Abstract
Background
Anti-sperm antibody (ASA) can decrease sperm motility and, therefore, it is a cause of male infertility. The aim of this study was to evaluate the effects of varicocelectomy on anti-sperm antibody in patients with varicocele.
Methods
This observational study was conducted on 90 patients with varicocele at Sina and Imam Khomeini hospitals during 2006 to 2009. All varicocelectomy candidates were selected for ASA assessment both in semen and serum before and after surgery. ASA level was measured using a direct method for semen and an indirect method of Sperm MAR test, for serum. Paired t-test and McNemar's test were used for data analysis, and p<0.05 was considered statistically significant.
Results
ASA level in semen was 13.7% before, and 15.7% after three month of varicocelectomy (p=0.881). Serum level of ASA before and after surgery were 13.6% and 21.7%, respectively (p=0.033). Three parameters including sperm count, motility and morphology showed recovery following, varicocelectomy, but only the difference in sperm motility was significant (p<0.05).
Conclusion
This study showed that varicocelectomy has no effect on semen ASA. Although serum antibody has been shown to increase after varicocelectomy but sperm motility will improve. Varicocelectomy seems to have a beneficial effect on semen parameters in infertile men with varicocele.
Keywords: Anti-sperm Antibody, Infertility, Sperm Motility, Varicocelectomy
Introduction
Varicocele is present in 11% of infertile men with normal and 24% of those with abnormal semen parameters. The most common finding in varicocele is reduced sperm motility (asthenospermia). Improvement of sperm motility indicates the first sign of success in varicocele treatment (1–3).
Immunological causes are responsible for more than 10% (4% to 15%) of infertility cases (1, 3). Anti-sperm antibodies (ASAs) are present in less than 2% of fertile and 10% of infertile men (4, 5). Anti-sperm antibodies affect sperm function differently as follows: impairment of sperm penetration into cervical mucus, inhibition of sperm capacitation, incomplete acrosomal reaction, disruption of sperm-egg binding, and disorder in egg fertilization (6).
A research conducted in Canada showed that direct immunobead test (IBT) is a reliable method for confirmation of ASAs (7).
Another study using immunobead test (IBT), in 30 patients, concluded that immunological mechanisms are not a major cause of infertility in men with varicocele (8). A study using direct immunobead assay showed that ASAs do not increase significantly in infertile men with varicocele compared to infertile men without the disease. In addition, ASAs do not play an important role in the infertility of men with varicocele (9).
In another study, the presence of ASAs was not shown to be an essential etiologic factor in the infertility of men with varicocele (10).
Measuring the level of ASAs before varicelectomy and approximately 3 and 6 months thereafter showed that surgical treatment does not affect the outcome (6). On the other hand, measurement of ASA level in 27 men before varicicelectomy and 6 months thereafter indicated an equivocal relationship among varicocele, ASAs, and infertility.
Varicocele can be accompanied by decreased or in some cases with increased ASA concentrations. The latter, however, has been shown to have no negative effect on semen parameters (9).
Although varicocele is suspected to be a possible etiologic factor in the development of ASAs, there is lack of comprehensive studies in this field. In addition, a clear cut-off point has not been presented for the exact determination of significant positivity for ASAs in serum or semen for clinical use.
The results of the aforementioned studies indicate that the relationship among varicocele, ASAs and infertility is equivocal; some suggest that varicicelectomy has no effect on ASA concentration, while others show the increase in ASAs has no effect on semen parameters. Therefore, we performed this study to gain more insight into the effect of varicicele on serum and semen level of ASAs. Therefore, we measured ASAs in the serum and semen of patients before and 3 months after varicicelectomy.
Methods
This is an observational study performed by measuring ASAs before and after varicocelectomy. Blood samples were randomly obtained from 95 men with clinical varicocele who had the indication for varicocelectomy. The patients were randomly recruited from urology outpatient clinics of Sina and Imam Khomeini hospitals in Tabriz, Iran during 2006 to 2009.
A written informed consent was obtained from all participants, before starting the study. Five patients were dropped out of the study after 3 months for different reasons, including being far from the clinic, having no access to telephone or being reluctant to participate in the study anymore. The patients were not included in the study if any of the following conditions were present: history of varicocelectomy, surgery for undescended testes (UDT), testicular torsion/ detortion (by operation or spontaneously), testicular biopsy, sexually transmitted diseases, testicular cancer, vasectomy, testcular trauma, history of anal sex, inguinal hernioraphy, immunosuppressed patients, use of immunosuppressive drugs, such as steroids or cytotoxic and chemopreventive drugs, and mal-nutrition.
Diagnosis of varicocele was made by a urologist. Examination was carried out with or without valsalva maneuver and the volume and consistency of testis and grade of varicocele were recorded. If the patient was obese or hypersensitive, or physical conditions did not let a reliable examination, Doppler sonography was performed, despite having a low sensitivity in confirming the diagnosis. Varicocele was regarded as a venous reflux detectable with valsalva maneuver and presence of more than 2 to 3 veins with a diameter of ≥3 mm.
Varicocele grading was done in accordance with the World Health Organization's criteria: grade I, veins of spermatic cords are palpable with val-salva maneuver; grade II, veins of spermatic cords are palpable without valsalva maneuver; grade III, veins of spermatic cords are visible through skin without valsalva maneuver (1).
We used a checklist to obtain demographic data (age and marital status), history of the disease and past medical histories. Semen analysis was performed according to the World Health Organization guide lines and reference values for human semen (11) as follows: seminal liquid volume (at least ≥2 ml), sperm count (at least 20 million/ml), total sperm count (at least 40 million/ejaculate), motility (at least 75%), vitality (if the percentage of viable sperm is more than 75%), and morphology; although there is no consensuses on morphological classification, but based on a rigid criterion, at least 4% of the sperms should have normal morphology.
Semen analysis was performed preoperatively and 3 months after the surgery according to WHO standard criteria. Serum samples were collected pre- and post-operatively and were stored at −70°C. The samples were later used to detect Anti-sperm antibodies by indirect method. Detection of sperm antibodies in the semen was also performed by direct method immediately after collection. To do this, we used SpermMAR kit for IgG (FertiPro N.V., Beerem, Belgium). In doing this test, latex particles coated with Antihuman antibodies were added to anti-sperm antibodies (ASAs) which cotated sperm in the specimens. Then, an anti-human globulin reagent (IgG) was added to the mixture and smeared. The mixed agglutination reaction between latex particles and motile sperm proved the presence of sperm antibodies (IgG) on the sperm (12).
Immunobead test is another method that could be employed in this regard (see Discussion) (13). Ranking is based on counting motile sperms bound to latex under a light microscope. The test was considered positive if more than 15% of motile sperms had adherent particles. The criterion which proved the presence of ASAs in serum was the formation of agglutination produced by a 1:16 dilution of serum in F-10 medium.
Shortly after laboratory evaluations, the patients underwent varicocelectomy. All patients were operated by the same surgical technique, open high retroperitoneal Palomo approach, so as to avoid the effect of different techniques on the study (14).
The results of biochemical tests were gathered pre-operatively and 3 months after varicocelectomy. Descriptive statistical testes (frequency, percentage, and means±SD), paired t-test, and Mc Nemar's test were used for the analysis. The data were analyzed using SPSS (version 14). A p<0.05 was considered statistically significant.
Results
The mean age of the patients was 24.98±5.38 years (ranging from 18 to 33 years) and 66.6% (60) were married. About 65% (59) of the patients had unilateral varicocele, 40 of whom had grade II varicocele and 35% (31) had bilateral varicocele, 21 of whom had grade II varicocele. Entirely, 67.7% of the participants had grade II varicocele. Moreover, 13.3% of the patients had grade I and 19% had grade III varicocele. A total of 59 patients (65.5%) underwent the operation for infertility treatment and 22 (24.4%) due to inguinal or scrotal pain. Nine patients were also operated when they were discovered to have varicocele during routine physical examination. Overall, 89.9% of the patients underwent varicocelectomy due to infertility or pain.
Six out of 22 patients having undergone the operation had mild to moderate recovery from pain. Comparing the three main indices of semen analysis (SA) pre- and post-operatively, a significant improvement was observed in the indices in terms of sperm count (p<0.005) and sperm motility (p<0.001), but this was not the case for impaired sperm morphology in the post-operative period (p=0.59) (Table 1). Before the surgery, only morphology was significantly different between two age groups (18 to 25 and 26 to 33) (p=0.014), but sperm motility became significantly different (p=0.048) between the two age groups post-operatively (Table 2).
Table 1.
Spermogram values before and after varicocelectomy
| Before varicocelectomy | After varicocelectomy | |
|---|---|---|
| Volume ( ml ) | 3.1±0.04 | 3.6±0.8 |
| Viscosity (High) (%) | 76.7 | 69.7 |
| Sperm count ( million/ml ) | 33.85±18.69 | 37.21±14.15 |
| Immotile sperm motility n (%) | 48 (80) | 27 (45) |
| Immotile sperm morphology n (%) | 15 (25) | 7 (12) |
| Liquefaction time ( min ) | 15-30 | 15-30 |
| pH | 7.35±0.11 | 7.35±0.11 |
Table 2.
Spermiogram values before and after varicocelectomy according to age
| Age | Before varicocelectomy | After varicocelectomy | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sperm count (million/ml) | Immotile sperm motility n (%) | Immotile sperm morphology n (%) | Sperm count (million/ml) | Immotile sperm motility n (%) | Immotile sperm morphology n (%) | |
| 18-25 | 35.51±14.32 | 26 (75) | 5 (15) | 40.25±11.10 | 10 (30) | 3 (8) |
| 26-33 | 30.11±16.54 | 21 (85) | 9 (36) | 34.47±13.85 | 15 (60) | 5 (20) |
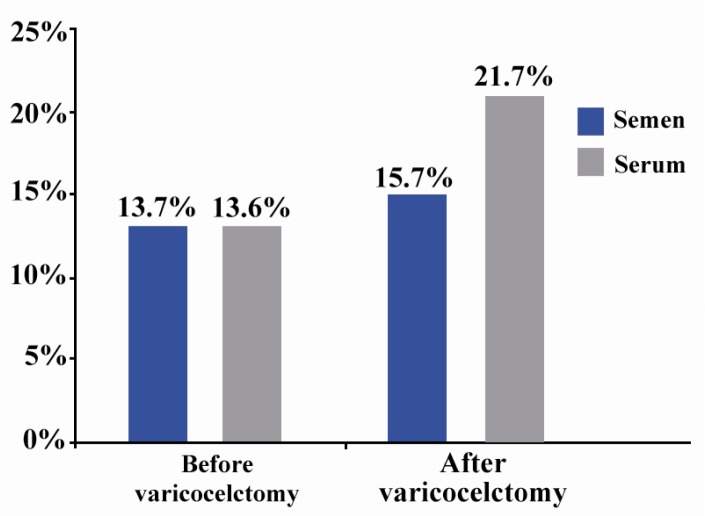
The following results were obtained regarding the association of semen analysis with the indications for operation (infertility, pain, other). At least, one of the three sperm parameters (count, motility or morphology) was impaired in 89% of the patients who were operated for infertility treatment. Moreover, two of the aforesaid parameters were also impaired in 56% of the patients in this group (Diagram 1). When ASA was present in the semen, it was positive in the serum too, although it significantly decreased after variccelectomy (p=0.033). Varicicelectomy also raised serum ASA significantly (p=0.033).
Diagram 1.
Percentage of cases with ASA in their serum and semen before and after varicocelectomy
Although most patients were negative for ASA in both semen and serum before or after the surgery, but the percentage of participants in whom semen and serum turned positive for ASA after surgery showed no significant difference between single or married participants.
Discussion
Among several studies published on the subject during 1990 to 2006, a study measured ASA before surgery and 3 and 6 months thereafter in 81 patients with a mean age of 28.7 years. Twenty-one out of 81 patients had weakly positive ASA before operation. After the operation ASA diminished in 15 and increased in three patients. ASA was negative in 61 patients before the surgery. Of these, 48 had slightly higher ASA concentrations after operation, although this increase was not statistically significant. Therefore, they concluded that varicocelectomy could reduce ASA concentration.
Our findings are consistent with those of a study in terms of pre- and post-operative sperm counts, but it is inconsistent in terms of morphology and motility before and after varicocelectomy (15).
Evaluation of semen analysis based on the indication for varicocelectomy showed sperm motility to be the most impaired index in patients undergoing the operation due to infertility or pain (15).
In the present study, 35% of the patients underwent bilateral varicocelectomy and the percentage reached 45% in men with infertility. In this study, the differences between the two groups with impaired and normal semen analysis and unilateral with bilateral varicocelectomy were significant (p=0.001). To best of our knowledge, there was no study in the literature to imply pain as an absolute indication for surgery. Only if other causes of scrotal or inguinal pain are ruled out, we can regard pain as an indication for varicocelectomy.
Our study is consistent with some, but not all studies evaluating serum and semen ASA in pre- and post-operative periods. Ozen et al. (16) evaluated 60 infertile patients with varicocele. They detected ASA by the immunofluorescent method in 24.6% of the patients. Golombo et al. (17) assessed ASAs by ELISA in 32 infertile patients with varicocele. ASA was positive in 90% of the patients, in contrast to 41% of the control group.
Using immunobead test, Knudson et al. (18) reported ASA levels in 32 infertile patients with varicocele. In this study, 28% had positive immunobead test results, among which IgG was found to be bound to the surface of the sperm in 100% and IgA in 86% of the cases. The investigators were not able to show any significant difference between pre- and post- varicocelectomy ASA concentrations.
Djaladat et al. (15) reported a 26% ASA positivity before the operation, but 6 months after the surgery, ASA concentration diminished in 15 but increased in three patients, while it underwent no changes in three other patients. The semen analysis of these patients showed that sperm morphology and count had been improved (p<0.05), whereas motility had not (p=0.02). They concluded that although varicocelectomy could diminish ASA concentration and improve semen parameters, but it has no adverse effects on the quality of semen parameters. In fact, ASA did not have much debilitating effect before surgery either. However, semen parameters showed improvement after surgery regardless of ASA concentration. In another study, it was shown that, ASA was positive in the semen of 30% to 50% of the cases, when it was positive in the serum too. This finding is consistent with other studies (19).
According to our study, in patients (71%) who had positive serum ASA post-operatively, it was positive in the semen too.
In our study, comparison of patients (13.6%) with positive serum ASA before with those (21.7%) after the operation showed statistically significant differences (p=0.033). But this was not true for patients (13.7%) with positive semen ASA before with those (15.7%) after the surgery (p=0.881).
Comparing patients who developed ASA after surgery with those whose ASA was negative after the operation, showed that only motility (p=0.035) was significantly different among the three semen parameters. Comparing patients with positive seminal versus serum ASA showed significant (p<0.001) differences in the percentage of impaired sperm motility. However, the difference was insignificant in terms of sperm morphology or count. In other words, varicocelectomy can lead to an improved sperm count and morphology but motility is impaired when it is accompanied by positive ASA. Although several etiologies have been postulated for the incidence of ASA, but only vasectomy and acute epididimitis are associated with remarkable ASA increase. The production of ASA after vasectomy is observed in 60% to 80% of cases, indicating presence of a time-dependent process. Manipulation of cord structures is not associated with the production of ASA (20) and even the effect of ASA on acrosomal reaction has been challenged in some studies (21). In addition to aforementioned indications, ASA should also be measured in patients with genitourinary disorders or in patients on chemopreventive therapies along with other parameters (22).
Regarding the 10% prevalence of ASA (IgG) in infertile men who have no other problems, sperm mixed agglutination reaction (MAR) should be considered as a routine test in the semen analysis of patients with varicocele (23).
Conclusion
ASA measurement is helpful in reaching a final decision in patients suspected of immunological causes of infertility; however, ASA positivity in the semen or in serum should not be considered as a problem as it only slightly affects sperm motility. Thus, considering the remarkable improvement in sperm motility after varicocelectomy, we should not abandon this surgical procedure in high risk patients with ASA positivity.
In our study, it was not possible to consider other preoperative treatment protocols along with varicocelectomy in patients with positive ASA. However interventional studies comparing use of preoperative cyclic anti-inflammatory steroid therapy accompanied by varicocelectomy with varicocelectomy without drug treatment is suggested.
To cite this article: Bonyadi MR, Madaen SK, Saghafi M. Effects of Varicocelectomy on Anti-sperm Antibody in Patients with Varicocele. J Reprod Infertil. 2013;14(2):73-78.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Rogenio A, Lobo A, Daniel R, Richard JR, Pavlson M. Role of immunology in infertility. In: Lobo A, Mishell DR, Paulsen RJ, Shoupe D, editors. Infertility, contraception and reproductive endocrinology. Massachusetts: Blackwell Science; 1997. pp. 675–9. [Google Scholar]
- 2.Bronson RA, O'Connor WJ, Wilson TA, Bronson SK, Chasalow FI, Droesch K. Correlation between puberty and the development of autoimmunity to spermatozoa in men with cystic fibrosis. Fertil Steril. 1992;58(6):1199–204. doi: 10.1016/s0015-0282(16)55569-1. [DOI] [PubMed] [Google Scholar]
- 3.Flickinger CJ, Baran ML, Howards SS, Herr JC. Sperm autoantigens recognized by autoantibodies in developing rats following prepubertal obstruction of the vas deferens. J Androl. 1996;17(4):433–42. [PubMed] [Google Scholar]
- 4.De Almeida M, Soumah A, Jouannet P. Incidence of sperm-associated immunoglobulins in infertile men with suspected autoimmunity to sperm. Int J Androl. 1986;9(5):321–30. doi: 10.1111/j.1365-2605.1986.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich A, Bonfig R, Wilbert DM, Strohmaier WL, Engelmann UH. Risk factors for antisperm antibodies in infertile men. Am J Reprod Immunol. 1994;31(2):69–76. doi: 10.1111/j.1600-0897.1994.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 6.Sinisi AA, Di Finizio B, Pasquali D, Scurini C, D'Apuzzo A, Bellastella A. Prevalence of antisperm antibodies by SpermMARtest in subjects undergoing a routine sperm analysis for infertility. Int J Androl. 1993;16(5):311–4. doi: 10.1111/j.1365-2605.1993.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 7.Haas GG, Jr, Lambert H, Stern JE, Manganiello P. Comparison of the direct radiolabeled antiglobulin assay and the direct immunobead binding test for detection of sperm-associated antibodies. Am J Reprod Immunol. 1990;22(3-4):130–2. doi: 10.1111/j.1600-0897.1990.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 8.Ohl DA, Naz RK. Infertility due to antisperm antibodies. Urology. 1995;46(4):591–602. doi: 10.1016/S0090-4295(99)80282-9. [DOI] [PubMed] [Google Scholar]
- 9.Oshinsky GS, Rodriguez MV, Mellinger BC. Varicocele-related infertility is not associated with increased sperm-bound antibody. J Urol. 1993;150(3):871–3. doi: 10.1016/s0022-5347(17)35636-7. [DOI] [PubMed] [Google Scholar]
- 10.Flickinger CJ, Howards SS, Bush LA, Baker LA, Herr JC. Antisperm autoantibody responses to vasectomy and vasovasostomy in Fischer and Lewis rats. J Reprod Immunol. 1995;28(2):137–57. doi: 10.1016/0165-0378(94)00904-l. [DOI] [PubMed] [Google Scholar]
- 11.Marc GO. Surgical management of male infertility and other scrotal disorders. In: Walsh PC, Retik AB, Vavghao ED, Wein AJ, editors. Campbell's Urology. Philadelphia: W.B. Saunders; 2002. pp. 1571–7. [Google Scholar]
- 12.Rajah SV, Parslow JM, Howell RJ, Hendry WF. Comparison of mixed antiglobulin reaction and direct immunobead test for detection of sperm-bound antibodies in subfertile males. Fertil Steril. 1992;57(6):1300–3. doi: 10.1016/s0015-0282(16)55091-2. [DOI] [PubMed] [Google Scholar]
- 13.Almagor M, Margalioth EJ, Yaffe H. Density differences between spermatozoa with antisperm autoantibodies and spermatozoa covered with anti-sperm antibodies from serum. Hum Reprod. 1992;7(7):959–61. doi: 10.1093/oxfordjournals.humrep.a137778. [DOI] [PubMed] [Google Scholar]
- 14.Cayan S, Kadioglu TC, Tefekli A, Kadioglu A, Tellaloglu S. Comparison of results and complications of high ligation surgery and microsurgical high inguinal varicocelectomy in the treatment of varicocele. Urology. 2000;55(5):750–4. doi: 10.1016/s0090-4295(99)00603-2. [DOI] [PubMed] [Google Scholar]
- 15.Djaladat H, Mehrsai A, Rezazade M, Djaladat Y, Pourmand G. Varicocele and antisperm antibody: fact or fiction? South Med J. 2006;99(1):44–7. doi: 10.1097/01.smj.0000197036.08282.70. [DOI] [PubMed] [Google Scholar]
- 16.Ozen H, Asar G, Gungor S, Peker AF. Varicocele and antisperm antibodiesm. Int Urol Nephrol. 1985;17(1):97–101. doi: 10.1007/BF02089408. [DOI] [PubMed] [Google Scholar]
- 17.Golomb J, Vardinon N, Homonnai ZT, Braf Z, Yust I. Demonstration of antispermatozoal antibodies in varicocele-related infertility with an enzyme-linked immunosorbent assay (ELISA) Fertil Steril. 1986;45(3):397–402. doi: 10.1016/s0015-0282(16)49224-1. [DOI] [PubMed] [Google Scholar]
- 18.Knudson G, Ross L, Stuhldreher D, Houlihan D, Bruns E, Prins G. Prevalence of sperm bound anti-bodies in infertile men with varicocele: the effect of varicocele ligation on antibody levels and semen response. J Urol. 1994;151(5):1260–2. doi: 10.1016/s0022-5347(17)35226-6. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert BR, Witkin SS, Goldstein M. Correlation of sperm-bound immunoglobulins with impaired semen analysis in infertile men with varicoceles. Fertil Steril. 1989;52(3):469–73. doi: 10.1016/s0015-0282(16)60921-4. [DOI] [PubMed] [Google Scholar]
- 20.Gubin DA, Dmochowski R, Kutteh WH. Multi-variant analysis of men from infertile couples with and without antisperm antibodies. Am J Reprod Immunol. 1998;39(2):157–60. doi: 10.1111/j.1600-0897.1998.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu RZ, Lu YL, Xu ZG, Zuo WJ, Xin JL, Wang ZS. [The effect of semen antisperm antibody on human sperm acrosin activity] Zhonghua Nan Ke Xue. 2003;9(4):252–3. Chinese. [PubMed] [Google Scholar]
- 22.Sinisi AA, D'Apuzzo A, Pasquali D, Venditto T, Esposito D, Pisano G, et al. Antisperm antibodies in prepubertal boys treated with chemotherapy for malignant or non-malignant diseases and in boys with genital tract abnormalities. Int J Androl. 1997;20(1):23–8. doi: 10.1046/j.1365-2605.1997.00101.x. [DOI] [PubMed] [Google Scholar]
- 23.Stedronska J, Hendry WF. The value of the mixed antiglobulin reaction (MAR test) as an addition to routine seminal analysis in the evaluation of the subfertile couple. Am J Reprod Immunol. 1983;3(2):89–91. doi: 10.1111/j.1600-0897.1983.tb00221.x. [DOI] [PubMed] [Google Scholar]