Abstract
Background
Apelin is a mitogenic peptide; it has functions in vessel formation and cell proliferation. In this study we aimed to evaluate the serum and tissue levels and local expression pattern of apelin in eutopic and ectopic endometrium from patients with and without endometriosis and to compare the proliferative and secretory phase differences.
Methods
Thirty women with endometriosis and 15 women without endometriosis undergoing surgery for benign indications as control group were included in the study. Serum and tissue concentrations and proliferative and secretory phase expression patterns of apelin were evaluated in the ectopic and eutopic endometrium using immunoassay and immunohistochemistry methods. The results were compared with Mann-Whitney U test. The p-values smaller than 0.05 were considered as statistically significant.
Results
Apelin expression was detected in eutopic and ectopic endometrium of women with endometriosis and endometrium of control group. Intense immunoreactivity of apelin was observed in glandular cells of eutopic and ectopic endometrial tissues of women with endometriosis and endometrium of control group during secretory phase (p<0.01). In both groups, tissue concentrations of apelin were higher than of the serum (p=0.03) but, there were no significant differences between the two groups for tissue and serum concentrations of apelin.
Conclusion
Apelin expression showed cyclic changes in eutopic and ectopic endometrium. Its expression may be related to menstrual changes of angiogenesis in endometrium of women.
Keywords: Angiogenesis, Apelin, Endometriosis, Endometrium, Expression pattern
Introduction
Endometriosis is characterized by the presence of endometrial stromal and glandular cells outside the uterine cavity, mainly in the pelvis (1). The exact etiology and pathophysiology of the disease is still not clear. The widely accepted theory is retrograde menstruation and implantation of endometrial cells on peritoneal surface (2–4). Endometriotic cells are capable of attaching to the peritoneal mesothelium, break the peritoneal lining, and destroy the extracellular matrix (ECM); thereby, they invade the surrounding tissue (5). ECM is involved in the regulation of these cellular events, and ECM invasion may be on the critical point of endometriosis development.
Apelin is a new investigated member of adipose tissue peptide (6) and apelin expression has been shown in different tissues and in the uterus (7–9). Apelin and its receptor, also known as APJ receptor, make function in vessel formation and cell proliferation (10, 11). Being regarded as a new mitogenic peptide for endothelial cells (12), apelin can promote cells from G0/G1 to S phase and stimulate epithelial proliferation. Apelin and APJ have been reported to be expressed in vascular muscle (13–16).
It has been suggested that the apelin/APJ system might be involved in the size-sensing mechanism of blood vessels which is an important event for supplying oxygen and nutrients in smaller vessels (17). Contribution of the apelin/APJ system to portosystemic collateralization and splanchnic neovascularization in portal hypertensive rats has been also reported (18).
Heinonen et al. reported significant correlation between apelin and TNF-alpha that apelin might play a role in inflammation (19). It was reported that apelin-APJ signaling was essential for embryonic angiogenesis and it was upregulated during tumor angiogenesis. Ectopic expression of apelin was sufficient to trigger premature angiogenesis. Apelin was non-mitogenic for in vitro culture of primary human endothelial cells but it promoted chemotaxis (20). The function of endometrial apelin is not known yet but, apelin may play a role in endometrial regeneration and endometriotic implantation via angiogenesis.
We aimed to investigate the expression pattern of apelin in eutopic and ectopic endometrial tissues according to possible angiogenic function of apelin.
Methods
Tissue and blood samples
Tissue samples were collected in accordance with the requirements of the Firat University Hospital Ethics Committee between November 2008 and September 2009. Eutopic and ectopic endometrial samples were obtained from 45 women aged 18 to 40 years, and a BMI of 18.5 to 24.9 kg/m 2 who were undergoing laparoscopy for benign indications. The participants had a regular 26 to 33 day menstrual cycle and had received no hormonal medication in the preceding 3 months. The women were classified into two groups; Group I: women who did not have any pelvic pathology confirmed by ultrasonography and laparoscopy (n=15), and Group II: women with endometriosis (ASRM-stage II and III; n=30).
Samples of endometriotic implants and endometriomas were obtained at laparoscopy, and the disease was confirmed by histological examination of the biopsies. Eutopic endometrium was obtained from endometrial biopsies during surgery. We histologically examined the sections of endometrium and endometriotic tissues taken from these samples and assessed them for proliferative or secretory endometrial morphological features, using the criteria proposed by Noyes et al. (21). Fortyfive endometrial samples (22 proliferative, 23 secretory) from 15 women with a normal pelvis and 30 women with endometriosis were used for immunohistochemical and enzyme immunoassay analysis. Samples of endometriosis (16 from women in the proliferative stage and 14 from women in the secretory stage of the menstrual cycle) were obtained from 15 ovarian endometriomas and 15 peritoneal implants. Semi-quantitative estimation of apelin staining was evaluated as an index of staining intensity and distribution. Moreover, peripheral venous blood samples (5 ml) were also collected for serum enzyme immunoassay. A written informed consent was obtained from each patient using consent forms.
Enzyme-Linked Immunosorbent Assay (ELISA)
Peripheral venous blood samples (5 ml) were collected after overnight fasting. After centrifugation of blood samples at 4000 rpm at room temperature for 10 min, 20–30 μl/ml aprotinin (400 kallikrein inactivator units, KIU) and 1/10 volume 1 N HCl were supplemented into serum samples. Samples were then stored at -20°C until analysis. Eutopic and ectopic endometrial tissue samples were divided into two parts: formaldehyde embedded tissues for immunohistochemical analysis and the remaining tissues for ELISA. Approximately, 15 mg tissue samples were boiled at 100°C for 5 min, then were squashed on iron mold and were homogenised in phosphate-buffered saline (PBS) containing 5% (w/v) dry milk. The homogenised samples were later centrifugated at 4000 rpm at room temperature for 10 min. For accurate quantification of apelin levels in tissue samples, 20–30 μl/ml aprotinin (400 kallikrein inactivator units, KIU) and 1/10 volume 1 N HCl were supplemented into tissue samples. Samples were then stored at -20°C until analysis. The extracted plasma and tissue samples were assayed for apelin concentration by Human Apelin kit (Active; Phoneix Pharmaceuticals, Germany).
Immunohistochemistry
Paraffin-embedded tissue samples were cut into 5 μm sections and mounted on Poly-L-Lysine slides (Lab Vision Corporation, USA). Slides were deparaffinized in xylene and rehydrated in a graded series of alcohol. Endogenous peroxidase activity was quenched by incubation in 3% H2O2 for 10 min and followed by a rinse with PBS. For antigen retrieval, slides were placed in 10 mmol/L citrate buffer (pH=6.0) and were microwaved twice for 5 min. Non-specific binding of the primary antibody was prevented by incubation in Ultra V Block (Lab Vision Corporation, USA). Sections were then incubated with rabbit polyclonal apelin antibody (ab59469, Abcam Inc., USA) as primary antibody for 30 min at 38°C. Thereafter, sections were washed with PBS and incubated in biotinylated goat antiserum (Lab Vision Corporation) for 10 min at 38°C. After rinsing in PBS again, the slides were incubated in streptavidin-biotin-peroxidase (SBP) (HistoMark, USA) for 10 min. As negative control, primary antibody was omitted. The slides were washed with PBS twice for 5 min, and then amino-ethylcarbazole (AEC) was used as chromogene for 10 minutes. Mayer's hematoxylin was used for counter staining.
We semiquantitatively evaluated the cytoplasmic intensity for apelin immunoreactivity in eutopic and ectopic endometrial tissues. The stromal and glandular cells positively stained using the following intensity categories: 0 (no staining), 1+ (weak but detectable staining), 2+ (moderate or distinct staining), and 3+ (intense staining). In each slide, five different areas were evaluated under ×60 magnification for each intensity within these areas were determined by two investigators blind to the type and source of the tissues. The average intensity of the two investigators was considered for each slide.
Statistical analysis
Power calculation was performed based on previous studies that involved semiquantification of tenascin expression by immunohistochemistry. A sample size of 15 in each group has a power of 80% to detect a difference between means of 22.07 with a significance level (alpha) of 0.05. The statistical power analysis was performed using G power 3 program. Statistical analysis was performed by Statistical Package for Social Sciences (SPSS), version 12.0 (SPSS, Chicago, Illinois, USA). Results were expressed as means and standard deviation. Differences between two groups for continuous variables were analyzed using Student's t-test. Differences in two groups for ordinal variables were analyzed using Mann-Whitney U test. The p-values smaller than 0.05 were considered as statistically significant.
Results
The age, BMI and waist/hip ratio of the participants, respectively, were as follows: 30.2±5.5 years, 22.1±1.8 kg/m 2, and 0.72±0.06 with no significant differences between two groups. The serum and tissue concentrations of apelin in both groups are presented in Table 1. There were no significant differences between serum and eutopic tissue levels of apelin in both groups. Serum apelin concentrations did not show cyclic changes, but both eutopic and ectopic endometrial tissue apelin concentrations increased during secretory, and decreased during proliferative phases. Concentrations of apelin were higher in tissue than serum (p=0.03). Eutopic endometrial tissue concentration of apelin did not differ between the two groups (p=0.43).
Table 1.
Serum and tissue concentrations of apelin
| Group 1 (controls, n=15) | Group 2 (Endometriosis, n=30) | p-value | |
|---|---|---|---|
| Serum apelin ( pg/ml ) | |||
| proliferative phase | 711±122 | 731±124 | 0.97 |
| secretory phase | 703±124 | 735±114 | 0.86 |
| p-value | NS | NS | |
|
| |||
| Eutopic endometrial apelin ( pg/mg ) | |||
| proliferative phase | 1317±299 | 1094±287 | 0.08 |
| secretory phase | 2499±208 | 2418±191 | 0.51 |
| p-value | |||
| SPP vs. EuPP | <0.05 | <0.05 | -- |
| SSP vs. EuSP | <0.05 | <0.05 | -- |
|
| |||
| Ectopic endometrial apelin ( pg/mg ) | |||
| proliferative phase | -- | 1189±134 | -- |
| secretory phase | -- | 2639±342 | -- |
| p-value | -- | -- | -- |
| EuPP vs. EcPP | -- | NS | -- |
| EuSP vs. EcSP | -- | NS | -- |
Note: The results were presented as mean±SD. SPP=serum proliferative phase; SSP=serum secretory phase;
EuPP=eutopic proliferative phase; EcPP=ectopic proliferative phase; EuSP=Eutopic secretory phase;
EcSP=ectopic secretory phase.
NS=Not significant
Immunohistochemical staining of apelin in eutopic endometrium of both groups were dominant in secretory phase (p<0.01), but comparison of eutopic endometrial tissue apelin staining did not show a significant difference between two groups (p=0.71).
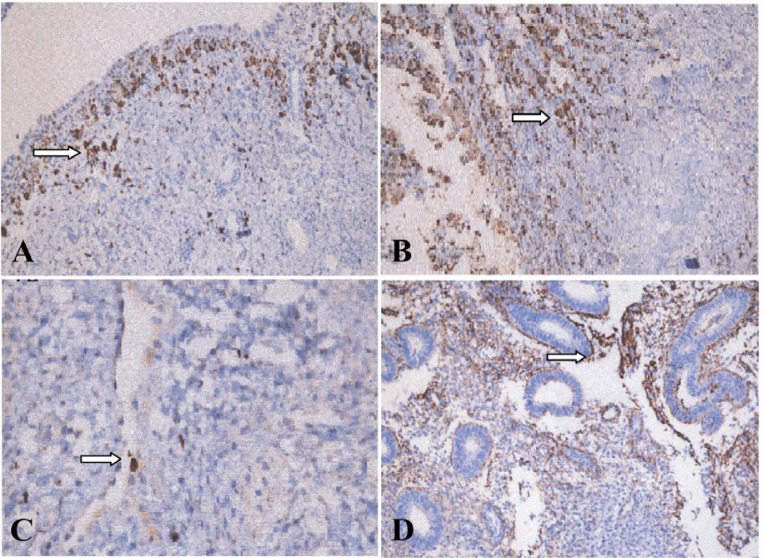
Upon immunohistochemical staining, intense immunoreactivity of apelin was observed in glandular cells of eutopic endometrial tissue of both groups during secretory phase (Figure 1). Moreover, endometriotic tissue showed intense apelin immunoreactivity in glandular cells during secretory phase (p<0.01). Stromal cells of ectopic and eutopic endometrial tissues showed weak staining for apelin; therefore, cyclic variation could not be analyzed in their expression levels. During secretory phase of menstrual cycle, apelin staining was intense in eutopic and ectopic endometrial tissue of women with endometriosis and in endometrium of non-endometriosis participants.
Figure 1.
Immunostaining of apelin in eutopic and ectopic endometria. In eutopic and ectopic endometrial glands, apelin was expressed diffusely in the secretory phase and downregulated in the proliferative phase. Stromal staining intensity of apelin was very low in both the eutopic and ectopic endometria (×200). A: Eutopic proliferative endometrium; B: Eutopic secretory endometrium; C: Ectopic proliferative endometrium; D: Ectopic secretory endometrium
Discussion
The present study most probably was the first to evaluate the expression of apelin in eutopic and ectopic endometrial tissues. Immunohistochemical and immunoassay results showed increased apelin expression during the secretory phase in glandular cells of eutopic and ectopic endometrium. However, the changes in stromal cells were less dynamic than those in glandular cells of both eutopic and ectopic endometrium. Increased local apelin concentration may indicate a paracrine function on endometrium.
The limitations of our study were as follows: Our small study population limited the statistical power of the analysis and immunohistochemical analysis of apelin could have been supported with APJ.
New vessel formation is involved in the pathogenesis of endometriosis (22), and it is thought that epithelial-mesenchymal interaction with the influence of sex steroids play a critical role in endometriosis (23–26). It is likely that the growth regulation of endometriotic cells is controlled by a complex interaction of ECM proteins and paracrine factors.
We hypothesized that apelin might play a role in the endometrial regeneration via angiogenesis process. Apelin mRNA and immunoreactive apelin were detected in various tissues and organs such as epithelial cells of the gastric mucosa, myocardium and endocardial endothelium, lungs, kidneys, adrenals, and the endothelium of large and small blood vessels (9, 27–29). Apelin has also been shown to be involved in the regulation of vessel formation and cell proliferation (11). Apelin causes endothelium dependent vasorelaxation by triggering the release of nitric oxide (30–32). Moreover, there are reports that apelin is a potent angiogenic factor inducing endothelial cell (EC) proliferation, EC migration, and the development of blood vessels in vivo (7, 33). Apelin was detected in the smooth muscle cells of intraluteal arterioles (8). Schilffarth et al. suggested that apelin might act as a chemo-attractant for sprouting endothelial cells (34). Apelin and APJ in the granulosa cells of bovine ovary may be involved in induction of cell apoptosis (35). Apelin has an important effect on activated T lymphocytes by supressing IFN-γ, IL-2 and IL-4 production (27).
Our study is most probably the first for describing apelin expression in ectopic and eutopic endometrium. Expression level of apelin was higher in secretory than proliferative endometrium and glandular cells more evidently showed intense expression than stromal cells. Similar expression patterns of apelin in eutopic and ectopic endometrium shows that endometriotic lesions share some of the characteristics of the cellular processes involved in the regeneration of endometrium. We observed that expression of apelin was similar in the eutopic endometrium of women with or without endometriosis. Presence of apelin in the ectopic endometrium may point to the expression of apelin by ectopic epithelium in the peritoneal environment of women susceptible to the disease.
Apelin may also play a spatio-temporal role for the maturation of blood vessels by transient expression in ectopic endometrium where angiogenesis is taking place. In addition, genetically engineered mouse models indicate that apelin/APJ regulates vascular stabilization and maturation in physiological and pathological angiogenesis (36). Thus, it is difficult to merely explain the proliferative potential of endometriotic epithelia in terms of quality or quantity of apelin.
Conclusion
In conclusion, our results point out secretory phase glandular cell dominancy of apelin expression in both of the eutopic and ectopic endometrium. Further studies are needed to investigate the possible effects of apelin on the formation of endometriotic lesions via their paracrine effects on vessel formation and ECM invasion.
Acknowledgement
We thank medical doctors in the Department of Gynecology of Firat University Hospital for their help during our study. This study was supported by Firat University Scientific Research Foundation.
To cite this article: Ozkan ZS, Cilgin H, Simsek M, Cobanoglu B, Ilhan N. Investigation of Apelin Expression in Endometriosis. J Reprod Infertil. 2013;14(2):50-55.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Lapp T. ACOG issues recommendations for the management of endometriosis. American College of Obstetricians and Gynecologists. Am Fam Physician. 2000;62(6):1431–4. [PubMed] [Google Scholar]
- 2.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 3.Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64(2):151–4. [PubMed] [Google Scholar]
- 4.Kruitwagen RF, Poels LG, Willemsen WN, de Ronde IJ, Jap PH, Rolland R. Endometrial epithelial cells in peritoneal fluid during the early follicular phase. Fertil Steril. 1991;55(2):297–303. doi: 10.1016/s0015-0282(16)54119-3. [DOI] [PubMed] [Google Scholar]
- 5.Hudelist G, Keckstein J, Czerwenka K, Lass H, Walter I, Auer M, et al. Estrogen receptor beta and matrix metalloproteinase 1 are coexpressed in uterine endometrium and endometriotic lesions of patients with endometriosis. Fertil Steril. 2005;84(Suppl 2):1249–56. doi: 10.1016/j.fertnstert.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Castan-Laurell I, Boucher J, Dray C, Daviaud D, Guigné C, Valet P. Apelin, a novel adipokine overproduced in obesity: friend or foe? Mol Cell Endocrinol. 2005;245(1-2):7–9. doi: 10.1016/j.mce.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, et al. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochem Biophys Res Commun. 2004;325(2):395–400. doi: 10.1016/j.bbrc.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Shirasuna K, Shimizu T, Sayama K, Asahi T, Sasaki M, Berisha B, et al. Expression and localization of apelin and its receptor APJ in the bovine corpus luteum during the estrous cycle and prostaglandin F2alpha-induced luteolysis. Reproduction. 2008;135(4):519–25. doi: 10.1530/REP-07-0409. [DOI] [PubMed] [Google Scholar]
- 9.Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, et al. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta. 2001;1538(2-3):162–71. doi: 10.1016/s0167-4889(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 10.Kunduzova O, Alet N, Delesque-Touchard N, Millet L, Castan-Laurell I, Muller C, et al. Apelin/APJ signaling system: a potential link between adipose tissue and endothelial angiogenic processes. FASEB J. 2008;22(12):4146–53. doi: 10.1096/fj.07-104018. [DOI] [PubMed] [Google Scholar]
- 11.Carpéné C, Dray C, Attané C, Valet P, Portillo MP, Churruca I, et al. Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem. 2007;63(4):359–73. [PubMed] [Google Scholar]
- 12.Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. Apelin (65-77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J. 2004;18(15):1909–11. doi: 10.1096/fj.04-1930fje. [DOI] [PubMed] [Google Scholar]
- 13.Katugampola SD, Maguire JJ, Matthewson SR, Davenport AP. [(125)I]-(Pyr(1))Apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. Br J Pharmacol. 2001;132(6):1255–60. doi: 10.1038/sj.bjp.0703939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept. 2004;118(3):119–25. doi: 10.1016/j.regpep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept. 2005;126(3):233–40. doi: 10.1016/j.regpep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto T, Kihara M, Ishida J, Imai N, Yoshida S, Toya Y, et al. Apelin stimulates myosin light chain phosphorylation in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2006;26(6):1267–72. doi: 10.1161/01.ATV.0000218841.39828.91. [DOI] [PubMed] [Google Scholar]
- 17.Takakura N, Kidoya H. Maturation of blood vessels by haematopoietic stem cells and progenitor cells: involvement of apelin/APJ and angiopoietin/Tie2 interactions in vessel caliber size regulation. Thromb Haemost. 2009;101(6):999–1005. [PubMed] [Google Scholar]
- 18.Tiani C, Garcia-Pras E, Mejias M, de Gottardi A, Berzigotti A, Bosch J, et al. Apelin signaling modulates splanchnic angiogenesis and portosystemic collateral vessel formation in rats with portal hypertension. J Hepatol. 2009;50(2):296–305. doi: 10.1016/j.jhep.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Heinonen MV, Laaksonen DE, Karhu T, Karhunen L, Laitinen T, Kainulainen S, et al. Effect of dietinduced weight loss on plasma apelin and cytokine levels in individuals with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009;19(9):626–33. doi: 10.1016/j.numecd.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Kälin RE, Kretz MP, Meyer AM, Kispert A, Heppner FL, Brändli AW. Paracrine and autocrine mechanisms of apelin signaling govern embryonic and tumor angiogenesis. Dev Biol. 2007;305(2):599–614. doi: 10.1016/j.ydbio.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 22.Braun DP, Ding J, Shaheen F, Willey JC, Rana N, Dmowski WP. Quantitative expression of apoptosis-regulating genes in endometrium from women with and without endometriosis. Fertil Steril. 2007;87(2):263–8. doi: 10.1016/j.fertnstert.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol. 2002;83(1-5):149–55. doi: 10.1016/s0960-0760(02)00260-1. [DOI] [PubMed] [Google Scholar]
- 24.Huet-Hudson YM, Chakraborty C, De SK, Suzuki Y, Andrews GK, Dey SK. Estrogen regulates the synthesis of epidermal growth factor in mouse uterine epithelial cells. Mol Endocrinol. 1990;4(3):510–23. doi: 10.1210/mend-4-3-510. [DOI] [PubMed] [Google Scholar]
- 25.Casimiri V, Rath NC, Parvez H, Psychoyos A. Effect of sex steroids on rat endometrial epithelium and stroma cultured separately. J Steroid Biochem. 1980;12:293–8. doi: 10.1016/0022-4731(80)90282-4. [DOI] [PubMed] [Google Scholar]
- 26.Cunha GR, Chung LW, Shannon JM, Taguchi O, Fujii H. Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog Horm Res. 1983;39:559–98. doi: 10.1016/b978-0-12-571139-5.50018-5. [DOI] [PubMed] [Google Scholar]
- 27.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta. 1999;1452(1):25–35. doi: 10.1016/s0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 28.De Falco M, De Luca L, Onori N, Cavallotti I, Artigiano F, Esposito V, et al. Apelin expression in normal human tissues. In Vivo. 2002;16(5):333–6. [PubMed] [Google Scholar]
- 29.Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept. 2004;118(3):119–25. doi: 10.1016/j.regpep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, et al. The novel peptide apelin lowers blood pressure via a nitric oxidedependent mechanism. Regul Pept. 2001;99(2-3):87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 31.Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279(25):26274–9. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 32.Zhong JC, Yu XY, Huang Y, Yung LM, Lau CW, Lin SG. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc Res. 2007;74(3):388–95. doi: 10.1016/j.cardiores.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Cox CM, D'Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol. 2006;296(1):177–89. doi: 10.1016/j.ydbio.2006.04.452. [DOI] [PubMed] [Google Scholar]
- 34.Schilffarth S, Antoni B, Schams D, Meyer HH, Berisha B. The expression of apelin and its receptor APJ during different physiological stages in the bovine ovary. Int J Biol Sci. 2009;5(4):344–50. doi: 10.7150/ijbs.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu T, Kosaka N, Murayama C, Tetsuka M, Miyamoto A. Apelin and APJ receptor expression in granulosa and theca cells during different stages of follicular development in the bovine ovary: Involvement of apoptosis and hormonal regulation. Anim Reprod Sci. 2009;116(1-2):28–37. doi: 10.1016/j.anireprosci.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Kidoya H, Takakura N. Biology of the apelin-APJ axis in vascular formation. J Biochem. 2012;152(2):125–31. doi: 10.1093/jb/mvs071. [DOI] [PubMed] [Google Scholar]