Abstract
Background
Due to increasing clinical demand for adipose tissue, a suitable cell for reconstructive adipose tissue constructs is needed. In this study, we investigated the ability of Human Endometrial-derived stem cells (EnSCs) as a new source of mesenchymal stem cells to differentiate into adipocytes. EnSCs are the abundant and easy available source with no immunological response, for cell replacement therapy.
Methods
Single-cell suspensions of EnSCs were obtained from endometrial tissues from 10 women experiencing normal menstrual cycles, and were cultured at clonal density (10 cells/cm 2) or limiting dilution. Endometrial mesenchymal stem cell markers were examined flow cytometry. These cells were treated with adipogenic-inducing medium for 28 days. The adipogenic differentiation of the EnSC was assessed by cellular morphology and further confirmed by Oil Red O staining and RT-PCR. The BM-MSC differentiated into adipocytes in the presence of adipogenic stimuli for 3 weeks.
Results
The flow cytometric analysis showed that the cells were positive for CD90, CD105, CD146 and were negative for CD31, CD34.We showed that the key adipocytes marker PPARa was expressed in mRNA level after 28 days post treatment (PT).
Conclusion
According to our finding, it can be concluded that EnSCs represent a useful in vitro model for human adipogenesis, and provide opportunities to study the stages prior to commitment to the adipocyte lineage.
Keywords: Adipocyte cell, Differentiation, Endometrial stem cell
Introduction
Large numbers of plastic and reconstructive surgical procedures are performed every year to repair soft tissue defects that result from deep burns, tumor resections and hereditary and congenital defects such as Romberg's disease and Poland syndrome (1). Despite the increasing clinical demand, the optimal strategy for the reconstruction of soft tissue defects remains a challenge in plastic and reconstructive surgery (2, 3). Cell replacement therapy is a promising strategy to cure such diseases. Stem cells are undifferentiated cells without mature tissue specific characteristics. They have the capacity to proliferate indefinitely (self renewal) or giving rise to tissue specific committed progenitors or differentiated cells (4, 5).
Recently, a number of attempts have been made in vitro and in vivo to differentiate adipose tissue using mesenchymal stem cells (6−11). The capacity of stem cells to differentiate into endothelial cells and adipocytes upon receiving proper stimuli may be promising for developing vascularised fat graft for reconstructive purposes (12).
Various stem cells such as mesenchymal stem cells/marrow stromal stem cells (MSC), hematopoietic stem cells (HSC), multipotent adult progenitor stem cells (MAPCs), umbilical cord blood stem cells (UCBSC), and embryonic stem cells (ES) have the potency to differentiate into the adipocyte cells (13−17). The human endometrium is a dynamic tissue, which undergoes cycles of growth and regression with each menstrual cycle. Endometrial regeneration also follows parturition and extensive resection and occurs in postmenpausal women taking estrogen replacement therapy. It is likely that adult stem/progenitor cells are responsible for this remarkable regenerative capacity (18−21).
It has been demonstrated that human endometrium contains a low number of EnSCs which seem to belong to the family of the mesenchymal stem cells. These cells are engaged in the monthly restructuring and remodeling of human endometrium (21−23).
Human endometrium is structurally and functionally divided into two major regions. The functionalis, comprising the upper two thirds contains glands and basalis containing the basal region of the glands. The functionalis is shed by each menses but basalis stable and used for generating the new functionalis each month (21). The human endometrium is a dynamic remodeling tissue undergoing more than 400 cycles of regeneration, differentiation and shedding during a woman's reproductive years (22). Each month 4–10 mm of mucosal tissue grows within 4–10 days in the proliferative stage of the menstrual cycle under the influence of increasing circulating estrogen levels. It has been hypothesized that adult stem or progenitor cells are responsible for the cyclic regeneration of the endometrial functionalis each month. These adult stem cells reside in the basalis, and are present in the atrophic endometrium of postmenopausal women (23).
Since endometrial stromal cells are easy to isolate, expand rapidly from patients without leading to major ethical and technical problems, and produce a higher overall clonogenicity, they have a unique potential as therapeutic agents as autologous graft (1, 18). Therefore, endometrium may be an alternative source of MSC-like cells for tissue engineering purposes, obtainable with no extra morbidity than that required for other sources of stem cells (22, 23). In the our previous study, we have shown EnSCs can differentiate to neural and adipocyte cells and we used oilred O staining for illustration of adipocyte differentiation (24). In this study we assayed PPARa specific marker for adipocyte with RT-PCR. The major aim of the present study was to obtaine growth curve and doubeling time for EnSCs, then to investigate the ability of EnSCs to differentiate in vitro toward adipocyte in the presence of adipogenic-promoting media. The adipogenic differentiation was demonstrated by cellular morphology, Oil Red O staining and RT-PCR for PPARa.
Methods
Isolation and cloning of human EnSCs
This study was down in cell culture laboratory, Department of Tissue Engineering, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences in early 2011.
Human endometrial tissues were obtained from Tehran reproductive aged women referred to the Imam Khomeini hospital for infertility treatment. A written informed consent form (According to instruction of Tehran University of Medical Sciences Research assistant) describing the procedures and aims of the study was obtained from each donor in compliance with regulations concerning the use of human tissues. Endometrial samples were obtained from the fundal region of the uterine cavity using an endometrial sampling device. The biopsy tissue was washed in Dulbecco's phosphate buffered saline (DPBS), minced and digested in Hank's balanced salt solution (HBSS) (Gibco, USA) containing 4-(2 hydroxyethyl)-1 piperazineethanesulfonic acid (HEPES) (25 mM), collagenase A (1 mg/ml, Gibco, USA) for 30–45 min at 37 °C with agitation. Resultant dispersed cell solutions were then passed through 70, 40 µm sieves (BD Biosciences, USA) to remove glandular epithelial components. The cells were then centrifuged and mononuclear cells were separated by Ficoll (Gibco, USA) and washed in PBS. The isolated cells were cultured in DMEM/ F12 medium (Gibco, USA) containing 10% FBS, 1% antibiotic penicillin/streptomycin (Gibco, USA) and 1% Glutamine (Gibco, USA) and then incubated at 37 °C in 5% CO2 (18, 24).
Imunophenotyping of EnSCs
To detect surface antigens, cells were characterized by flow cytometry after passage three. First, cells were washed with HBSS + 2% BSA twice and incubated with the specific antibody conjugated with fluorescein isothiocyanate (FITC) or phyco erythrin (all from Santa Cruz) at concentrations recommended by the respective manufacturers. Cells were incubated for 20 min and analyzed by flow cytometry (Partec, Germany). The antibodies used were: SH2 (CD105, endoglin), CD90 (Thy-1) (mesenchymal markers), CD146 (endometrial stem cell marker), CD34 (hematopoietic marker), CD31 (endothelial marker), and FITC conjugated mouse IgG1, PE-conjugated mouse IgG1 were used for negative control.
Population doubling numbers (PDN) and time (PDT) and growth curve
The number of population doublings (PDN) and the time required by cells for each population doubling (PDT) were calculated by hemocytometer counts for each passage according to the following formulae and growth curve was obtained after 7 days:
PDN = log (N1/N0)x 3.31
PDT = CT/PDN
Where N1 is the cell number at the end of cultivation period, N0 is the cell number at culture initiation, and CT is the cell culture time.
Adipogenic differentiation
Cells derived from whole isolates of endometrium were expanded and passaged in DMEM with 10% FBS. Adipogenic differentiation was induced in the third passage cells by plating the EnSCs at 2×104 cells per cm2, allowing the cells to reach confluence and then incubating for a further 48 hr. The media was then changed to DMEM supplemented with 10% FBS as described above and the following hormones were added: insulin (10 g/ml), dexamethasone (1 M), indomethacin (200 M) and isobutylmethylxanthine (0.5 mM) all from Sigma, USA (18). Media were changed every 4 days and differentiation medium every 3–4 days for 28 days.
Morphological observation
Cells were observed under a phase-contrast microscope to evaluate their overall appearance. Microphotographs were taken with 10x objective (TS-100 Nikon, Japan).
Oil Red O Staining
Oil Red O Stain was used to confirm the presence of lipid in differentiaed cells. Cells were washed with PBS, fixed in 2% paraformaldehyde, 0.2% gluteraldehyde in PBS for 15 min and then rinsed with PBS. Then they were stained with Oil Red O (reconstituted in iso-propanol) (Sigma, USA) for 10 min and rinsed in 60% isopropanol followed by PBS (10). Lipid droplets were visualized in red under light microscopy.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
RT-PCR analysis was done to monitor the expression of activation of peroxisome proliferator activated receptor- PPAR during the programming of EnSCs into adipocytes cell lineage. First, whole total RNA was extracted from differentiated cells 28 post treatment by TRIZOL Reagent (Invitrogen, USA) according to the manufacturers instructions. Subsequently, 5 g of total RNA was transcribed into cDNA by using Moloney-murine leukemia virus (MMLV) Superscript II reverse transcriptase (Promega) and random hexamer primers. The specific oligonucleotides for RT-PCR are listed in Table 1. Each RT-PCR analysis was done on three independent samples.
Table 1.
Primers used in RT-PCR for adipocyte markers
| Gene | Sequence | Annealing temperature (°C) | Length (bp) | Gene bank code |
|---|---|---|---|---|
| PPARα | ||||
| F 5’–CCGCCTCCTTCGGCGTTC–3’ | 58 | 149 | NM_001001928.2 | |
| R 5’–AGCTCCAAGCTACTGTGGTGACA–3’ | ||||
| β- actin | ||||
| F 5’–CGTGACATTAAGGAGAAG–3’ | 56 | 202 | NM_001101 | |
| R 5’–TGATGGAGTTGAAGGTAF–3’ | ||||
Results
Characterization of isolated human EnSCs
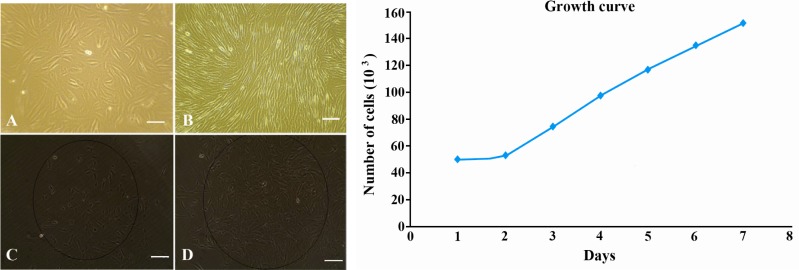
Human EnSCs could be isolated easily by their adherence to plastic flask. After plating for 24 hr, some adherent MSCs appeared in flask, which was heterogeneous in appearance. About 10 days later, these cells developed to many clusters, and could be used for subculture. These cells are relatively elongated or spindle-shaped cells (Figure 1A and 1B). In order to determine a clonal population of cells, we derived cell lines by single-cell plating in 24 well plates, which revealed clonogenic potential (Figure 1C and 1D). The cells grew at a doubling time of approximately one doubling every 49.9 hr based on quantification of cell number using microscope counting and growth curve obtained after 7 days (Figure 1).
Figure 1.
Morphology of Cultured EnSCs; A: Morphology of freshly isolated EnS cells; B: Fibroblast-like morphology of EnSCs after 2 weeks cell culture; C: Clonal population of EnSCs after plating in 24 well plate 1 week after cloning; D: The same population 2 weeks after cloning. Growth curve for EnSCs after 7 days cell culture (×100 magnification)
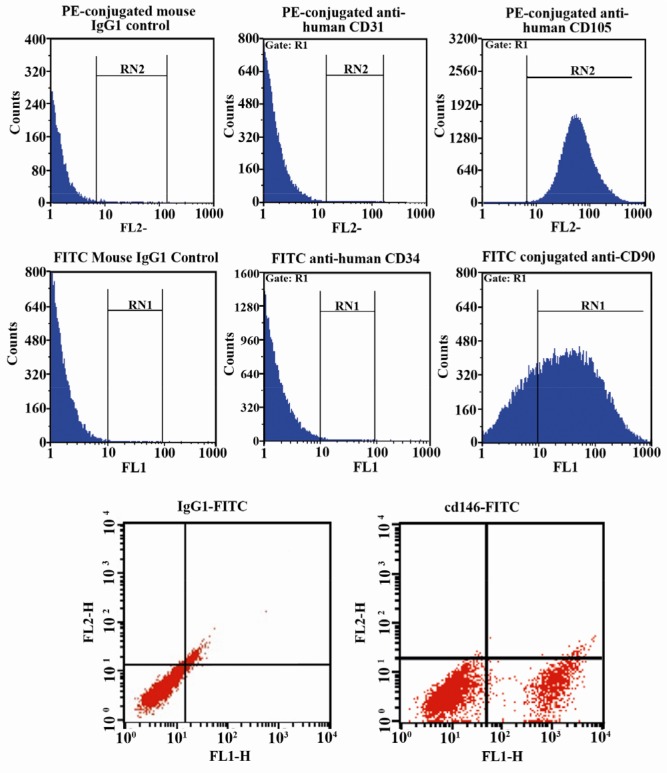
The immunophenotype was based on the flow cytometry analysis of a subset of mesenchymal stem cell markers (CD146, CD90 and CD105), hematopoietic marker (CD34) and endothelial marker (CD31). The flow cytometric analysis showed that isolated cells were positive for CD146, CD90, CD105 and were negative for CD31, CD34 (Figure 2).
Figure 2.
Flow cytometric analysis of isolated EnSCs for mesenchymal stem cell markers (CD90, CD105 and CD146), hematopoietic marker (CD34), endothelial marker (CD31). As shown in figure 2 the isolated cells are positive for CD90, CD105 and CD146 and are negative for CD31, CD34
Analysis of adipocytes differentiation
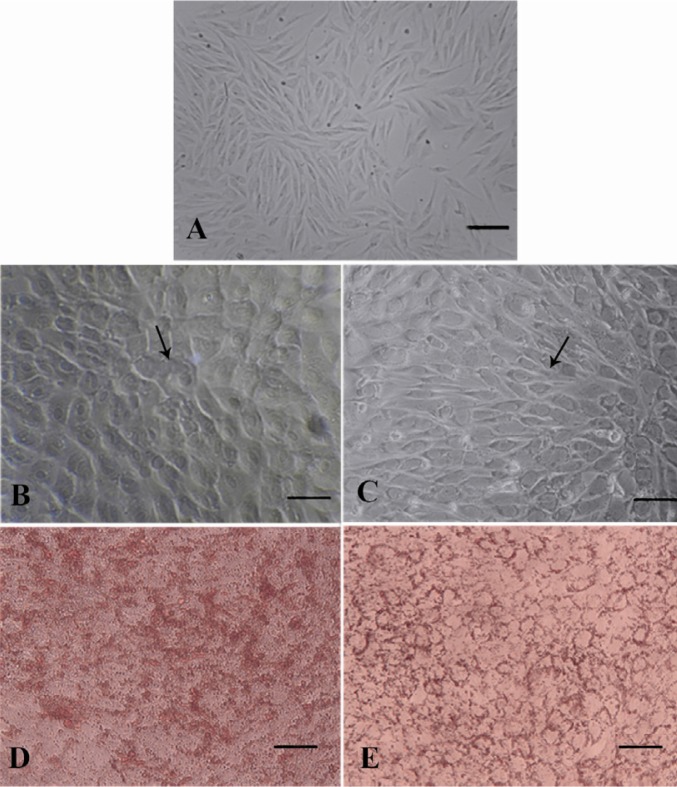
After only 12 days of adipogenic induction, small lipid droplets (arrows in Figure 3) were observed within EnSCs treated with differentiation-promoting medium (Figure 3B) whereas there was no lipid droplet in non-treating group at day 12 (Figure 3A). At day 21, in the presence of differentiation medium the size of the lipid droplets increased to occupy most of the cytoplasm, consistent with differentiation of EnSC into adipocytes (Figure 3C). Adipogenic differentiation was further confirmed by Oil Red O staining at the end of the experiment (28 days). Lipid droplets in differentiating EnSC were positively stained with Oil Red O in the presence of differentiation medium (Figure 3D and 3E). To investigate the expression of adipocyte marker in the level of mRNA, RT-PCR was carried out (Figure 4). The PPAR gene as a specific marker of adipocyte was expressed in the level of mRNA in 28 days PT.
Figure 3.
EnSCs differentiate into adipocytes. EnSCs before (A) and after differentiation into adipocytes at 12 days PT (B) and at 21 days PT (C), as demonstrated by light microscopy (A,B,C), Oil Red O staining to demonstrate lipid accumulation at 28 days PT (D,E). Arrows in B and C show adipocyte cells (×100 magnification)
Figure 4.
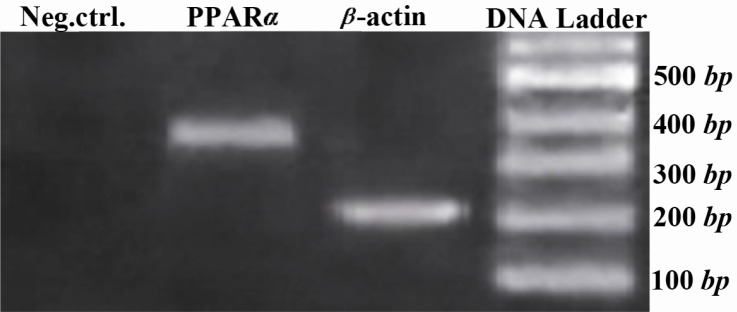
Adipocyte-related gene expression analysis of EnSCs 28 days PT using RT-PCR. β-actin was used as internal standard
Discussion
The EnSCs are new source of mesenchymal stem cells (21–25). In the present study, we isolated, characterized and differentiated EnSCs to adipocyte. Our findings showed that EnSCs expressed CD146, CD105 and CD90. In our previous study, we didn't survey CD146 (24), but in this study we have shown that EnSCs are positive for CD146 in agreement with Schwab et al. and Gargget et al. works (20, 23). We obtained growth curve and PDT = 49.9 for these cells. The result showed that the EnSCs in presence of adipogenic-inducing medium obtained an adipocyte fate 28 days PT. The EnSCs-derived adipocyte cells could express adipocyte marker such as PPAR, associating with remarkable morphological modifications. These data support the possibility of wider applications of EnSCs in cell therapy of soft tissue defects that result from deep burns and tumor resections and congenital defects. Previous studies concerning long-term follow up of animals treated with endometrial regenerative cells, and the karyotypic normality of these cells after extended passage (68 doublings) confirmed lack of tumorigenicity (26−28).
Endometrial MSC were recently isolated from human endometrium by their coexpression of two perivascular cell markers, CD146 and PDGF-receptor-β (PDGF-Rβ) (20–23). The CD146+ PDGF-Rβ+ cells underwent multilineage differentiation into adipogenic, myogenic, chondrogenic and osteoblastic lineages when cultured in appropriate induction media (20, 23). We found that single, freshly isolated endometrial stem cells self-renewal, have high proliferative potential, and undergo adipogenic differentiation media can differentiate into adipocyte cells in vitro, suggesting that they are similar to bone marrow MSCs. This suggests that they are responsible for monthly endometrial tissue regeneration, preparing the endometrium for steroid hormone-initiated differentiation into a receptive environment for embryo implantation. Both epithelial progenitor cell and MSC-like populations were identified. The entire endometrial functionalis layer, which is shed each month during menstruation, is likely replenished from these endometrial stem cells, supposed to reside in the basalis (23). Endometrial stem cells demonstrated substantial proliferative capacity (49.9 PDs), greater than most human bone mar-row, dental pulp, and adipose CFU-F (20 PDs) and fetal muscle cells (40 PDs) (29–32).
EnSCs should also contribute to the development of novel regenerative therapies for reconstruction of soft tissue defects after tumor resections, extensive deep burns and lipodystrophy. Adipose tissue engineering strategies have commonly involved the use of seeding preadipocytes on appropriate polymeric scaffolds. Recently, a number of attempts have been made in vitro and in vivo to engineer adipose tissue using mesenchymal stem cells (33–35). Vashi et al. 2008, show that bone marrow mesenchymal stem cells (BM-MSC) can use for adipose tissue engineering. They used pluronic F12 hydrogel in vitro for differentiation of BM-MSCs to adipocytes (36). We purpose that EnSCs may apply in scaffolds for tissue engineering. In our study, the data clearly demonstrated EnSCs can be differentiated into adipocytes phenotype in vitro. We have shown that after 12 days of induction, small lipid droplets appeared within EnSCs treated with differentiation medium, and the size of the lipid droplets increased at 21 days PT. Besides the morphological evidence, we have also demonstrated that the adipocyte-like phenoltypes derived from EnSCs express PPAR in mRNA level in 28 PT.
It may be concluded that the EnSCs in the plastic and reconstructive surgical procedures for repairing soft tissues defects are more convenient than other sources of stem cells due to the following properties. First, obtaining bone marrow stem cells in the clinic is invasive, because of the requirement for anesthesia whereas EnSCs can be obtained by a simple, safe and painless procedure such as Pop smears, in contrast to bone marrow aspiration. Second, EnSCs produce a higher overall clonogenicity of 1.25% in comparison to the clonogenic activity of stromal cells in bone marrow. Third, bone marrow MSCs are not perfect seeding cells for the elderly patients since these cells lose their differentiation capacity significantly with increased donor age. Fourth, karyotypic normality of the endometrial stromal cells after extended passage (68 doublings) demonstrated lack of tumorigenicity (37, 38).
Conclusion
Adult human endometrium contains rare epithetlial progenitors and MSCs, likely responsible for its immense regenerative capacity, which may provide a readily available source of MSCs for cell-based therapies. We speculate that endometrial adult stem cells can differentiate into adipocytes cell when they are exposed to adipogenic induction media. The EnSCs are attractive alternative candidate for repairing soft tissue defects, because they exhibit several important and potential advantages over other stem cells and EnSCs have provided potential alternative cells for adipose tissue engineering. The underlying mechanisms of these differences are unclear and further studies are needed to determine whether this may be of importance in further understanding of determinants of cell fate within the adipocyte lineage.
Acknowledgement
We thank Tehran University of Medical Sciences Research assistant for supported this work with grant number (No. 89-02-87-9704), and Tehran University of Medical Sciences and Research Center for Science and Technology in Medicine and Iranian Council of Stem Cell Technology.
To cite this article: Ai J, Shahverdi AR, Ebrahimi Barough S, Mohseni Kouchesfehani H, Heidari S, Roozafzoon R, et al. Derivation of Adipocytes from Human Endometrial Stem Cells (EnSCs). J Reprod Infertil. 2012;13(3):151-157.
Conflict of Interest
Authors declare no conflict of interest.
References
- 1.Langstein HN, Robb GL. Reconstructive approaches in soft tissue sarcoma. Semin Surg Oncol. 1999;17(1):52–65. doi: 10.1002/(sici)1098-2388(199907/08)17:1<52::aid-ssu7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Hedrick MH, Daniels EJ. The use of adult stem cells in regenerative medicine. Clin Plast Surg. 2003;30(4):499–505. doi: 10.1016/s0094-1298(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 3.Vashi AV, Keramidaris E, Abberton KM, Morrison WA, Wilson JL, O'Connor AJ, et al. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro. Biomaterials. 2008;29(5):573–9. doi: 10.1016/j.biomaterials.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Conrad C, Huss R. Adult stem cell lines in regenerative medicine and reconstructive surgery. J Surg Res. 2005;124(2):201–8. doi: 10.1016/j.jss.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Pelled G, G T, Aslan H, Gazit Z, Gazit D. Mesenchymal stem cells for bone gene therapy and tissue engineering. Curr Pharm Des. 2002;8(21):1917–28. doi: 10.2174/1381612023393666. [DOI] [PubMed] [Google Scholar]
- 6.Alhadlaq A, Tang M, Mao JJ. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: implications in soft tissue augmentation and reconstruction. Tissue Eng. 2005;11(3-4):556–66. doi: 10.1089/ten.2005.11.556. [DOI] [PubMed] [Google Scholar]
- 7.Choi YS, Park SN, Suh H. Adipose tissue engineering using mesenchymal stem cells attached to injectable PLGA spheres. Biomaterials. 2005;26(29):5855–63. doi: 10.1016/j.biomaterials.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Neubauer M, Hacker M, Bauer-Kreisel P, Weiser B, Fischbach C, Schulz MB, et al. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng. 2005;11(11-12):1840–51. doi: 10.1089/ten.2005.11.1840. [DOI] [PubMed] [Google Scholar]
- 9.Hong L, Peptan I, Clark P, Mao JJ. Ex vivo adipose tissue engineering by human marrow stromal cell seeded gelatin sponge. Ann Biomed Eng. 2005;33(4):511–7. doi: 10.1007/s10439-005-2510-7. [DOI] [PubMed] [Google Scholar]
- 10.Morganstein DL, Wu P, Mane MR, Fisk NM, White R, Parker MG. Human fetal mesenchymal stem cells differentiate into brown and white adipocytes: a role for ERRalpha in human UCP1 expression. Cell Res. 2010;20(4):434–44. doi: 10.1038/cr.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 12.Gomillion CT, Burg KJ. Stem cells and adipose tissue engineering. Biomaterials. 2006;27(36):6052–63. doi: 10.1016/j.biomaterials.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–62. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 14.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116(5):639–48. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 15.Dani C, Smith AG, Dessolin S, Leroy P, Staccini L, Villageois P, et al. Differentiation of embryonic stem cells into adipocytes in vitro. J Cell Sci. 1997;110(Pt 11):1279–85. doi: 10.1242/jcs.110.11.1279. [DOI] [PubMed] [Google Scholar]
- 16.Xiong C, Xie CQ, Zhang L, Zhang J, Xu K, Fu M, et al. Derivation of adipocytes from human embryonic stem cells. Stem Cells Dev. 2005;14(6):671–5. doi: 10.1089/scd.2005.14.671. [DOI] [PubMed] [Google Scholar]
- 17.De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89(2-3):267–70. doi: 10.1016/s0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 18.Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70(6):1738–50. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 19.Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 2008;17(3):303–11. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- 20.Schwab KE, Hutchinson P, Gargett CE. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23(4):934–43. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- 21.Gargett CE, Chan RW, Schwab KE. Endometrial stem cells. Curr Opin Obstet Gynecol. 2007;19(4):377–83. doi: 10.1097/GCO.0b013e328235a5c6. [DOI] [PubMed] [Google Scholar]
- 22.Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136–45. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taherian MZ, Ai J, Ebrahimi Barough S, Yazdani BF, Rezayat Sorkhabadi SM, Vasei M, et al. Human endometrial stem cells as a new source for programming to neural cells. Cell Biol Int Rep. 2012;19(1):7–14. doi: 10.1042/CBR20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimitrov R, Timeva T, Kyurkchiev D, Stamenova M, Shterev A, Kostova P, et al. Characterization of clonogenic stromal cells isolated from human endometrium. Reproduction. 2008;135(4):551–8. doi: 10.1530/REP-07-0428. [DOI] [PubMed] [Google Scholar]
- 26.Matthai C, Horvat R, Noe M, Nagele F, Radjabi A, van Trotsenburg M, et al. Oct-4 expression in human endometrium. Mol Hum Reprod. 2006;12(1):7–10. doi: 10.1093/molehr/gah254. [DOI] [PubMed] [Google Scholar]
- 27.Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13(1):87–101. doi: 10.1093/humupd/dml045. [DOI] [PubMed] [Google Scholar]
- 28.Bühring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007;1106:262–71. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 29.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 30.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116(Pt 9):1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 32.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 33.Deslex S, Negrel R, Vannier C, Etienne J, Ailhaud G. Differentiation of human adipocyte precursors in a chemically defined serum-free medium. Int J Obes. 1987;11(1):19–27. [PubMed] [Google Scholar]
- 34.Björntorp P, Karlsson M, Pettersson P. Expansion of adipose tissue storage capacity at different ages in rats. Metabolism. 1982;31(4):366–73. doi: 10.1016/0026-0495(82)90112-3. [DOI] [PubMed] [Google Scholar]
- 35.Grégoire FM, Johnson PR, Greenwood MR. Comparison of the adipoconversion of preadipocytes derived from lean and obese Zucker rats in serum-free cultures. Int J Obes Relat Metab Disord. 1995;19(9):664–70. [PubMed] [Google Scholar]
- 36.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22(11):2903–11. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 37.Khazaei M, Esfandiari N, Gotlieb L, Casper RF. Angiogenesis following three-dimensional culture of isolated human endometrial stromal cells. Int Fertil Steril. 2004;82(Suppl 2):S61–2. [Google Scholar]
- 38.Ai J, Mehrabani D. Are endometrial stem cells novel tools against ischemic heart failure in women? A hypothesis. Iran Red Crescent Med J. 2010;12(1):73–5. [Google Scholar]