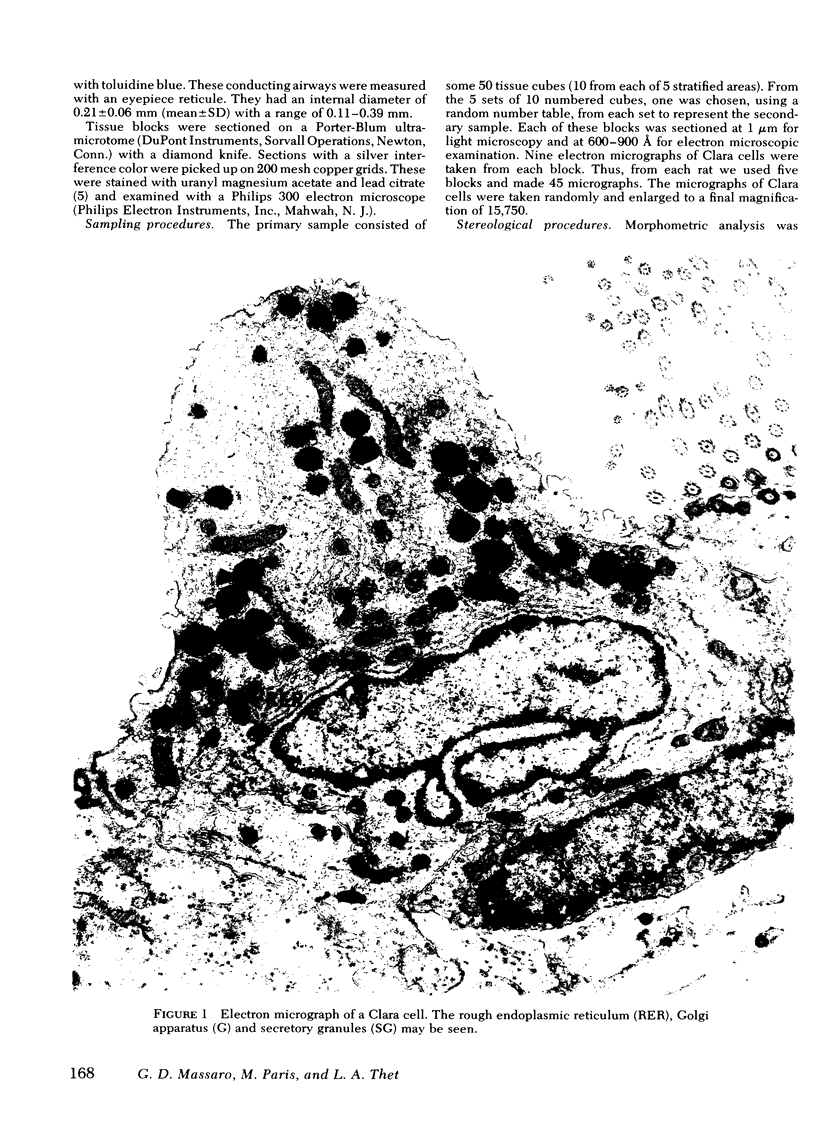
Abstract
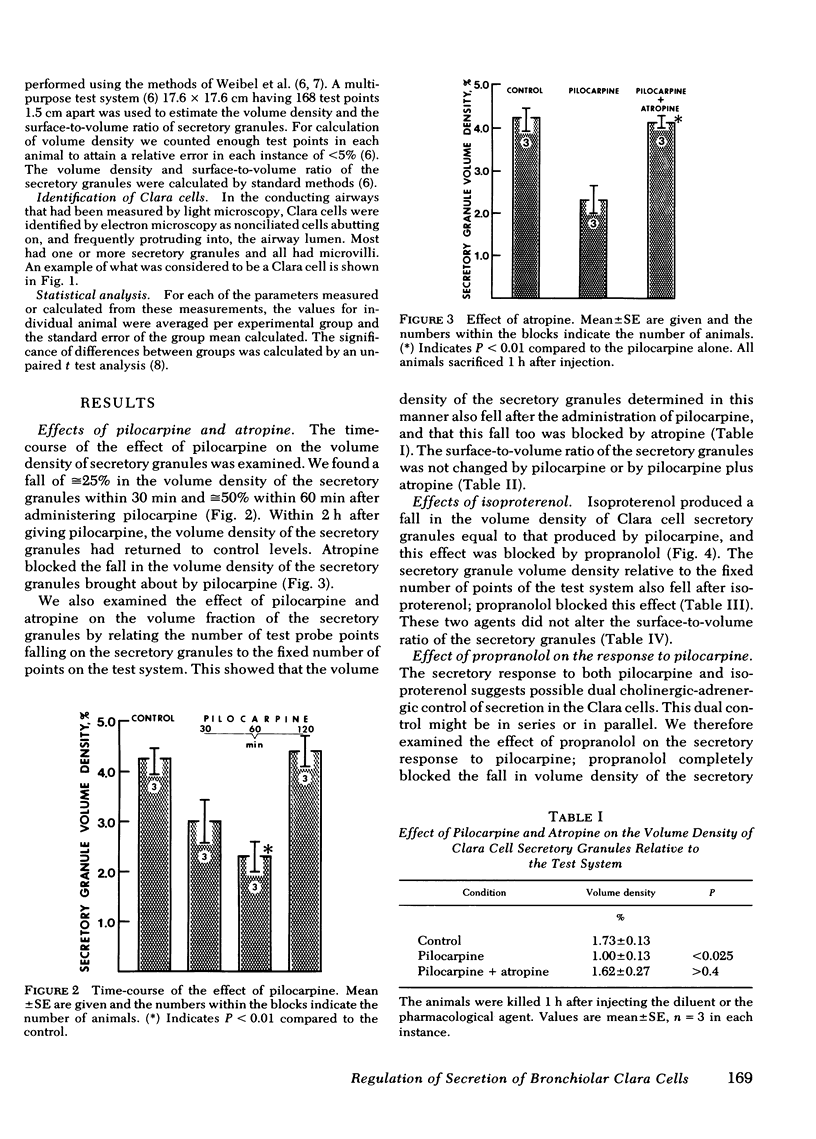
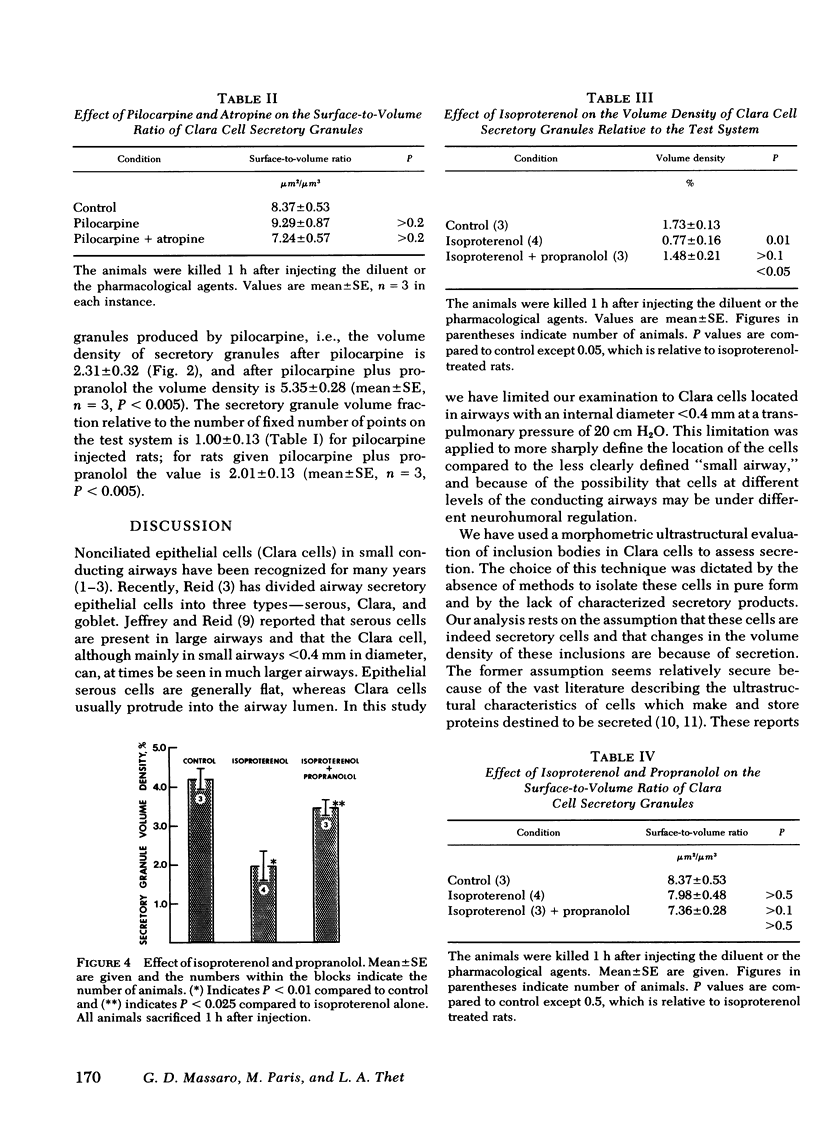
We used ultrastructural morphometric methods to study the in vivo regulation of secretion in bronchiolar Clara cells of rats. The Clara cells studied were located in airways with an internal diameter of 0.21 +/- 0.06 mm (mean +/- SD) at a transpulmonary pressure of 20 cm H2O. We found that pilocarpine caused a 50% decrease in the volume density of secretory granules of Clara cells in 60 min and that atropine blocked this effect. Isoproterenol produced a similar fall in volume density and this was blocked by propranolol. Propranolol also blocked the effect of pilocarpine. The fall in volume density of the secretory granules produced by pilocarpine and by isoproterenol occurred without any change in the surface-to-volume ratio of the granules. This indicates the change in volume density reflected a decrease in number rather than in size of the secretory granules. The observation that propranolol blocks the secretory response to pilocarpine as well as the response to isoproterenol suggests a dual in series cholinergic adrenergic regulation of secretion in bronchiolar Clara cells in rats.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feldberg W., Minz B., Tsudzimura H. The mechanism of the nervous discharge of adrenaline. J Physiol. 1934 Jun 9;81(3):286–304. doi: 10.1113/jphysiol.1934.sp003136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry C. B. Cholinergic link hypothesis in adrenergic neuroeffector transmission. Physiol Rev. 1966 Jul;46(3):420–456. doi: 10.1152/physrev.1966.46.3.420. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K., Reid L. New observations of rat airway epithelium: a quantitative and electron microscopic study. J Anat. 1975 Nov;120(Pt 2):295–320. [PMC free article] [PubMed] [Google Scholar]
- Massaro D., Kelleher K., Massaro G., Yeager H., Jr Alveolar macrophages: depression of protein synthesis during phagocytosis. Am J Physiol. 1970 Jun;218(6):1533–1539. doi: 10.1152/ajplegacy.1970.218.6.1533. [DOI] [PubMed] [Google Scholar]
- Massaro G. D., Massaro D. Granular pneumocytes. Electron microscopic radioautographic evidence of intracellular protein transport. Am Rev Respir Dis. 1972 Jun;105(6):927–931. doi: 10.1164/arrd.1972.105.6.927. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Reid L. M. Secretory cells. Fed Proc. 1977 Dec;36(13):2703–2707. [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K. Pilocarpine stimulation of the bronchiolar Clara cell secretion. Lab Invest. 1977 Nov;37(5):447–452. [PubMed] [Google Scholar]