Abstract
Background
Chlamydia trachomatis is the most reported bacterial sexually transmitted disease, especially among young women worldwide. The aim of this study was comparison the prevalence of Chlamydia trachomatis infection in woman with tubal infertility by means of PCR and cell culture techniques.
Methods
Fifty-one women with confirmed TFI were enrolled in this study in (avicenna infertility Clinic) between January 2010 and January 2011. Cervical swab and cytobrush specimens were collected from each patient by gynecologists and sent to laboratory in transport media. Detection of Chlamydia trachomatis in samples was performed using PCR and bacteria culture in MacCoy cell line. The data were analyzed by Fisher's exact test and independent t-test. Statistical significance was established at a p-value <0.05.
Results
A significant relation was observed between increased the age of first intercourse and chlamydial infection. Six (11.7%) samples had positive PCR result, whereas cell culture results were positive in only 2 (3.9%) samples. A significant relation was also identified between the duration of infertility and infection (p < 0.05) by PCR versus cell culture method.
Conclusion
The results showed that PCR is a rapid method, compared to cell culture for detecting Chlamydial organism. It also became clear that the age at first intercourse is important to predict the likelihood of Chlamydia trachomatis.
Keywords: Cell culture, Chlamydia trachomatis, Cytobrush, PCR, Swab
Introduction
Chlamydia trachomatis (C. trachomatis) is an obligate intracellular human pathogen which is responsible for the most reported bacterial sexually transmitted disease worldwide. Genital chlamydial infection has been identified as a major public health and there is an estimated annual incidence of around 92 million cases of chlamydial infections in the world (1). Although, infection with this organism can be asymptomatic in up to 80% of women (2), it may give rise to urethral syndrome, salpingitis, pelvic inflammatory disease (PID), tubal factor infertility and chronic pel vic pain (3).
Infection of the female reproductive tract with C. trachomatis is one of the leading global causes of tubal factor infertility (4), and leading causes of female factor infertility.
In order to reduce the rate of PID and prevent development of reproductive sequelae, early diagnosis and treatment of Chlamydial infection can be of great importance (5). Since the prevalence of chlamydial diseases is on the rise, development of sensitive, specific, and rapid methods to diagnose this infections is highly favored.
Cell culture, cytological tests for the detection of cytoplasmic inclusion bodies, direct immunofluorescence (DFA), enzyme-linked immunosorbent assay (ELISA), DNA hybridization techniques and polymerase chain reaction (PCR) are several laboratory methods which are used for the diagnosis of C. trachomatis (6).
Studies indicate that PCR has a sensitivity of 97% to 100% and a specificity of 98% for detecting C. trachomatis. The sensitivity and specificity of cell culture are 85% and 100%, respectively (7, 8). Therefore, cell culture is the gold standard for the diagnosis of C. trachomatis.
In this study, we used a nucleic acid amplification method (PCR) to detect and evaluate the prevalence of C. trachomatis infection in woman with tubal infertility using cytobrush and cervical swab samples.
Methods
Patients and samples
We recruited 51 women with tubal factor infertility, confirmed by laparos-copy and hysterosalpingography, attending Avicenna Infertility clinic in Tehran, Iran between January 2010 and January 2011. Patients were 19–48 years old (mean 33.3±SD years) and their age at first intercourse was 23.5±7.2 ranging from (10–43 years).
Cytobrush and cervical swab samples were obtained from each participant by the gynecologists. Swab and cytobrush samples were placed in PBS and 2sp (sucrose-phosphate) transport media, respectively, and were sent to laboratory under suitable protective (4°C) condition. The demographic data and medical histories were collected by direct interviews with the patients.
DNA extraction and PCR
DNA was extracted from cervical swabs using DNA extraction kit (QIAGEN, Germany) according to the manufacturer's instructions. All the extracted DNA was stored at −20°C until analyzing by PCR. Primers for amplification of orf8 gene were:
Forward 5’-CTAGGCGTTTGTACTCCGTCA-3’ and reverse 5’-TCCTCAGGAGTTTATGCACT-3’. After setting up, PCR was performed. The amplification program consisted of a first cycle of a 5 min denaturation at 94°C, followed by 37 cycles, each lasting 40 s at 94°C, 45 s at 61.6°C, and 89 s, at 72°C, with a final extension for 5 min at 72°C.
The volume of each PCR tube was 50 µl containing 5 µl DNA, 25 mmol MgCl2, 25 mmol dN TP, 1 mmol forward and reverse primers and 1.5 unit Taq polymerase. The amplified PCR product (5 µl) was analysed by electrophoresis on 1.5% w/v agarose gel containing 0.5 µg/ml of ethidium bromide.
Culture on McCoy cell line
Cytobrush samples were used for isolation of C. trachomatis in cycloheximide treated McCoy cell monolayer, which was grown on cover slip cultures in 12–well plates using RPMI supplement with 10% fetal bovine serum (FBS), 2 mM L-glutamine and antibiotics. Plates were incubated at 37°C for 2–3 days till adequate growth of bacteria appeared.
After vigorous vortexing, 200 µl of each cytobrush specimen was inoculated on the cells. The plates were centrifuged for 1 hr (1500 g, 30°C) and after replacement of medium, they were incubated at 37°C for 48–72 hr the monolyers were fixed in methanol and inclusion bodies were detected by Giemsa staining.
Data analysis
The data were analyzed by SPSS version 16 software. Chi square (χ2), Fisher's exact test, and independent t-test, were used for significance analysis. A p-value smaller than 0.05 was regarded as statistically significant.
Results
Fifty-one infertile women who had failed to have children after one year of intercourse without contraception were enrolled in this study. Most of the participants belonged to the 30–35 year-old age group. About 67.4% of the aptients had a history of vaginal infection; 86.0% had primary infertility, and 64.7% were infertile for more that five years.
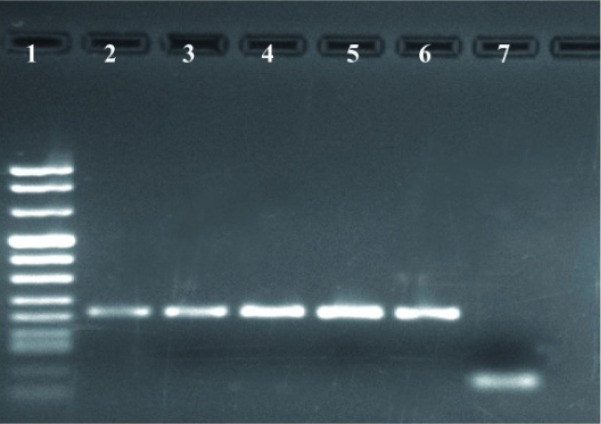
We amplified a fragment with a length of 200 bp during PCR and visualized it on 1.5% agarose gel (Figure 1). Cell cultures were performed in Mac Coy cell line (Figure 2).
Figure 1.
Detection of C. trachomatis from cervical swab specimens by PCR using orf8 primers. Lane 1, DNA size markers; Lane 2-5, positive patients for C. trachomatis; Lane 6, positive control; Lane 7, negative control
Figure 2.
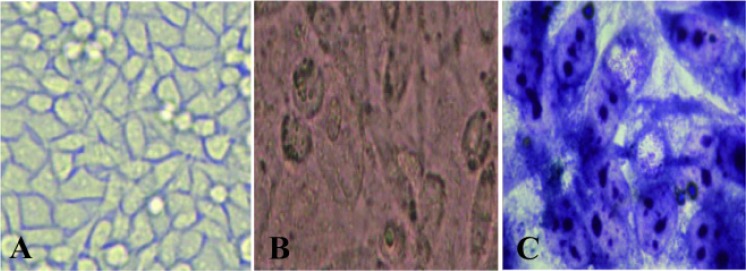
MacCoy cell line; A: before inoculation of specimen; B: after inoculation and C: after Gimsa staining
According to the result presented in Table 1, of 51 samples, 6 (11.7%) were positive by PCR and 2 (3.9%) by cell culture (Table 1).
Table 1.
PCR and cell culture results in TFI patients
| Cell culture | Total | |||
|---|---|---|---|---|
|
| ||||
| + | - | |||
| PCR | + | 2 (3.9%) | 4 (7.8%) | 6 (11.7%) |
| - | 0 (0.0%) | 45 (88.2%) | 45 (88.2%) | |
| Total | 2 (3.9%) | 49 (96%) | 51 (100%) | |
The mean age at first intercourse was 30.67±9.89 and 22.72±6.45 in positive and negative PCRs, respectively, (p < 0.05). Moreover, a significant relation was seen between the age at first intercourse and the risk of infection (p < 0.05). About 18.8% of patients with an infertility duration less than 5 years versus 9.1% of those with an infertility duration more than 5 years had positive PCR results (p < 0.05), while such a relation was not observed for cell culture.
T-test showed no significant relationship between the patients’ age and C. trachomatis infection, but the possibility of infection increased significantly with increased age of marriage (p < 0.05).
Infertility duration had a certain correlation with PCR results but it was not significant (p = 0.056) and showed no relationship with results obtained from cell culture.
Chi-squarea and Fisher's exact test were used to evaluate the relationship between symptoms of genital infections with PCR and culture results. None of the symptoms such as genital discharge, dysuria, genital itching or pelvic showed any significant association with the infection (Table 2).
Table 2.
Frequency of symptoms in women with tubal factor infertility
| Symptoms | n | (%) |
|---|---|---|
| Genital | 36 | 70.58 |
| Discharge | 18 | 35.29 |
| Genital itching | 22 | 43.13 |
| Genital | 12 | 23.52 |
| Irritation | 18 | 35.29 |
| Dysuria | -- | -- |
| Pelvic pain | -- | -- |
Discussion
Genital chlamydial infection, caused by the sexually transmitted bacterium C. trachomatis, is currently the most prevalent sexually transmitted infection throughout the world (1). This infection has been associated with a wide spectrum of complications.
C. trachomatis is among the most common microorganisms interfering with female fertility and is considered the most important cause of tubal obstruction and PID (9).
Therefore, screening and treatment of chlamydial infection in order to reduce its transmission and prevent PID and its long-term sequelae including infertility, chronic pain, PID and ectopic pregnancy are of great value.
About 10% to 20% of female infertility is associated with tubal factor infertility. Although C. trachomatis infection will effectively clear in most women, but infection persists in some and may ascend to the upper genital tract and increase the risk of tubal factor subfertility (10). In the current study, we evaluated women with tubal factor infertility for C. trachomatis infection by PCR and cell culture.
Based on several investigations, prevalence of C. trachomatis infection varies with the population under study and the type and sensitivity of the detection methods used. For example, the prevalence of chlamydial DNA in fresh tissue specimens from 14 women with tubal factor infertility was investigated in UK. C. trachomatis DNA was detected in 43% of these patients (11). The prevalence was 12% in our study, which is comparable with the results of other studies carried out in Iran, suggesting the lower incidence of this infection in our community.
Assessment of C. trachomatis infection in Iranian women with Cervicitis was done earlier by direct fluorescent antibody (DFA) and PCR techniques. About 15.5% of 142 samples were positive for Chlamydia based on PCR results, while DFA results showed a 14.1% positivity (12). These data are comparable with PCR results of the current study, although the values had been higher for cell culture. This shows that PCR and DFA methods are more sensitive than cell culture.
Of 110 Indian women with primary and secondary infertilities, C. trachomatis was detected in 25 (22.72%) cases by cell culture method (13), while we detected the organisms this way in only 4% of the cases. This difference could be due to different sample sizes and populations and perhaps better cell-culture techniques used in their study.
Endocervical swabs from 109 women attending gynecology and infertility clinics in Gaza were analyzed using PCR and enzyme immunoassay techniques. The results showed that overall prevalence rate of C. trachomatis to be 20.2% (14).
In another study, the rate of C. trachomatis infection evaluated by PCR was 27.2% and 18.9% in asymptomatic and symptomatic women, respectively (15).
Conclusion
In the present study we used PCR and cell culture techniques to detect C. trachomatis in cervical swabs and cytobrush specimens. Based on PCR results, the prevalence of C. trachomatis in the study population was 12%, while cell culture detected the organism in only 4% of the cases.
In agreement with several other studies, our results showed that PCR is more sensitive than cell culture in detecting chlamydial infections.
Although culture was earlier considered the gold standard, its sensitivity is as low as 75% to 85% even in expert laboratories and it takes 3 to 6 days to complete (16). But nucleic acid amplification methods, such as PCR, have high sensitivity and specificity and the results could be obtained within a short time. Furthermore in this method specimens will be stable during transport and can be subjected to delays between collection and processing without significant loss of sensitivity (17).
Since undetected and untreated chlamydial infections may lead to many reproductive sequelae, such as infertility, the results of the current study suggest that all infertile women should referred to infertility clinics and be screened for C. trachomatis in our community.
To cite this article: Hajikhani B, Motallebi T, Norouzi J, Bahador A, Bagheri R, Asgari S, et al. Classical and Molecular Methods for Evaluation of Chlamydia trachomatis Infection in Women with Tubal Factor Infertility. J Reprod Infertil. 2013;14(1):29–33.
Conflict of Interest
There is no conflict of interest for authors.
References
- 1.Beagley KW, Timms P. Chlamydia trachomatis infection: incidence, health costs and prospects for vaccine development. J Reprod Immunol. 2000;48(1):47–68. doi: 10.1016/s0165-0378(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 2.Patel AL, Sachdev D, Nagpal P, Chaudhry U, Sonkar SC, Mendiratta SL, et al. Prevalence of Chlamydia infection among women visiting a gynaecology outpatient department: evaluation of an in-house PCR assay for detection of Chlamydia trachomatis. Ann Clin Microbiol Antimicrob. 2010;9:24. doi: 10.1186/1476-0711-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morré SA, Rozendaal L, van Valkengoed IG, Boeke AJ, van Voorst Vader PC, Schirm J, et al. Urogenital Chlamydia trachomatis serovars in men and women with a symptomatic or asymptomatic infection: an association with clinical manifestations? J Clin Microbiol. 2000;38(6):2292–6. doi: 10.1128/jcm.38.6.2292-2296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cetin MT, Vardar MA, Aridogan N, Köksal F, Kiliç B, Burgut R. Role of Chlamydia trachomatis infections in infertility due to tubal factor. Indian J Med Res. 1992;95:139–43. [PubMed] [Google Scholar]
- 5.Murawski M, Matusiak M, Gryboś M. Chlamydia trachomatis as an etiological factor of marital infertility--is a routine diagnostics worth to perform? Wiad Lek. 2007;60(9-10):445–8. Polish. [PubMed] [Google Scholar]
- 6.Watson EJ, Templeton A, Russell I, Paavonen J, Mardh PA, Stary A, et al. The accuracy and efficacy of screening tests for Chlamydia trachomatis: a systematic review. J Med Microbiol. 2002;51(12):1021–31. doi: 10.1099/0022-1317-51-12-1021. [DOI] [PubMed] [Google Scholar]
- 7.Olafsson JH, Davídsson S, Karlsson SM, Pálsdóttir R, Steingrímsson O. Diagnosis of Chlamydia trachomatis infection in high-risk females with PCR on first void urine. Acta Derm Venereol. 1996;76(3):226–7. doi: 10.2340/0001555576226227. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox MH, Reynolds MT, Hoy CM, Brayson J. Combined cervical swab and urine specimens for PCR diagnosis of genital Chlamydia trachomatis infection. Sex Transm Infect. 2000;76(3):177–8. doi: 10.1136/sti.76.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkowska-Trojniel M, Zdrodowska-Stefanow B, Ostaszewska-Puchalska I, Zbucka M, Wołczyński S, Grygoruk C, et al. Chlamydia trachomatis urogenital infection in women with infertility. Adv Med Sci. 2009;54(1):82–5. doi: 10.2478/v10039-009-0007-6. [DOI] [PubMed] [Google Scholar]
- 10.den Hartog JE, Morré SA, Land JA. Chlamydia trachomatis-associated tubal factor subfertility: Immunogenetic aspects and serological screening. Hum Reprod Update. 2006;12(6):719–30. doi: 10.1093/humupd/dml030. [DOI] [PubMed] [Google Scholar]
- 11.Barlow RE, Cooke ID, Odukoya O, Heatley MK, Jenkins J, Narayansingh G, et al. The prevalence of Chlamydia trachomatis in fresh tissue specimens from patients with ectopic pregnancy or tubal factor infertility as determined by PCR and in-situ hybridization. J Med Microbiol. 2001;50(10):902–8. doi: 10.1099/0022-1317-50-10-902. [DOI] [PubMed] [Google Scholar]
- 12.Zaeimi Yazdi J, Khorramizadeh MR, Badami N, Kazemi B, Aminharati F, Eftekhar Z, et al. Comparative assessment of Chlamydia trachomatis infection in Iranian women with cervicitis: A cross-sectional study. Iran J Public Health. 2006;35(2):69–75. [Google Scholar]
- 13.Malik A, Jain S, Hakim S, Shukla I, Rizvi M. Chlamydia trachomatis infection & female infertility. Indian J Med Res. 2006;123(6):770–5. [PubMed] [Google Scholar]
- 14.El Qouqa IA, Shubair ME, Al Jarousha AM, Sharif FA. Prevalence of Chlamydia trachomatis among women attending gynecology and infertility clinics in Gaza, Palestine. Int J Infect Dis. 2009;13(3):334–41. doi: 10.1016/j.ijid.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Jenab Anahita, Golbang N, Golbang P, Chamani-Tabriz L, Roghanian R. Diagnostic Value of PCR and ELISA for Chlamydia trachomatis in a Group of Asymptomatic and Symptomatic Women in Isfahan, Iran. Inter J Fertil Steril. 2009;2(4):193–8. [Google Scholar]
- 16.Jaschek G, Gaydos CA, Welsh LE, Quinn TC. Direct detection of Chlamydia trachomatis in urine specimens from symptomatic and asymptomatic men by using a rapid polymerase chain reaction assay. J Clin Microbiol. 1993;31(5):1209–12. doi: 10.1128/jcm.31.5.1209-1212.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puolakkainen M, Hiltunen-Back E, Reunala T, Suhonen S, Lähteenmäki P, Lehtinen M, et al. Comparison of performances of two commercially available tests, a PCR assay and a ligase chain reaction test, in detection of urogenital Chlamydia trachomatis infection. J Clin Microbiol. 1998;36(6):1489–93. doi: 10.1128/jcm.36.6.1489-1493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]