Abstract
Background
The present study was conducted in order to evaluate the effects of bilateral uterine artery ligation (BUAL) on the ovarian follicular fate, and alterations in carbohydrate, lipid, lipase and serum levels of F9SH, LH, prolactin, estrogen and progesterone.
Methods
Twenty-four mature female rabbits divided into two test and control-sham groups. The animals underwent ovariohystrectomy on days 23, 43 and 63 after BUAL. Later serum and tissue samples were processed for histological and bio-chemical analyses. Two-way ANOVA test was used for statistical analyses and p<0.05 was considered as significant.
Results
The ovaries from the case groups exhibited markedly increased atretic follicles, which were characterized by early antrum formation, ooplasmic vacoulation, granulosa cells dissociation and oocyte deformation. Lipid foci were remarkably present in the cytoplasm of oocytes, granulosa and theca cells in BUAL rabbits. Smaller sized atretic follicles showed higher lipid reactions than large ones. The PAS reaction was highly positive in zona pellucida (ZP), basement membrane, granulosa cells and follicular fluid of atretic follicles. Early atresiated follicles showed remarkable reaction sites for lipase. Significant (p<0.05) increase in serum levels of FSH, LH, progesterone, and prolactin was revealed in BUAL rabbits compared to the control group while serum levels of estrogen decreased time-dependently in the test groups.
Conclusion
The current study suggests the critical role of the uterine artery in controlling ovulation and follicular growth. Moreover atresia processes might relate to lipid accumulation in the cells along with attenuation of lipase activity.
Keywords: Atresia, Follicle, Gonadal hormones, Ligation, Uterine artery
Introduction
In rabbits, the ovaries possess a dual blood supply, including the ovarian and uterine arteries. The bifurcation of the uterine artery creates the utero-ovarian branch, which supplies the tip of the uterine horns and the oviduct; it also forms an anastomosis with a primary branch of the ovarian artery. Thus, there is a link between the uterus and ovary by the vascular junction (1). Previous studies have shown that the uterine artery is the major source of the ovarian blood flow in cycling rhesus monkeys (2), and supplies the major portion of the ovarian blood as a second origin after the ovarian artery (1–5). It is very important to understand the contribution of this anastomosis in supplying blood to the ovaries. Any disturbance that influences this correlation, leads to an adverse effect on the ovarian follicular growth and hormonal balance (3).
Different methods such as complete hysterectomy, unilateral hysterectomy, tubal ligation, and total salipingectomy can destroy different parts of the tube and block the correlation between the uterine and the ovary (3–6), leading to reduced ovarian blood supply, and ultimately a significant reduction in ovulation rate (7, 8). For example, selective and mass uterine artery ligations are surgical options for avoiding hysterectomy in non-controllable postpartum hemorrhage (9). At the same time, this option can result in short corpus luteum life span and also cause abnormal or pathological hormonal changes in animals (4, 10, 11) and humans (5, 6). On the other hand, technological advances and increasing social acceptance has made tubal sterilization the most commonly chosen form of female contraception, especially during the last decade of their reproductive life (2, 6, 12). The possible adverse effects of tubal sterilization on the ovarian function has been understood in recent years (2, 13).
Studies investigating on the possible cause of ovarian disturbance have especially focused on the ovarian arterial blood supply at the main arterial level (such as the ovarian artery). However, the ovarian stroma obtains its blood from anastomosis, as well as the ovarian artery.
The fate of follicular atresia due to surgical or other pathological disorders is not fully understood. Hence, the purpose of this study was to evaluate the follicular fate, relevant hormonal changes and the possible relationship between the rate of atresia and follicle size after bilateral uterine artery ligation (BUAL) in mature rabbits as a laboratory animal model. Furthermore, any association between the time of ligation and the rate of possible follicular atresia in rabbits were also the subject of this study.
As special attention is paid to atretic follicle formation pathways, this study also undertaken to develop a new approach in characterizing atrectic follicles with a focus on granulosa cell dissociation (GCD), early antrum formation, and GC luteinization and floatation in the antrum which resemble macrophages.
Carbohydrates are the main sources of energy in follicular cells. In normal follicles, carbohydrates play a critical role in cellular biological activity such as granulosa cell proliferation atretic-follicle cells show different patterns of cytoplasmic carbohydrate concentration, lipid ratio and lipase activity, during the early stages of atresia, thus, the histochemical and biochemical changes of these cells were examined too.
Methods
Animals and experimental design
Twenty-four 6 to 8 month old rabbits (Albino-albino) were obtained from the animal center of the Faculty of Veterinary Medicine affiliated to Urmia University in Urmia, Iran. The animals were acclimatized in an environmentally-controlled room (temperature 17-23°C; relative humidity 50-70%; alternating 12 hr light (dark cycles). Food and water were given ad libitum. The rabbits were later assigned into test and control-sham operated groups. The test group was subdivided into three groups of A, B, and C (n=6). The test subgroups were named according to the day the study terminated, e.g. day 23 (group A), day 43 (group B) and day 63 (group C) after the uterine artery ligation.
To determine day 0 of menstrual cycle, the vulva of animals was observed daily for color intensity which was based on rapid but consistent changes to reddish and/or purple color (4). Arterial ligation was conducted on day 0 in the test groups. This study was approved by the Animal Care and Ethics Committee of the Faculty.
Surgical method for bilateral uterine artery ligation
The test and control-sham groups were anesthetized by 35 mg/kg of 5% Ketamin (Trittau, Germany), 5 mg/kg of 2% Xylazine (Woerden, The Netherlands) intra-peritoneally. After anesthesia, the rabbits were positioned in dorsal recumbency, with hind limbs restrained and extended. A midline incision (2-3 cm) was made through the skin, between the ambilicus and the cranial rim of the pelvis (Pelvix symphisis).
The isolated opposite structures were carefully inspected. The uterine arteries were bilaterally ligated (by 2-0 surgical silk) close to the splitting region of the uterine horns. No arterial ligation was done in the control-sham rabbits.
Ovariohysterectomy
On days 23, 43 and 63 after the ligation, ovariohysterectomy was performed in the test groups A, B and C, respectively after animals were anesthetized. After making the incision, body of the uterus was isolated and clamps were placed. The ovarian pedicles were isolated and ligated and the uterine artery and vessels supplying the broad ligament were also ligated. Finally, the organs were dissected out and the remaining stumps were checked for hemorrhage, before placing them back through the incision.
Histomorphological analyses
On days 23, 43 and 63 after operation, the ovaries were removed and fixed in 10% formalin for 4 weeks. Ultimately, overies were dissected free from per-ovarian tissues. Samples were processed through paraffin embedding and blocks were cut by rotary microtome serially and stained with hematoxylin-eosin and toluidine-blue techniques. For histomorphometric analyses, follicles were characterized in terms of their sizes to under 100, 101-200, 201-300, 301-400 and larger than 400 µm. Follicular morphology was examined by microscope under 400× magnification. Follicles with a complete layer of flattened granulosa cells, oocytes with cytoplasm and a normal nucleus were considered as normal follicles. Abnormal follicles were classified as follows: granulosa cells dissociation (GC D), early antrum formation, GC luteinization and floatation in antrum resembling macrophages. Follicular count was estimated by counting follicles in all slides.
Serum sampling and hormonal assays
Blood samples from corresponding animals were collected from auricular marginal vein and the serum was separated by centrifugation (3000 g for 5 min) and subjected to assessment for serum LH, FSH, progesterone, estrogen and prolactin concentrations. FSH and LH levels were measured by radioimmunoassay according to the manufacturer's instructions using WHO. IRP78/549 and WHO 80/552 kits, respectively. Progesterone and estrogen were measured by electrochemilunescence and prolactin by immunoradiometric methods.
The intra-assay coefficient variance for FSH, LH, estradiol and progesterone were, 3.56% (for 10 times), 2.64% (for 10 times), 5.9% (for 10 times) and 4.8% (for 10 times), respectively. Inter-assay coefficients variances of 8.98% (for 10 times), 7.52% (for 10 times), 5.9% (for 10 times) and 9.9% (for 10 times) were also calculated for FSH, LH, estrogen and progesterone, respectively.
Lipids ratio analyses
To examine the lipid profile of the samples, the Sudan Black B (Pajohesh Asia Kits, Iran) technique was performed. In this procedure, fresh tissue specimens were sectioned by cryostat microtome (Bright 00361, England) and stained by different techniques separately. In brief, the frozen sections of the prepared specimens were picked-up on clean glass slides. The slides were fixed in fresh 6% formalin. Then, the slides were washed well in tap water, rinsed in distilled water and the excess water was drained. Later, the slides were transferred in to propylene glycol for 5 min and passed to Sudan Black B solution. The slides were, then, placed in 85% propylene glycol for 7 min. Rinsing the slides in distilled water, the nuclear fast-red staining solution was used for contrast. Scores of 1+, 2+, 3+ and 4+ were, respectively, considered for atretic follicles with 25, 50, 75 and 100 percent positivity for lipid staining.
Carbohydrates ratio assay
To evaluate the carbohydrate ratio, the PAS technique (Pajohesh Asia Kits, Iran) was employed. In brief, the paraffin sectioned specimens were deparraffinized and hydrated. The hydrated slides were oxidized in 5% periodic acid solution for 5 min. After rinsing in distilled water, the slides were placed in Schiff reagent for 15 min before being washed with luke warm water. After 5 min, the slides counterstained with Meyer's hematoxylin. The pattern for evaluation of carbohydrate ratio was the same as the one performed for lipid staining.
Lipase activity evaluation
In order to show lipase activity in the ovarian tissue, the fresh unfixed and chilled specimens were sectioned with cryostat microtome, and stained using a specific lipase staining technique (Pajohesh Asia Kits, Iran). In brief, specimens from the frozen section were picked-up on glass slides and fixed by cold 6% formalin. The slides were then rinsed in cold distilled water for 1 hour and incubated for 12 hours in incubation solution (85 tween, 5% barbiturate buffer, 10% calcium chloride and distilled water). After incubating the slides, they were stained with ammonium sulfide. Meyer's hema-toxylin was used for contrast staining. The method for evaluating lipase enzyme ratio was the same as mentioned above.
Statistical analysis
All results are presented as mean±SD. Differences between quantitative histological and hematological data on days 23, 43 and 63 of the treatment were analyzed with two-way ANOVA, followed by Bonferroni test, using Graph Pad Prism, 4.00. A p<0.05 was considered as statistically significant.
Results
BUAL increased the rate of atresia
Histological studies showed that ovaries from the control-sham group contained follicles in various developmental stages including primary, secondary and tertiary follicles with different sizes (>100 µm to <400 µm), whereas there was no large antral follicle (>400 µm) in the test groups. BUAL resulted in a significant (p<0.05) decline in follicular size in the test groups in comparison to the control sham group. In the animals undergoing BUAL, the cortex of ovaries was covered with small antral atretic follicles (<100-200 µm). Accordingly atresia occurred mostly in <100 µm and 201- 300 µm follicles. The rate of atrectic follicles (AFs) significantly (p<0.05) increased on animals which terminated after day 63 vs. the control sham rabbits. On day 23 after operation, follicles <100 µm and on days 43 and 63, follicles 101 µm to 200 µm in size showed the highest rate of atresia representing 39.04%, 40.90% and 38.46% of the entire follicles, respectively.
Comparing the rate of normal follicles between the control-sham and test groups revealed a significant (p<0.05) decrease in the number of such follicles in the test groups. This reduction was time dependently progressive, as on day 63 the lowest number of normal follicles were observed. The highest rate of normal follicles between the test groups was observed in group A, 23 days after BUAL. The data for atretic and normal follicles are depicted in Table 1.
Table 1.
The number of normal and atretic follicles with different sizes after bilateral uterine artery ligation
| Atretic Follicles (NO) | ||||
|---|---|---|---|---|
|
| ||||
| Follicles (µm) | Control-sham | 23 days after BUAL | 43 days after BUAL | 63 days after BUAL |
| <100 | 40.5±2.27a | 41.6±2.27a | 45.2±2.29b | 46.1±1.85b |
| 101-200 | 39.81± 1.07a | 40.81± 1.07a | 45.18 ±2.52b | 50.45± 3.35c |
| 201-300 | 11.27±1.00a | 12.36±1.12a | 14.18±1.77b | 24.09±1.75c |
| 301-400 | 12.00 ±0.77a | 12.90±0.83a | 8.18±0.75b | 10.63±0.67c |
| >400 | 2.80 ±0.78 | No follicles were observed in this size | ||
|
| ||||
| Normal Follicles (NO) | ||||
|
| ||||
| <100 | 132.20 ± 2.20a | 130.50± 2.71a,b | 126.10±1.91b | 121.80 ±2.34c |
| 101-200 | 19.41±1.83a | 18.41±1.83a | 10.41±2.50b | 8.33±1.07b |
| 201-300 | 9.36 ±1.28a | 8.36±1.28a | 3.72±1.42b | 2.18±1.07b |
| 301-400 | 8.81±0.98a | 7.81±0.98a | 1.54±0.68b | 0.33±0.67c |
| >400 | 0.27±0.46 | No follicles were observed in this size | ||
BUAL: Bilateral Uterine Artery Ligation.
numbers with different superscript letters in each row differ significantly, p<0.05
Histological examinations also demonstrated early antrum formations in 200 µm follicles, that had a significant (p<0.01) increase in the test groups during the study period. In light microscopy analyses, follicles with 3 to 4 GC layers showed early antrum formation. Follicles ≤100 µm diameter in size showed no signs of early antrum formation either in the test or in the control-sham groups. Ooplasmic vacoulation and GC pyknosis remarkably increased in follicles less than 200 µm in size in comparison to other sizes of follicles in the test groups. GC pyknosis and ooplasmic vacoulation were significantly (p<0.05) enhanced time -dependently in the test groups in comparison to the control-sham group.
GC dissociation was another criterion to evaluate the atretic follicles. According to this index, follicles between 301-400 µm in diameter showed a significant (p<0.05) decrease in GC dissociation on day 43, while in other sizes of atretic follicles GC dissociation increased in the test groups compared to the control-sham groups (Figure 1).
Figure 1.
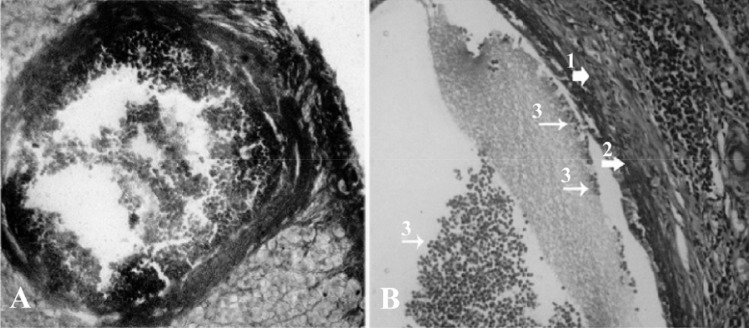
Cross-section of the ovary 63 days after bilateral uterine artery ligation, A: a low magnification view of atretic follicles (301–400 µM) with dissociated granulosa cells; B: a high magnification view of atretic follicles (301–400 µM) with dissociated GCs; (1) theca externa (2) theca interna (3); note the granulosa cells which are floating in the antrum, toluidine-blue staining, (600×)
Presence of GCs which were floating in the antrum and had the appearance of macrophages, was considered another property of atretic follicles. In histological analyses, of the test groups small follicles (pre-antral), showed low and large follicles (antral) showed high rates of floating GCs. Floating GCs increased time-dependently in 300-400 µm follicles. Follicles under 100 µm in diameter showed the lowest percentage of floating GCs in the antrum. There was a significant (p< 0.05) increase in atretic follicles (<100 µm) with floating GCs in the antrum on day 23 in comparison to the control-sham group. On day 63, however, this situation was found to have substantially increased compared to other groups. Surprisingly, the percentage of follicles under 100 µm in diameter showing this property was very low in comparison to the other sizes. Statistical comparison of all sizes in terms of floating GC in the antrum revealed significant differences (p<0.05) between various sizes of the follicles. The data for percentage of atretic follicles with granulosa cells dissociation and floatation in antrum are presented in Table 2.
Table 2.
The percentage of atretic follicles in different sizes with dissociated and floating granulosa cells after bilateral uterine artery ligation
| Dissociated granulosa cells (%) | ||||
|---|---|---|---|---|
|
| ||||
| Follicles (µm) | Control-sham | 23 days after BUAL | 43 days after BUAL | 63 days after BUAL |
| <100 | 24.92±0.40a | 26.40±0.46b | 30.79±0.55c | 32.76±0.71d |
| 101-200 | 15.16±0.67a | 17.72±0.50b | 20.44±0.63 c | 24.14±0.62d |
| 201-300 | 18.62±0.59a | 16.85±0.76b,d | 15.33±0.57c | 17.35±0.67d |
| 301-400 | 12.33±0.43a | 12.09±0.52a | 10.82±0.38b | 13.53±0.46c |
| >400 | 0.50±0.03 | No follicles were observed in this size | ||
|
| ||||
| Floating granulosa cells (%) | ||||
|
| ||||
| <100 | 00.00±00.00a | 1.98±0.23b | 2.53±0.30c | 3.98±0.42d |
| 101-200 | 20.84±0.43a | 22.39±0.59b | 24.38±0.61c,d | 25.66±0.67d |
| 201-300 | 24.07±0.40a | 24.27±0.44a | 26.18±0.46b | 29.55±0.97c |
| 301-400 | 26.44±0.40a | 27.69±0.90a | 29.14±0.28b | 32.52±1.62c |
| >400 | 1.10±0.02 | No follicles were observed in this size | ||
BUAL: Bilateral Uterine Artery Ligation.
numbers with different superscript letters in each row differ significantly, p<0.05
GC luteinization was considered as another marker for atresia. In microscopic investigation luteinization was observed in most of the follicles (antral and pre-antral) in the test groups. Moreover, the cells in the theca layer and cumulus cells showed luteinization in most of the follicles, as well. Observations demonstrated no significant differences, between the percentage of luteinization in <100, 101-200 and 201-300 µm follicles in the control-sham group except follicles with 301-400 µm diameter on day 23. GC luteinization increased in larger follicles of the test groups in comparison to the control-sham group in a time dependent manner. Deformation of the oocyte was considered as the final morphometric character is tics for atresia. Follicles between 201-300 µm in diameter showed the highest rate of oocyte deformation in atretic follicles in the test groups. Oocyte distortion was frequently observed in the test groups, especially, on day 63. Follicles <200 µm showed lower rates of oocyte deformation in comparison to the control-sham group. Over time, this situation was also observed in larger follicles of the test groups (Figure 2). The data for granulosa cells luteinization and oocyte deformation are indicated in Table 3.
Figure 2.
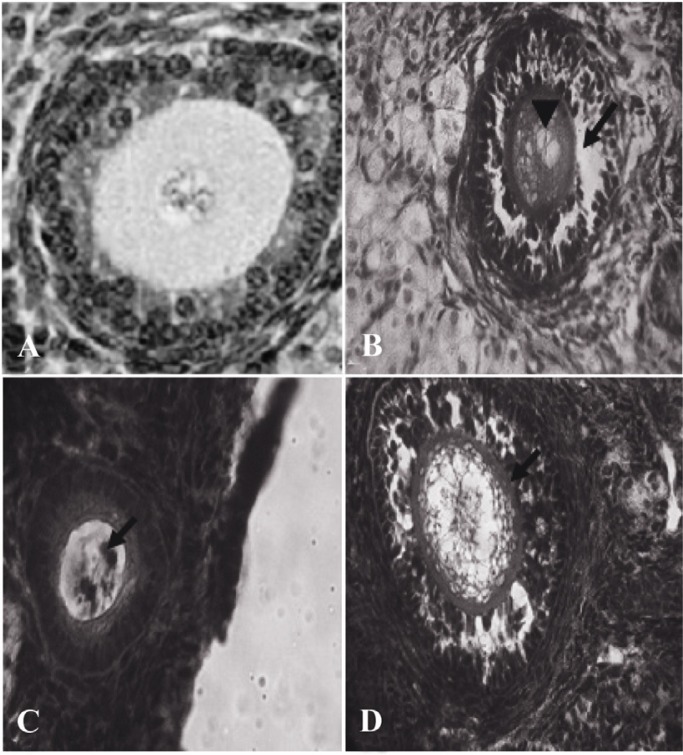
Atretic follicles belong to the ovary 63 days after BUAL bilateral uterine artery ligation. A: Normal follicle from the control-sham group; B: Atretic follicle (201-300 µm) form with GCs dissociation (Arrow) around the vacuolated oocyte (Arrow head); C: Atretic follicle (101–200 µm) with pyknosis of the oocyte and D: Atretic follicle (301–400 µm) with increased thickness of ZN (Arrow). H&E staining, (A: 600×, B: 400×, C: 100× and D: 400×)
Table 3.
The percentage of atretic follicles with luteinized granulosa cells and deformed oocytes after bilateral uterine artery ligation
| Granulosa cells luteinization (%) | ||||
|---|---|---|---|---|
|
| ||||
| Follicles (µm) | Control-sham | 23d ays after BUAL | 43 days after BUAL | 63 days after BUAL |
| <100 | 2.95±0.57a | 3.23±0.60a | 17.31±0.73b | 19.20±0.60c |
| 101-200 | 11.24±1.18a | 12.25±0.68a | 30.23±3.34b,d | 33.28±2.09d |
| 201-300 | 10.82±0.60a | 11.48±0.82a | 38.44±2.90b | 45.19±2.22c |
| 301-400 | 5.11±0.21a | 6.29±0.50b | 20.60±0.59c | 24.85±0.43d |
| >400 | 13.21±1.02 | No follicles were observed in this size | ||
|
| ||||
| Oocyte deformation (%) | ||||
|
| ||||
| <100 | 20.29±0.46a | 8.23±0.96b,c | 7.41±0.94b,c | 8.21±0.20c |
| 101-200 | 42.78±1.25a | 20.90±0.65b | 17.47±0.34c | 15.80±0.54d |
| 201-300 | 24.45±0.31a | 35.95±0.81b | 41.47±0.84c | 58.45±1.35d |
| 301-400 | 19.74±0.68a | 22.38±0.74b | 23.42±0.57b,c | 24.52±0.65c |
| >400 | 1.23±0.02 | No follicles were observed in this size | ||
BUAL: Bilateral Uterine Artery Ligation.
numbers with different superscript letters in each row differ significantly, p<0.05
BUAL altered serum hormones concentrations
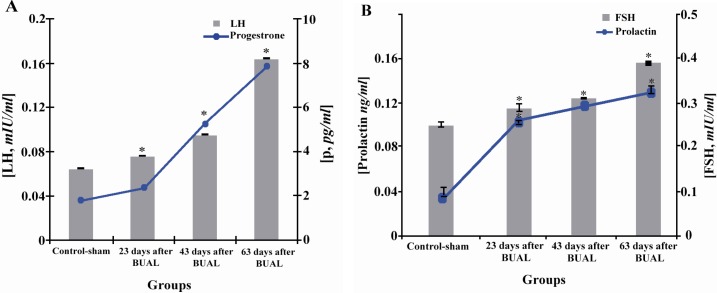
Hormonal analyses showed significant (p<0.05) increases in FSH, LH, progesterone and prolactin concentrations in the test groups in comparison to the control-sham group. Moreover, the mentioned increase was remarkable on day 63. Blood levels of estrogen were 7.43±0.36 pg/ml in the control sham group, while it was under the limit of detection (5.00 pg/ml) in the test groups. This situation shows that estrogen decreased over time in the test groups. Progesterone level increased considerably on day 63 after the operation (Figures 3A, 3B and 3C).
Figure 3.
Effect of BUAL on the alterations of A: LH and progesterone and B: FSH and prolactin levels in serum, *s represent significant differences (p<0.05) between the BUAL-induced groups and the control-sham group. All data are presented as Mean±SD and N=6 in each individual group
BUAL induced pathological alterations in lipid and carbohydrate ratios and lipase activity
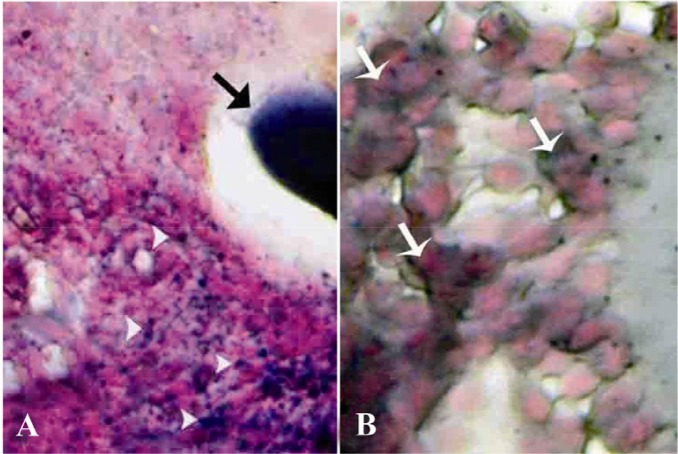
Special staining of BUAL ovaries for lipid detection revealed lipid accumulation in follicular granulosa, theca cells, oocytes and macrophages. The granulosa cell reaction for Sudan-black staining in early atretic follicles was significantly (p<0.05) stronger than that of the mature follicles. The majority of the ovarian parenchymal cells in BUAL ovaries showed remarkable positive reaction to lipid staining (Figures 4A and 4B). These cells were observed in the form of huge dense bluish black colonies by Sudan-black B staining. In comparison to the control-sham group, the distribution of Sudan black B positive cells was higher in BUAL induced rabbits. Accordingly, histo-chemical analyses of the ovaries after 63 days showed the highest rate of lipid staining in ovarian parenchymal cells in animals undergoing BUAL. In contrast to atretic follicles (AFs), normal follicles were negative for lipid staining. By atresia progressing, granulosa cells had higher saturated lipids depicted by staining in comparison to the theca cells. Comparing the reactions for lipid staining between different stages of atresia showed that, the oocytes of AFs in first stages exhibited stronger reaction versus later stages.
Figure 4.
Cross-section from ovary 63 days after bilateral uterine artery liagtion, A: An early atretic follicle; note dense lipidophilic reaction of the oocyte (arrow) and lipid positive ovarian interstitial cells (arrow heads); B: showing intensely lipid positive reacted granulosa cells (arrows). Sudan Black-B staining technique, (A, 400 × and B, 1000 Head ×)
PAS reaction was positive in ZP, basement membrane of granulose cells, stroma of follicles, the follicular fluid and granulosa cells of AFs. In contrast to AFs, normal follicles showed a very weak PAS reaction. Small AFs, which were committed to precautions atresia, showed strong PAS reaction in the antrum. The data showing degree of histochemical reaction are presented in Table 4.
Table 4.
The intracytoplasmic carbohydrate, lipid content, and lipase activity in follicled with different sizes after bilateral uterine artery ligation
| Reaction degree for carbohydrate supplement | ||||
|---|---|---|---|---|
|
| ||||
| Follicles (µm) | Control-sham | 23 days after BUAL | 43 days after BUAL | 63 days after BUAL |
| <100 | 1+ | 3+ | 4+ | 4+ |
| 101-200 | 2+ | 2+ | 4+ | 3+ |
| 201-300 | 1+ | 3+ | 3+ | 4+ |
| 301-400 | 2+ | 2+ | 2+ | 3+ |
| >400 | 3+ | |||
| Degree of reaction for lipid foci accumulation | ||||
| <100 | 3+ | 3+ | 4+ | 4+ |
| 101-200 | 2+ | 3+ | 4+ | 4+ |
| 201-300 | 3+ | 3+ | 3+ | 3+ |
| 301-400 | 2+ | 2+ | 4+ | 3+ |
| >400 | 1+ | |||
| Degree of reaction for lipase activity | ||||
| <100 | 2+ | 2+ | 4+ | 4+ |
| 101-200 | 1+ | 2+ | 4+ | 3+ |
| 201-300 | 2+ | 3+ | 2+ | 2+ |
| 301-400 | 1+ | 1+ | 1+ | 1+ |
| >400 | 1+ | |||
BUAL: Bilateral Uterine Artery Ligation
Granulosa and theca cells were hyper-reactive for lipase. However, by the progression of atresia, this reaction became weak. By contrast, the interstitial tissue cell showed poor lipase activity. The theca layers of AFs showed quite stronger reaction for lipase in comparison to the granulosa cells (Figures 5A and 5B).
Figure 5.
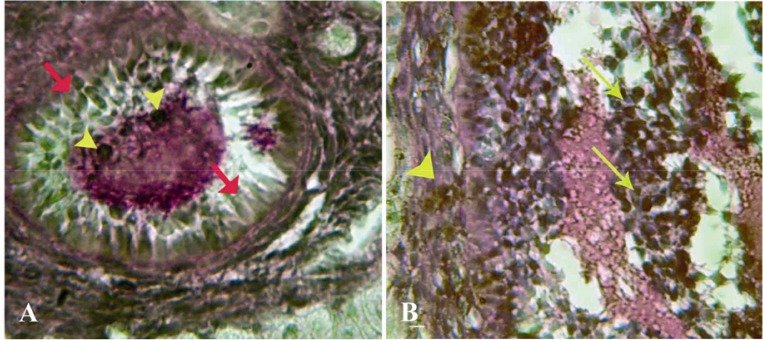
Cross-section from ovary 63 days after bilateral uterine artery ligation, A: granulosa cells (arrow) and the oocyte (arrow head) are presented with dense reaction for lipase enzyme; B: High magnification from granulosa cells and theca layer, note lipase positive sites in granulosa cells (arrows) and in theca cells (arrow head). Lipase enzyme staining, (A, 600× and B, 1000 ×)
Discussion
The primary purpose of the current study was to show the histological and histochemical alterations of ovaries following BUAL and also to eval uate the follicular fate and/or growth. We showed a significant enhancement of atretic follicle formation in animals undergoing BAUL that was accompanied by a remarkable decrease in the rate of normal follicles. Moreover, the early antrum formation and changes in GCs, including dissociation, floatation, and leutinization, were also found as other biomarkers of this type of atresia. More over, the present study showed hormonal changes after BUAL in rabbits, including considerable increase in FSH, LH, and progesterone levels and decrease in serum estradiol levels.
Previous studies in rats showed that BUAL leads to decreased ovulation (14, 15). During ovulation, distinct homodynamic and structural changes occur in the ovary. An elevation in intra-follicular blood flow (16), increase in capillary permeability (17), and breakdown of extra-cellular matrix at the apex of the follicle (18) are major components of the ovulatory process. The ovarian blood flow is comparatively high and increases several folds during ovulation (19, 20).
Recent studies indicate a rapid and persistent reduction of blood flow after both the ovarian and uterine artery ligation, independent of the stage of ovulation (10).
The atresia process can be demonstrated by characteristics such as GC pyknosis (21), dissociation of GCs from basal membrane (5), GC floatation in the antrum (22), segmentation and deformation or annihilation of oocyte nucleus (23–26).
There are controversies concerning the initial sign of atresia, as some scientists believe that primary signs of atresia starts from the oocyte itself (27, 28), while others think that this process begins with GCs first, and then extends to other parts (29, 30). Nevertheless, follicular atresia is a process that can occur in various phases (31). One of the main messages of the current study is that most of the atretic follicles showed various signs of atresia, particularly in large follicles. Occasionally, some follicles showed more than four essential character is tics of atresia at the same time. The most important point, however, was that the majority of follicles showed atresic characteristics, mostly in GCs. However, our histochemical study showed much more considerable pathological alterations in lipid and carbohydrate ratios in these cells in comparison to oocytes in AFs. Thus, it may let us draw early conclusion as BUAL induces atresia firstly in GCs and then it extends to other parts of AFs.
In normal conditions, secondary follicles have 2-8 GC layers without any antrum formation (26, 32). In accordance to this classification, our histological examinations showed early antrum formation in 200 µm atretic follicles in the test groups. To this end, it may indicate that this particular disorder occurs in secondary follicles after BUAL, as well. On the other hand small AFs showed higher PAS reaction in different compartments of AFs, for example in ZP, basement membrane of granulose cells, stroma of follicles, the follicular fluid and granulosa cells.
It is known that ooplasmic vacuolation and GC pyknosis are observed simultaneously with early antrum formation in atretic follicles (21, 33). Our data, therefore, is in harmony with previous studies where we showed the co-occurrence of early antrum formation and ooplasmic vacoulation in secondary follicles.
Lipid staining showed heterochromatic nucleus with positive lipid reaction foci in the cytoplasm of GCs in AFs. This finding is supported by previous reports that showed cytoplasmic lipid accumulation with marginal vacuolation in cystic and/ or atretic follicles (34, 35).
According to Pulumba et al. atresia is an apoptotic reaction (36). Similar to other types of cells, apoptotic GCs show chromatin lysis, cytoplasmic vacoulation, pyknosis, fragmentation and disappearance of nucleus (31, 37). In the present study we showed that follicles under 200 µm in diameter had increased percentage of GC pyknosis in the test groups in comparison to the control-sham group. By the time this reaction occurred on day 63, high numbers of GCs demonstrated all the mentioned characteristics. It is a clue for the concept that 63 days after BUAL, a massive cell destruction and a probable increase in androgenic hormones could cause remarkable follicular atresia. From cytological point of view, GC pyknosis, chromatolysis, dissociation and their floating in antrum are essential to describe a real atresia (33, 37). Morphological analyses revealed that GC dissociation was evident in both small-sized and antral follicles. Furthermore, special lipid staining showed that in the first stages of atresia, follicles had higher lipid accumulation in cytoplasm and lower cytoplasmic carbohydrate ratio. This finding corroborates the theory exhibited in previous reports (38). This observation may suggest that following chromatolysis and degeneration of the nucleus in both GCs and oocytes, intensive deficiency in proteins and in the metabolism of carbohydrates, as an essential source of energy, will occur. Thus, cell dissociation and floatation in antrum will follow.
One of the signs of atretic follicles is oocyte deformation. In atretic follicles, oocytes show pyknosis and have unclear zona pellucida (32, 39). Our investigation showed oocyte deformation in atretic follicles with centric nucleus and none continues zona pellucida in PAS staining. Although there were an increasing percentage of atretic follicles with oocyte deformation, GCs, however, showed higher rates of degeneration.
Recently, Dahn et al. reported that macrophages have a critical role in the regulation of ovarian function by phagocytosis of cellular residues (18). Previous studies reported that ovarian macrophages had different origin and they could originate from follicular GCs (35, 39). Our histological observations demonstrated no macrophages adjacent to normal follicles, while they were localized densely around and/or in the antrum of the AFs with densely lipid stained cytoplasm. Accordingly, by the time macrophages increased in number in 63 days a large macrophage population with dark stained lipid droplets in the cytoplasm were observeable in the test group. Thus, we may hypothesis that in response to high cell destruction in the atretic follicles, the population and activity of the macrophages were increased.
Previous reports indicated that serum FSH levels increased significantly in the hysterectomized group compared to the control group six months after the operation in human beings (4). In another study on human beings undergoing tubular sterilization, serum FSH and LH levels increased time dependently (2). By contrast, Zeleziket et al. reported that mean FSH, LH, and estradiol values, and the ovarian volume remain unchanged after both total salpingectomy and abdominal hysterectomy (32). In accordance with previous studies, our biochemical analyses revealed that in the case of uterine artery disruption, serum levels of FSH and LH increased time-dependently in the test groups, while it was in normal levels in the control-sham rabbits. The possible explanation would be that, the increased GC degeneration following uterine artery disruption and severe loss in biological activity of these cells, the feedback response for of FSH and LH surge was deactivated and in turn it resulted in hormone increase.
Previous studies reported that estradiol levels decreased in women who had previously undergone tubal sterilization (8, 38). In addition, the authors suggested a theory of localized hypertension and tissue damage to explain the poor ovarian function when utero-ovarian connection was disrupted (39). In this regard, other researchers believe that the changes observed in estradiol secretion may relate to the changes in autocrine and endocrine factors such as inhibin, which mediates the regulatory mechanisms about FSH (8, 40). Our finding about reduction in serum estradiol may associate with the increase in degenerated GCs in the test groups.
In the present study, serum levels of progesterone increased in the test groups. It seems that the ovaries in the test groups actively engage in producing enough progesterone necessary for a normal cycle in a compensatory manner. However, because of abnormal androgenic interferences, progesterone level increased over time in the test rabbits. Another reason for this elevation could be the increase in luteinization rate in the atretic follicles.
Conclusion
Considering the increased rates of atresia in different sizes of follicles (pre-antral and/or antral) and absence of >400 µm follicles in BUAL induced animals, it seems that BUAL could result in a significant follicular atresia. Accumulation of carbohydrate in follicular cells and basement membrane of atretic follicles was remarkable. Increase in follicular lipid droplets in the first stages of atresia may result in enhancement of lipase activity, which declines by time.
Acknowledgement
The authors wish to thanks Mr. Ali Jafari the staff of pathology division in Emam hospital.
To cite this article: Akhtari K, Razi M, Malekinejad H. Uterine Artery Interruption: Evidence for Follicular Growth and Histochemical and Biochemical Changes. J Reprod Infertil. 2012;13(4):193-203.
Conflict of Interest
Authors declare no conflict of interest.
References
- 1.Kuscu E, Duran HE, Zeyneloglu HB, Demirhan B, Bagis T, Saygili E. The effect of surgical sterilization on ovarian function: a rat model. Eur J Obstet Gynecol Reprod Biol. 2002;100(2):204–7. doi: 10.1016/s0301-2115(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 2.Kelekci S, Yorgancioglu Z, Yilmaz B, Yasar L, Savan K, Sonmez S, et al. Effect of tubal ligation on ovarian reserve and the ovarian stromal blood supply. Aust N Z J Obstet Gynaecol. 2004;44(5):449–51. doi: 10.1111/j.1479-828X.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- 3.Bulent Tiras M, Noyan V, Ozdemir H, Guner H, Yildiz A, Yildirim M. The changes in ovarian hormone levels and ovarian artery blood flow rate after laparoscopic tubal sterilization. Eur J Obstet Gynecol Reprod Biol. 2001;99(2):219–21. doi: 10.1016/s0301-2115(01)00410-9. [DOI] [PubMed] [Google Scholar]
- 4.Ozdamar S, Ulger H, Sorkun HC, Müderris I. Effects of hysterectomy on ovarian morphology and serum FSH level in rats. Maturitas. 2005;52(1):60–4. doi: 10.1016/j.maturitas.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Thonse VR, Judson K, Vang R. Utero-ovarian anastomosis: histopathologic correlation after uterine artery embolization with or without ovarian artery embolization. J Vasc Interv Radiol. 2007;18(1 Pt 1):31–9. doi: 10.1016/j.jvir.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Petri Nahás EA, Pontes A, Nahas-Neto J, Borges VT, Dias R, Traiman P. Effect of total abdominal hysterectomy on ovarian blood supply in women of reproductive age. J Ultrasound Med. 2005;24(2):169–74. doi: 10.7863/jum.2005.24.2.169. [DOI] [PubMed] [Google Scholar]
- 7.Ahn EH, Bai SW, Song CH, Kim JY, Jeong KA, Kim SK, et al. Effect of hysterectomy on conserved ovarian function. Yonsei Med J. 2002;43(1):53–8. doi: 10.3349/ymj.2002.43.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Kelekci S, Yorgancioglu Z, Yilmaz B, Yasar L, Savan K, Sonmez S, et al. Effect of tubal ligation on ovarian reserve and the ovarian stromal blood supply. Aust N Z J Obstet Gynaecol. 2004;44(5):449–51. doi: 10.1111/j.1479-828X.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- 9.Camprubí M, Ortega A, Balaguer A, Iglesias I, Girabent M, Callejo J, et al. Cauterization of meso-ovarian vessels, a new model of intrauterine growth restriction in rats. Placenta. 2009;30(9):761–6. doi: 10.1016/j.placenta.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Zackrisson U, Mikuni M, Peterson MC, Nilsson B, Janson PO, Brännström M. Evidence for the involvement of blood flow-related mechanisms in the ovulatory process of the rat. Hum Reprod. 2000;15(2):264–72. doi: 10.1093/humrep/15.2.264. [DOI] [PubMed] [Google Scholar]
- 11.Wehrenberg WB, Dierschke DJ, Wolf RC, Meyer RK. The effect of ligating the ovarian and uterine arteries on ovarian function in cyclic rhesus monkeys. Biol Reprod. 1979;20(3):596–600. doi: 10.1095/biolreprod20.3.596. [DOI] [PubMed] [Google Scholar]
- 12.Luukkainen T. Contraception after thirty-five. Acta Obstet Gynecol Scand. 1992;71(3):169–74. doi: 10.3109/00016349209009913. [DOI] [PubMed] [Google Scholar]
- 13.Hulka JF, Phillips JM, Peterson HB, Surrey MW. American association of gynecologic laparoscopic 1993 membership survey. J Reprod Med. 1990;35:5846. [PubMed] [Google Scholar]
- 14.Battaglia C, Pasini A, Mancini F, Persico N, Burnelli R, Cicognani A, et al. Utero-ovarian ultra sonographic and Doppler flow analyses in female childhood cancer survivors with regular menstruation and normal circulating follicle-stimulating hormone levels. Fertil Steril. 2006;85(2):455–61. doi: 10.1016/j.fertnstert.2005.07.1299. [DOI] [PubMed] [Google Scholar]
- 15.Sumiala S, Pirhonen J, Tuominen J, Mäenpää J. Increased uterine and ovarian vascular resistance following Filshie clip sterilization: preliminary findings obtained with color Doppler ultrasonography. J Clin Ultrasound. 1995;23(9):511–6. doi: 10.1002/jcu.1870230902. [DOI] [PubMed] [Google Scholar]
- 16.Campbell S, Bourne TH, Waterstone J, Reynolds KM, Crayford TJ, Jurkovic D, et al. Transvaginal color blood flow imaging of the periovulatory follicle. Fertil Steril. 1993;60(3):433–8. [PubMed] [Google Scholar]
- 17.Robker RL, Russell DL, Espey LL, Lydon JP, O’ Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A. 2000;97(9):4689–94. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahm-Kähler P, Löfman C, Fujii R, Axelsson M, Janson PO, Brännström M. An intravital microscopy method permitting continuous long-term observations of ovulation in vivo in the rabbit. Hum Reprod. 2006;21(3):624–31. doi: 10.1093/humrep/dei394. [DOI] [PubMed] [Google Scholar]
- 19.Acosta TJ. Studies of follicular vascularity associated with follicle selection and ovulation in cattle. J Reprod Dev. 2007;53(1):39–44. doi: 10.1262/jrd.18153. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka N, Espey LL, Okamura H. Increase in ovarian blood volume during ovulation in the gonadotropin-primed immature rat. Biol Reprod. 1989;40(4):762–8. doi: 10.1095/biolreprod40.4.762. [DOI] [PubMed] [Google Scholar]
- 21.Fénichel P, Gobert B, Carré Y, Barbarino-Monnier P, Hiéronimus S. Polycystic ovary syndrome in autoimmune disease. Lancet. 1999;353(9171):2210. doi: 10.1016/S0140-6736(99)00256-1. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Yamanouchi K, Nishihara M. Expression of ski in the granulosa cells of atretic follicles in the rat ovary. J Reprod Dev. 2006;52(6):715–21. doi: 10.1262/jrd.18051. [DOI] [PubMed] [Google Scholar]
- 23.Corner GW. On the origin of the corpus luteum of the sow from both granulosa and theca interna. Am J Anat. 1919;26(1):116–83. [Google Scholar]
- 24.Gunin AG, Sharov AA. Role of mast cells in oestradiol effects on the uterus of ovariectomized rats. J Reprod Fertil. 1998;113(1):61–8. doi: 10.1530/jrf.0.1130061. [DOI] [PubMed] [Google Scholar]
- 25.Junqueira LC, Carneiro J, Kelley RO. Basic histology. 4th ed. California: Lange Medical Publication; 1992. pp. 123–4. [Google Scholar]
- 26.Najati V, Sadrkhanlou RA, Hasanzadeh SH, Farshid AA. Histological and histochemical and electronmicroscopic study on PCO induce immature rat ovary and endometrium. Iran Vet Res J. 2005;7:176–80. [Google Scholar]
- 27.Henmi H, Endo T, Nagasawa K, Hayashi T, Chida M, Akutagawa N, et al. Lysyl oxidase and MMP-2 expression in dehydroepiandrosterone induced polycystic ovary in rats. Biol Reprod. 2001;64(1):157–62. doi: 10.1095/biolreprod64.1.157. [DOI] [PubMed] [Google Scholar]
- 28.Dvorak AM. New aspects of mast cell biology. Int Arch Allergy Immunol. 1997;114(1):1–9. doi: 10.1159/000237635. [DOI] [PubMed] [Google Scholar]
- 29.Faulds G. Mechanism of catecholamine resistance in polycystic ovarian syndrome reviewed. Women Health Wkly. 2003;1:6–7. [Google Scholar]
- 30.Jefferson WN, Padilla-Banks E, Newbold RR. Lactoferrin is an estrogen responsive protein in the uterus of mice and rats. Reprod Toxicol. 2000;14(2):103–10. doi: 10.1016/s0890-6238(00)00061-7. [DOI] [PubMed] [Google Scholar]
- 31.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensi-tivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047–52. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 32.Zeleznik AJ, Hutchison JS, Schuler HM. Interference with the gonadotropin-suppressing actions of estradiol in macaques overrides the selection of a single preovulatory follicle. Endocrinology. 1985;117(3):991–9. doi: 10.1210/endo-117-3-991. [DOI] [PubMed] [Google Scholar]
- 33.Sumiala S, Pirhonen J, Tuominen J, Mäenpää J. Increased uterine and ovarian vascular resistance following Filshie clip sterilization: preliminary findings obtained with color Doppler ultrasonography. J Clin Ultrasound. 1995;23(9):511–6. doi: 10.1002/jcu.1870230902. [DOI] [PubMed] [Google Scholar]
- 34.Lee GY, Croop JM, Anderson E. Multidrug resistance gene expression correlates with progesterone production in dehydroepiandrosterone-induced polycystic and equine chorionic gonadotropin stimulated ovaries of prepubertal rats. Biol Reprod. 1998;58(2):330–7. doi: 10.1095/biolreprod58.2.330. [DOI] [PubMed] [Google Scholar]
- 35.Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppä L, et al. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;84(8):2744–50. doi: 10.1210/jcem.84.8.5921. [DOI] [PubMed] [Google Scholar]
- 36.Carmona F, Cristóbal P, Casamitjana R, Balasch J. Effect of tubal sterilization on ovarian follicular reserve and function. Am J Obstet Gynecol. 2003;189(2):447–52. doi: 10.1067/s0002-9378(03)00487-3. [DOI] [PubMed] [Google Scholar]
- 37.Abbott DH, Barnett DK, Levine JE, Padmanabhan V, Dumesic DA, Jacoris S, et al. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod. 2008;79(1):154–63. doi: 10.1095/biolreprod.108.067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ying SY, Becker A. Periodical relationship between the secretion of foolicle stimulatory hormone and production of inhibin in rat. J Reprod Fertil. 1991;27:281–2. [Google Scholar]
- 39.Batth BK, Parshad RK. Mast cell dynamics in the house rat (Rattus rattus) ovary during estrus cycle, pregnancy and lactation. Eur J Morphol. 2000;38(1):17–23. doi: 10.1076/0924-3860(200002)38:01;1-#;ft017. [DOI] [PubMed] [Google Scholar]
- 40.Brinton LA, Gammon MD, Coates RJ, Hoover RN. Tubal ligation and risk of breast cancer. Br J Cancer. 2000;82(9):1600–4. doi: 10.1054/bjoc.1999.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]