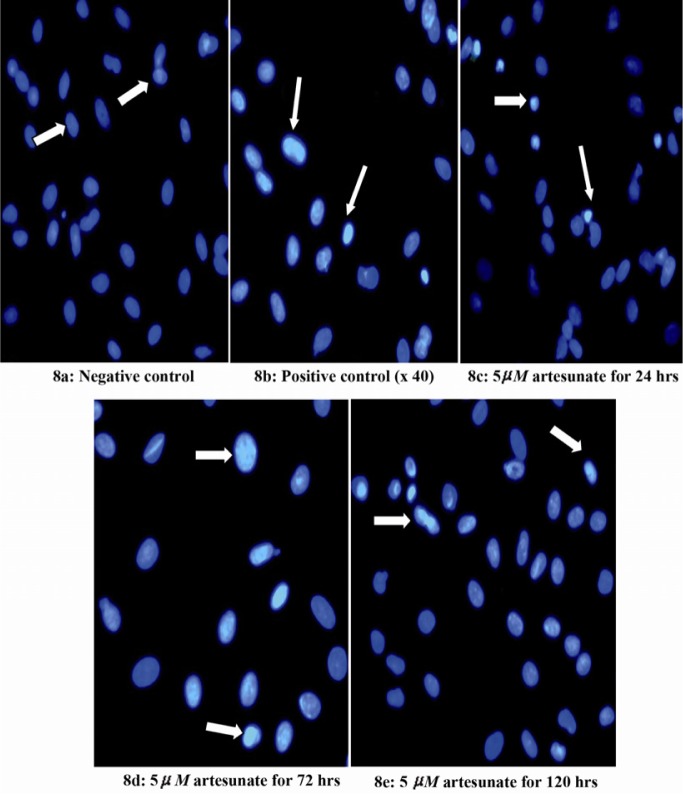
There was a dose and duration dependent fragmentation in the nuclei of Sertoli cells treated with different doses of artesunate; with 10 µM showing the greatest effect and 2.5 µM showing the least at 120 hours (Figures 7–9).
Figure 7.
Effects of 10 μM artesunate on ds-DNA integrity of Sertoli cells. (7a) Negative control (untreated) (7b) Positive control (N-9 treated) (7c) 10 μM of artesunate for 24 hours (7d) 10 μM of artesunate for 72 hours (7e) 10 μM of artesunate for 120 hours. (Arrows showing Sertoli cell nucleus; Mag. x 40)
Figure 9.
Effects of 2.5 μM artesunate on ds-DNA integrity of Sertoli cells. (9a) Negative control (untreated) (9b) Positive control (N-9 treated) (9c) 2.5 μM of artesunate for 24 hours (9d) 2.5 μM of artesunate for 72 hours (9e) 2.5 μM of artesunate for 120 hours. (Arrows showing Sertoli cell nucleus; Mag. x 40)
Figure 8.
Effects of 5 μM artesunate on ds-DNA integrity of Sertoli cells. (8a) Negative control (untreated) (8b) Positive control (N-9 treated) (8c) 5 μM of artesunate for 24 hours (8d) 5 μM of artesunate for 72 hours (8e) 5 μM of artesunate for 120 hours. (Arrows showing Sertoli cell nucleus; Mag. x 40)
Effects of artesunate on gene expression
Glial cell line-derived neurotrophic factor cultured Sertoli cells treated with 5 µM, 2.5 µM and 1.25 µM of artesunate when compared with the gene expression in control Sertoli cells. Similarly, transferrin gene in Sertoli cells treated with artesunate showed normal expressions when compared with the controls (Figures 10–11).
Figure 10.

GDNF gene expression in cultured Sertoli cells. 1=Artesunate (5 μM); 2=Artesunate (2.5 μM); 3= Artesunate (1.25 μM); 4=Control
Figure 11.
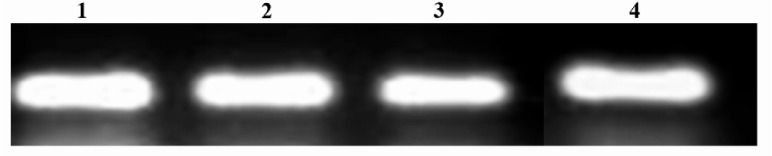
Transferrin gene expression in cultured Sertoli cells. 1=Artesunate (5 μM); 2=Artesunate (2.5 μM); 3= Artesunate (1.25 μM); 4=Control
Discussion
The results of this study showed that short-term administration of artesunate did not cause any significant changes in the body regarding the relative organ weights of the treated rats. This implies that short-term oral administration of this drug had no negative effects on somatic growth. However, there were significant reductions in these parameters with prolonged administration of the drug. It has been reported that a change in relative or absolute weight of an organ after drug administration is an indication of the toxic effects of that drug (42–43). Moreover, the weight of male reproductive organs usually provides a useful fertility/reproductive risk assessment in experimental studies (28). Testicular size is the best primary assessment for spermatogenesis since the tubules and germinal elements account for approximately 98% of the testicular weight (44). Thus the decrease observed in testicular weight can be linked to the degeneration of tubules and loss of germinal elements which were observed in this study. These finding are similar to an earlier report on artemether (16) and that of Okanlawon and Ashiru (45) who reported that administration of chloroquine to rats for 7 weeks significantly reduced their body and organ weights.
The progressive sperm motility and viability of rats treated with artesunate significantly decreased in both short- and long-term treatments. It is known that sperm cells gain their motility as they move through different regions of the epididymis. As the spermatozoa moves through the corpus epididymis, their motility increases sharply and continues to improve through the cauda epididymis and vas deferens (46). These effects on motility and viability observed in this study were also reported by Adeeko and Dada (13) in which chloroquine inhibited epididymal sperm motility. This study further reported that chloroquine accumulated in the epididymis and also in the epididymal sperm thereby interfering with the acquisition of motility. In their experiment on developing a male contraceptive using pyrimethamine and dapsone Consentino et al. (15) reported that pyrimethamine caused infertility in the treated mice. Recently, Obianime and Aprioku (21) also reported significant decreases in sperm motility of rats treated with artesunate and artemisinin-based combination drugs. Artesunate destroys Plasmodium by the generation of free radicals alkylating the parasite's membranes. The significant reduction observed in the progressive sperm motility and viability of rats treated with artesunate could be due to the free radical generating capacity of this drug. Free radicals have been implicated in male infertility by decreasing sperm motility (47). The reactive oxygen species and lipid peroxides may have serious deleterious effects on sperm motility. Arrest of sperm motility is probably due to the chain of reactions induced by reactive oxygen species which can affect sperm axoneme function (48). Short term administration of artesunate did not significantly reduce the daily sperm production of the treated rats, however a significant decrease was observed in the daily sperm production of rats for a longer duration of artesunate administration. This was also observed in the sperm morphology of the treated rats. A similar finding was reported by Otubanjo and Mosuro (49) in mice treated with sulphamethoxypyridazine: pyrimethamine (Metakelfin).
Spermatogenesis is influenced and achieved by the interplay of reproductive hormones secreted by the hypothalamo-pituitary-testicular axis and relating testosterone, gonadotrophin releasing, luteinizing and follicle stimulating hormones. In this study, the concentrations of luteinizing hormone, follicle stimulating hormone and testosterone showed no significant changes in both short-term and long-term experiments. Follicle stimulating hormone is known to stimulate mitotic division and proliferation of Sertoli and germ cells (50). The increased follicle stimulating hormone concentration observed with long-term administration of artesunate could be related to the decrease recorded in the testosterone concentration. This will remove the inhibitory effect caused by the negative feedback of testosterone on gonadotropin production. It could also be due to the need for growth and proliferation of germ and Sertoli cells necessary for spermatogenesis, which had earlier been probably arrested due to the adverse effects caused by artesunate administration.
Histological sections of the testis and epididymis of rats treated with artesunate showed visible lesions and degenerative changes. Testicular histology showed degeneration of the plasmalemma with vacuolization of the seminiferous tubules. This might imply that these drugs have been able to permeate through the blood-testis barrier and cause arrest of spermatogenesis (51). Histology of the epididymis showed a visible reduction in the sperm content. This could explain the decrease observed in the sperm count of rats treated with artesunate. However, the histological section of the testis and epididymis of rats in the recovery groups showed regeneration of the germinal epithelium and germ cells, re-building of different layers within the seminiferous tubules of the testis and thus re-establishment of spermatogenesis. Moreover, their epididymal histology showed a visible restoration of sperm content. A similar finding was also reported by Raji et al. (16), and Okanlawon and Ashiru (45) in their experiments on artemether and chloroquine, respectively. In another study, Asuquo et al. (52) also reported degenerative changes in the seminiferous tubules of chloroquine phosphate-treated rats. In the recovery experiments, withdrawal of drug administration for 6 weeks resulted in recovery from the deleterious effects induced by artesunate. It could be inferred that the toxic effects are not permanent but reversible when the drug is withdrawn.
The results obtained in the present study suggest that artesunate impaired reproductive function of treated male albino rats, although artesunate did not have any adverse effect on fertility as the litter size from untreated female rats mated with artesunate-treated male rats produced the same number of pups as with the controls.
Sertoli cells are the somatic cells of the testis that are essential for spermatogenesis. They facilitate the progression of sperm cells to spermatozoa (53). The results obtained from Sertoli cell viability study showed a dose and duration dependent decrease in the number of viable Sertoli cells treated with artesunate in vitro. A reduction in the number of viable Sertoli cells due to drug toxicity will definitely expose germ cells and the developing spermatocytes and spermatids within the seminiferous tubules of the testis to the drug's toxic effects. Sertoli cells also constitute the blood-testis barrier (54). Thus damage to Sertoli cells may lead to the impairment of male reproduction. This could explain why artesunate was probably able to permeate the blood-testis barrier and thus affect spermatogenesis as evidenced by the degeneration in testicular histological studies and also the reduction observed in the sperm count in this study.
The molecular basis of Sertoli cell damage by artesunate in this study showed a dose and duration dependent degeneration of the double-stranded deoxyribonucleic acid (ds-DNA) related to Sertoli cells of the treated rats. It was observed that the greatest occurrence of nuclear fragmentation and chromatin condensation was noticed in Sertoli cells treated with 10 µM of artesunate for 120 hours. A similar effect was seen in Nonoxynol-9 treated Sertoli cells which served as the positive controls. Nonylphenol-9 has been shown to induce adverse oxidative stress in rat Sertoli cells (36). Based on the results obtained from the determination of the double- stranded deoxyribo-nucleic acid integrity, the expressions of two Sertoli cell genes were thus determined in this study. These genes were transferrin and Glial cell line-derived neurotropic factor (G.D.N.F.). Transferrin gene codes the formation of the iron transport glycoprotein, transferrin which is important in the delivery of iron to germinal cells within the adluminal compartment of the seminiferous tubules (55, 56). GDNF is a strong neurotropic factor which is expressed in the testis. It plays an important role in the proliferation of Sertoli cells (57). The obtained results showed normal DNA expression of both transferrin and glial cell line-derived neurotropic factor genes of the drug co-cultured Sertoli cells in all different doses of the drug used in this study. This shows that although artesunate caused some nuclear fragmentation and chromatin condensation, the DNA expressions of transferrin and GDNF genes remained unchanged at various tested doses.
Conclusion
Results of both the in vivo and in vitro studies showed that artesunate caused reversible adverse effects on male reproductive functions in a dose and duration dependent manner. The study of biological phenomena in-vivo is often complicated by various interactions operative within a living organism. Although highly artificial, tissue culture models provide valuable systems in which the environmental conditions can be controlled and the effects of various factors on a specific cell type can be directly investigated, however, morphological and functional characteristics are frequently subject to alteration in culture due to changes in pH, temperature, culture medium and atmospheric conditions, but care was taken to maintain these factors at physiological conditions suitable to the cultured cells in this study. The present in-vitro studies were, therefore, supported at least and in part by the in-vivo experiments. Moreover, the long term use of artesunate might have to be done with caution because the greatest adverse effect was observed during the long-term administration of the drug. Sertoli cells are very important in spermatogenesis because they nourish the germ cells and form the blood-testis barrier which protects the germ cells and developing spermatocytes from direct contact with the external environment. However, if this barrier is breached, there would be a greater tendency for the infiltration of toxic substances into the interior of the seminiferous tubules, thereby, affecting the process of spermatogenesis. Further studies could be aimed at co-culturing Sertoli cells with germ cells and then treating them with artesunate. This will shed more light as to how these drugs affect the protective function of Sertoli cells on germ cells.
Acknowledgement
The expert advice by Professor Ademowo, O.G. (IAMRAT UCH, Ibadan, Nigeria), Dr. Gupta, G. and Dr. Maikhuri, J.P. (Division of Endocrinology, Central Drug Research Institute, Lucknow, India) are gratefully acknowledged.
This work was partly supported by a predoctoral fellowship grant from the Third World Academy of Science in conjunction with the Council of Scientific and Industrial Research given to Akinsomisoye Olumide Stephen.
To cite this article: Akinsomisoye OS, Yinusa R. Long-Term Administration of Artesunate Induces Reproductive Toxicity in Male Rats. J Reprod Infertil. 2011;12(4):249–260.
Conflict of Interest
The authors declare no conflict of interest regarding the relevant research and the present article.
References
- 1.Breman JG, Alilio MS, Mills A. Conquering the in-tolerable burden of malaria: what's new, what's needed: a summary. Am J Trop Med Hyg. 2004;71(2 Suppl):1–15. [PubMed] [Google Scholar]
- 2.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415(6872):680–5. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 3.Rønn AM, Msangeni HA, Mhina J, Wernsdorfer WH, Bygbjerg IC. High level of resistance of Plasmodium falciparum to sulfadoxine-pyrimethamine in children in Tanzania. Trans R Soc Trop Med Hyg. 1996;90(2):179–81. doi: 10.1016/s0035-9203(96)90129-7. [DOI] [PubMed] [Google Scholar]
- 4.Ogutu BR, Smoak BL, Nduati RW, Mbori-Ngacha DA, Mwathe F, Shanks GD. The efficacy of pyrimethamine-sulfadoxine (Fansidar) in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children. Trans R Soc Trop Med Hyg. 2000;94(1):83–4. doi: 10.1016/s0035-9203(00)90450-4. [DOI] [PubMed] [Google Scholar]
- 5.Djimdé A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourté Y, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344(4):257–63. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 6.Hien TT. An overview of the clinical use of artemisinin and its derivatives in the treatment of falciparum malaria in Viet Nam. Trans R Soc Trop Med Hyg. 1994;88(Suppl 1):S7–8. doi: 10.1016/0035-9203(94)90461-8. [DOI] [PubMed] [Google Scholar]
- 7.Harinasuta T, Karbwang J. Qinghaosu: a promising antimalarial. J Am Med Assoc SEA. 1994;34:7–8. [Google Scholar]
- 8.Heppner DG, Ballou WR. Malaria in 1998: advances in diagnosis, drugs and vaccine development. Curr Opin Infect Dis. 1998;11(5):519–30. [PubMed] [Google Scholar]
- 9.Akanbi OM, Odaibo AB, Afolabi KA, Ademowo O G. Effect of self-medication with antimalarial drugs on malaria infection in pregnant women in South-Western Nigeria. Med Princ Pract. 2005;14(1):6–9. doi: 10.1159/000081915. [DOI] [PubMed] [Google Scholar]
- 10.Jaeger A, Sauder P, Kopferschmitt J, Flesch F. Clinical features and management of poisoning due to antimalarial drugs. Med Toxicol Adverse Drug Exp. 1987;2(4):242–73. doi: 10.1007/BF03259868. [DOI] [PubMed] [Google Scholar]
- 11.Agboruche RL. In-Vitro toxicity assessment of antimalarial drug toxicity on cultured embryonic rat neurons, macrophage (RAW 264.7), and kidney cells (VERO-CCl-81) FASEB J. 2009;23(Suppl):529. [Google Scholar]
- 12.Izunya AM, Nwaopara AO, Oaikhena GA. Effect of chronic oral administration of chloroquine on the weight of the heart in wistar rats. Asian J Med Sci. 2010;2(3):127–31. [Google Scholar]
- 13.Adeeko AO, Dada OA. Chloroquine reduces fertilizing capacity of epididyma sperm in rats. Afr J Med Med Sci. 1998;27(1-2):63–4. [PubMed] [Google Scholar]
- 14.Orisakwe OE, Obi E, Orish VN, Udemezue OO. Effect of halofantrine on testicular architecture and testosterone level in guinea pigs. Eur Bull Drug Res. 2003;11:105–9. [Google Scholar]
- 15.Cosentino MJ, Pakyz RE, Fried J. Pyrimethamine: an approach to the development of a male contraceptive. Proc Natl Acad Sci U S A. 1990;87(4):1431–5. doi: 10.1073/pnas.87.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raji Y, Osonuga IO, Akinsomisoye OS, Osonuga OA, Mewoyeka OO. Onadotoxicity evaluation of oral artemisinin derivative in male rats. J Med Sci. 2005;5(4):303–6. [Google Scholar]
- 17.Nontprasert A, Nosten-Bertrand M, Pukrittayakamee S, Vanijanonta S, Angus BJ, White NJ. Assessment of the neurotoxicity of parenteral artemisinin derivatives in mice. Am J Trop Med Hyg. 1998;59(4):519–22. doi: 10.4269/ajtmh.1998.59.519. [DOI] [PubMed] [Google Scholar]
- 18.Nontprasert A, Pukrittayakamee S, Dondorp AM, Clemens R, Looareesuwan S. White NJ. Neuro-pathologic toxicity of artemisinin derivatives in a mouse model. Am J Trop Med Hyg. 2002;67(4):423–9. doi: 10.4269/ajtmh.2002.67.423. [DOI] [PubMed] [Google Scholar]
- 19.Genovese RF, Newman DB, Brewer TG. Behavioral and neural toxicity of the artemisinin antimalarial, arteether, but not artesunate and artelinate, in rats. Pharmacol Biochem Behav. 2000;67(1):37–44. doi: 10.1016/s0091-3057(00)00309-9. [DOI] [PubMed] [Google Scholar]
- 20.Genovese RF, Petras JM, Brewer TG. Arteether neurotoxicity in the absence of deficits in behavioral performance in rats. Ann Trop Med Parasitol. 1995;89(4):447–9. doi: 10.1080/00034983.1995.11812975. [DOI] [PubMed] [Google Scholar]
- 21.Obianime AW, Aprioku JS. Comparative study of artesunate, ACTs and their combinants on the spermatic parameters of the male guinea pig. Niger J Physiol Sci. 2009;24(1):1–6. [PubMed] [Google Scholar]
- 22.Lou XE, Zhou HJ. [Effects of artesunate on progestrone estrogen content and decidua in rats] Yao Xue Xue Bao. 2001;36(4):254–7. Chinese. [PubMed] [Google Scholar]
- 23.Rath B, Jena J, Samal S, Rath B. Reproductive profile of artemisinins in albino rats. Indian J Pharmacol. 2010;42(3):192–3. doi: 10.4103/0253-7613.66846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark RL. Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester. Reprod Toxicol. 2009;28(3):285–96. doi: 10.1016/j.reprotox.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Heywood R. Long-term toxicity. In: Balls M, Riddell RJ, Worden AN, editors. Animals and alternatives in toxicity testing. London: Academic Press; 1983. pp. 79–93. [Google Scholar]
- 26.Walker SR, Schuetz E, Schuppan D, Gelzer J. A comparative retrospective analysis of data from short- and long-term animal toxicity studies on 40 pharmaceutical compounds. Arch Toxicol Suppl. 1984;7:485–7. doi: 10.1007/978-3-642-69132-4_102. [DOI] [PubMed] [Google Scholar]
- 27.Meneguz A, Fortuna S, Lorenzini P, Volpe MT. Influence of urethane and ketamine on rat hepatic cytochrome P450 in vivo. Exp Toxicol Pathol. 1999;51(4-5):392–6. doi: 10.1016/S0940-2993(99)80027-X. [DOI] [PubMed] [Google Scholar]
- 28.Raji Y, Ifabunmi OS, Akinsomisoye OS, Morakinyo AO, Oloyo AK. Gonadal responses to antipsychotic drugs: chlorpromazine and thioridazine reversibly suppress testicular functions in male rats. Int J Pharmacol. 2005;1(3):287–92. [Google Scholar]
- 29.World Health Organization. 2nd ed. London: Cam-bridge University Press; 1987. Laboratory manual for Examination of Human Semen and Semen-Cer-vical Mucus Interaction; pp. 1–10. [Google Scholar]
- 30.Farag AT, Eweidah MH, El-Okazy AM. Reproductive toxicology of acephate in male mice. Reprod Toxicol. 2000;14(5):457–62. doi: 10.1016/s0890-6238(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 31.Cooke PS, Porcelli J, Hess RA. Induction of increased testis growth and sperm production in adult rats by neonatal administration of the goitrogen propylthiouracil (PTU): the critical period. Biol Reprod. 1992;46(1):146–54. doi: 10.1095/biolreprod46.1.146. [DOI] [PubMed] [Google Scholar]
- 32.Blazak WF, Trienen KA, Juniewicz PE. Application of testicular sperm head counts in the assessment of male reproductive toxicity. In: Chapin RE, editor. Methods in Toxicology: Male Reproductive Toxicology. San Diego: Academic Press; 1993. pp. 86–94. [Google Scholar]
- 33.Raji Y, Udoh US, Mewoyeka OO, Ononye FC, Bolarinwa AF. Implication of reproductive endocrine malfunction in male antifertility efficacy of Azadirachta indica extract in rats. Afr J Med Med Sci. 2003;32(2):159–65. [PubMed] [Google Scholar]
- 34.Akpantah AO, Oremosu AA, Ajala MO, Noronha CC, Okanlawon AO. The effect of crude extract of Garcinia Kola seed on the histology and hormonal milieu of male sprague-dawley rats' reproductive organs. Niger J Health Biomed Sci. 2003;2:40–6. [Google Scholar]
- 35.Kelly CW, Janecki A, Steinberger A, Russell LD. Structural characteristics of immature rat Sertoli cells in vivo and in vitro. Am J Anat. 1991;192(2):183–93. doi: 10.1002/aja.1001920207. [DOI] [PubMed] [Google Scholar]
- 36.Gong Y, Han XD. Nonylphenol-induced oxidative stress and cytotoxicity in testicular Sertoli cells. Reprod Toxicol. 2006;22(4):623–30. doi: 10.1016/j.reprotox.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Hill J, Holland M, Kurihara Y, Loveland KL. Bovine sertoli cells colonize and form tubules in murine hosts following transplantation and grafting procedures. J Androl. 2008;29(4):418–30. doi: 10.2164/jandrol.107.004465. [DOI] [PubMed] [Google Scholar]
- 38.Jackson JR, Gilmartin A, Imburgia C, Winkler JD, Marshall LA, Roshak A. An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res. 2000;60(3):566–72. [PubMed] [Google Scholar]
- 39.Jain JK, Li A, Nucatola DL, Minoo P, Felix JC. Nonoxynol-9 induces apoptosis of endometrial explants by both caspase-dependent and -independent apoptotic pathways. Biol Reprod. 2005;73(2):382–8. doi: 10.1095/biolreprod.104.037168. [DOI] [PubMed] [Google Scholar]
- 40.Muguruma M, Yamazaki M, Okamura M, Moto M, Kashida Y, Mitsumori K. Molecular mechanism on the testicular toxicity of 1,3-dinitrobenzene in Sprague-Dawley rats: preliminary study. Arch Toxicol. 2005;79(12):729–36. doi: 10.1007/s00204-005-0006-8. [DOI] [PubMed] [Google Scholar]
- 41.Snedecor GW, Cochran WG. Statistical methods. 7th ed. Ames: Iowa State University Press; 1980. p. 215. [Google Scholar]
- 42.Simmons JE, Yang RS, Berman E. Evaluation of the nephrotoxicity of complex mixtures containing organics and metals: advantages and disadvantages of the use of real-world complex mixtures. Environ Health Perspect. 1995;103(Suppl 1):67–71. doi: 10.1289/ehp.95103s167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maina MB, Garba SH, Jacks TW. Histological evaluation of the rats testis following administration of a herbal tea mixture. J Pharmacol Toxicol. 2008;3:464–70. [Google Scholar]
- 44.Sherines RJ, Howards SS. Male fertility. In: Har-rison JH, Gittes RF, Perimutter AD, Stamey TA, Walsh PC, editors. Campbell's Urology. Philadelphia: Saunders WB. Co; 1987. p. 715. [Google Scholar]
- 45.Okanlawon AO, Ashiru OA. Sterological estimation of seminiferous tubular dysfunction in chloroquine treated rats. Afr J Med Med Sci. 1998;27(1-2):101–6. [PubMed] [Google Scholar]
- 46.van Der Horst G, Seier JV, Spinks AC, Hendricks S. The maturation of sperm motility in the epididymis and vas deferens of the vervet monkey, Cercopithecus aethiops. Int J Androl. 1999;22(3):197–207. doi: 10.1046/j.1365-2605.1999.00171.x. [DOI] [PubMed] [Google Scholar]
- 47.Griveau JF, Dumont E, Renard P, Callegari JP, Le Lannou D. Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human spermatozoa. J Reprod Fertil. 1995;103(1):17–26. doi: 10.1530/jrf.0.1030017. [DOI] [PubMed] [Google Scholar]
- 48.de Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl. 1992;13(5):368–78. [PubMed] [Google Scholar]
- 49.Otubanjo OA, Mosuro AA. An in vivo evaluation of the induction of abnormal sperm morphology by sulphamethoxypyridazine: pyrimethamine (Metakelfin) Pak J Biol Sci. 2007;10(1):156–9. doi: 10.3923/pjbs.2007.156.159. [DOI] [PubMed] [Google Scholar]
- 50.Almirón I, Chemes H. Spermatogenic onset. II. FSH modulates mitotic activity of germ and Sertoli cells in immature rats. Int J Androl. 1988;11(3):235–46. doi: 10.1111/j.1365-2605.1988.tb00998.x. [DOI] [PubMed] [Google Scholar]
- 51.Baldessarini RJ. Drugs and the treatment of psychiatric disorders. In: Gilman AG, Goodman LS, editors. The pharmacological basis of therapeutics. New York: MacMillan; 1980. pp. 301–417. [Google Scholar]
- 52.Asuquo OR, Igiri AO, Olawoyin OO, Eyong EU. Correlation of histological and histometric changes in rats testes treated with chloroquine phosphate. Niger J Physiol Sci. 2007;22(1-2):135–9. doi: 10.4314/njps.v22i1-2.54885. [DOI] [PubMed] [Google Scholar]
- 53.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9(4):411–6. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 54.Buzzard JJ, Wreford NG, Morrison JR. Marked extension of proliferation of rat Sertoli cells in culture using recombinant human FSH. Reproduction. 2002;124(5):633–41. doi: 10.1530/rep.0.1240633. [DOI] [PubMed] [Google Scholar]
- 55.Skinner MK, Griswold MD. Secretion of testicular transferrin by cultured Sertoli cells is regulated by hormones and retinoids. Biol Reprod. 1982;27(1):211–21. doi: 10.1095/biolreprod27.1.211. [DOI] [PubMed] [Google Scholar]
- 56.Sylvester SR, Russell LD, Griswold MD, editors. The Sertoli cell. Clearwater, FL: Cache River Press; 1993. Secretion of transport and building proteins; pp. 201–16. [Google Scholar]
- 57.Hu J, Shima H, Nakagawa H. Glial cell line-derived neurotropic factor stimulates sertoli cell proliferation in the early postnatal period of rat testis development. Endocrinology. 1999;140(8):3416–21. doi: 10.1210/endo.140.8.6922. [DOI] [PubMed] [Google Scholar]