Abstract
The broad spectrum kinase inhibitor sunitinib is a first-line therapy for advanced clear cell renal cell carcinoma (ccRCC), a deadly form of kidney cancer. Unfortunately, most patients develop sunitinib resistance and progressive disease after about 1 year of treatment. In this study, we evaluated the mechanisms of resistance to sunitinib to identify the potential tactics to overcome it. Xenograft models were generated that mimicked clinical resistance to sunitinib. Higher microvessel density was found in sunitinib-resistant tumors, indicating that an escape from antiangiogenesis occurred. Notably, escape coincided with increased secretion of interleukin-8 (IL-8) from tumors into the plasma, and coadministration of an IL-8 neutralizing antibody resensitized tumors to sunitinib treatment. In patients who were refractory to sunitinib treatment, IL-8 expression was elevated in ccRCC tumors, supporting the concept that IL-8 levels might predict clinical response to sunitinib. Our results reveal IL-8 as an important contributor to sunitinib resistance in ccRCC and a candidate therapeutic target to reverse acquired or intrinsic resistance to sunitinib in this malignancy.
Introduction
Sunitinib is currently considered the standard of care for first-line treatment of metastatic clear cell renal cell carcinoma (ccRCC), a disease which has traditionally had a very poor patient survival rate. Sunitinib is a small molecule inhibitor of multiple receptor tyrosine kinases (RTK), including vascular endothelial growth factor receptors (VEGFR-1, VEGFR-2, and VEGFR-3), platelet-derived growth factor receptors (PDGFR-α and PDGFR-β), FLT3, the stem cell growth factor receptor KIT, and RET (1). It may inhibit tumor angiogenesis through targeting of both VEGF and PDGF receptors; this antiangiogenic effect is believed to play a critical role in sunitinib activity against ccRCC (1). In terms of an antiangiogenic effect on ccRCC, the action of sunitinib against VEGFR has received particular attention (2). ccRCCs are highly vascularized tumors thought to be highly dependent on VEGF-mediated angiogenesis. In addition to sunitinib, a number of antiangiogenic therapies which target the VEGF pathway have shown efficacy in the treatment of ccRCC (3, 4). The importance of VEGF signaling for ccRCC growth is also supported by the high frequency of von Hippel-Lindau (VHL) gene mutations found in ccRCC. The VHL gene product regulates VEGF expression through suppression of the HIF transcription factor. Loss-of-function mutations in VHL lead to unregulated activation of HIF and overexpression of VEGF and other proangiogenic factors (5). Despite the efficacy of sunitinib in the treatment of ccRCC, the development of ccRCC resistance to sunitinib treatment is of major clinical concern. Studies have shown that roughly 40% of patients who receive sunitinib for treatment of advanced ccRCC show an initial positive response to treatment; however, the vast majority of these patients exhibit progressive disease after 1 year of treatment (4).
The aim of this study was to evaluate the mechanism of ccRCC resistance to sunitinib treatment and to identify potential targets to overcome sunitinib resistance. Our results implicate interleukin-8 (IL-8) as one of the contributors to sunitinib resistance in ccRCC.
Materials and Methods
Reagents
Sunitinib was provided by Pfizer Global Pharmaceuticals. The monoclonal IL-8 neutralizing antibody was purchased from R&D Systems (MAB208, clone 6217.111). The mouse IgG control was obtained from Innovative Research (IR-MS-GF). The polyclonal IL-8 antibody used for immunohistochemistry was obtained from Santa Cruz Biotechnology (sc-7922).
Cells and cell culture
A-498 and 786-O RCC cell lines were obtained from the American Type Culture Collection. SN12C cells were kindly provided by Dr. George Vande Woude (Van Andel Research Institute). The cells were maintained in DMEM or RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 100 IU/mL of penicillin, and 100 μg/mL of streptomycin (Invitrogen) in a humidified incubator containing 5% CO2 at 37°C.
Human ccRCC samples
Human ccRCC tumor sections used for IL-8 immunohistochemical staining were provided by Spectrum Health (Grand Rapids, MI) and Cleveland Clinic (Cleveland, OH). These samples were obtained with the approval from the Van Andel Research Institute Institutional Review Board in Grand Rapids, MI. Written informed consent from patients were also obtained.
Establishment of sunitinib-resistant xenograft models
All animal studies were in compliance with Van Andel Research Institute Institutional Animal Care and Use Committee policies. Six-week-old female BALB/c nu/nu nude mice (Charles River) were given s.c. injections of 3 × 106 A-498, 786-O, or SN12C cells in the right flank. Tumor size was measured twice or thrice per week using digital calipers (Mitutoyo) with an accuracy of ±0.02 mm, and tumor volume was calculated as length × width × height × 0.5. Tumor growth ratio was determined by dividing the tumor volume measured at an indicated time by the tumor volume at the start of sunitinib treatment. Tumor growth ratios for each treatment group are presented as mean ± SD. Sunitinib-resistant tumors were established in xenograft models using two dosing strategies. To directly mimic the treatment regimen for human ccRCC (4 wk on and 2 wk off), we treated A-498 and SN12C xenograft mice with an intermittent dosing schedule with modification (3–4 wk on and 3–4 wk off). For 786-O xenografts, a continuous dosing strategy was used in which sunitinib was given daily without a break. Xenograft tumors that either did not respond to treatment or that progressed on treatment after an initial response were considered to display phenotypic resistance. In detail, phenotypic sensitivity of each individual mouse to sunitinib treatment was defined by a long-term trend toward tumor stasis (tumor volume increase of <25%) or regression. In contrast, tumors that increased >25% of initial volume when treatment began, and which showed a long-term trend toward continued growth, were considered sunitinib-resistant. Due to the time needed for drug treatment to affect tumor volume, we waited until the last week of each round of drug treatment to determine sensitivity or resistance. At this time point, mice were classified as “sensitive” or “resistant” and plotted accordingly.
Sunitinib was administered by oral gavage as a citratebuffered (pH 3.5) solution once daily, at the dosages of 40 mg/kg (for A-498 and 786-O xenografts), or 80 mg/kg (for SN12C xenografts), respectively. At the same time, one vehicle control group received citrate buffer (pH 3.5) only. Treatment began when the average tumor volume reached 200 to 300 mm3. Plasma samples were collected before, during, and after the course of sunitinib treatment and stored at −80°C for further studies.
Twenty-four hours after the last treatment, tumors were removed, cleaned from adjacent tissues, fixed in 4% paraformaldehyde and paraffin-embedded, and then 4-μm-thick sections were prepared. Some sections were stained with H&E and the others were used for subsequent immunohistochemical analysis.
Sequencing of RTK genes
DNA extracted from the corresponding control, sensitive, and resistant xenograft tumors as well as the parental cell line (SN12C) were sent to Wellcome Trust Sanger Institute (UK) for RTK gene sequencing as described (6).
Microvessel density determination
For analysis of microvessel density (MVD), CD34 staining of tumors was performed and quantified as previously described (7).
Cytokine screening
The levels of 89 cytokines (human MAP service version 1.6, 89 antigens) in the plasma samples collected from resistant, sensitive, and control tumor–bearing mice were analyzed by antibody array analysis (Rules-Based Medicine).
IL-8 ELISA
The IL-8 level in plasma from xenograft mice was determined using an ELISA kit (D8000C, R&D Systems).
Neutralizing IL-8 antibody treatment
A-498 xenografts were treated with sunitinib on an intermittent dosing schedule until the emergence of phenotypic resistance. Neutralizing IL-8 antibody or control IgG was delivered by i.p. injection every other day for a total of seven times; 100 μg of antibody was given per mouse during each injection. 786-O xenografts were treated continuously with sunitinib until the emergence of phenotypic resistance. Neutralizing IL-8 antibody or control IgG was delivered by i.p. injection every other day for a total of eight times; 100 μg of antibody was given per mouse during each injection.
Immunohistochemistry for human IL-8
The immunohistochemical staining for IL-8 on human ccRCC sections was performed on an automatic stainer (Discovery XT, Ventana). Briefly, 5-μm tissue sections of a representative tumor block was antigen-retrieved in Tris/Borate/EDTA buffer (pH 8.0–8.5; Ventana, 950-124) at 95°C for 44 min. The sections were then incubated sequentially with a polyclonal rabbit anti–IL-8 antibody (sc-7922, Santa Cruz Biotechnology) at 1:50 dilution, secondary antibody, and chromogenic substrate (ChromoMap DAB, Ventana, 760-159). This antibody has also been tested in immunohistochemical staining by others (8, 9). The immunostaining was evaluated by a genitourinary pathologist (M. Zhou). The cytoplasmic and membranous staining was scored as 0 (negative), 1 (weakly positive), and 2 (strongly positive). In addition, the percentage of cells with each staining grade was also recorded. A final composite score was calculated by adding the products of each of the IL-8 intensities (0–3) and the percentage of cells displaying that respective staining intensity. For example, if 80% of the cells in a tumor had a staining intensity of 3 and 20% of the cells had a staining intensity of 2, then the IL-8 composite score was (80 × 3) + (20 × 2) = 280.
Statistical analysis
All values are expressed as mean ± SD unless otherwise specified. Values were compared using Student’s t test. P < 0.05 was considered significant.
Results
Reacquisition of angiogenesis and elevated plasma IL-8 levels are associated with the sunitinib-resistant phenotype in an intermittent dosing animal model
To establish a sunitinib-resistant ccRCC xenograft model, we first used a 3/4-weeks-on and 3/4-weeks-off dosing strategy, which mimicked the clinical regimen (4-weeks-on and 2-weeks-off) given to patients with metastatic ccRCC with modifications (10, 11). Based on previous efficacy studies, we used the minimal dosage of sunitinib which could cause stasis effects in ccRCC xenografts (Supplemental Fig. S1). A-498 ccRCC xenografts were treated with 40 mg/kg of sunitinib under a 3-weeks-on and 4-weeks-off regimen, and SN12C ccRCC xenografts were treated with 80 mg/kg of sunitinib under a 4-weeks-on and 4-weeks-off regimen. Notably, A-498 cells contained a loss-of-function mutation of VHL as occurs in a majority of human ccRCC cases, whereas SN12C cells contained wild-type VHL. Phenotypic sensitivity of each individual mouse to sunitinib treatment was defined by a longterm trend toward tumor stasis (tumor volume increase of <25%) or regression. In contrast, tumors that increased by >25% of the initial volume when treatment began, and which showed a long-term trend toward continued growth, were considered sunitinib-resistant. All of the A-498 xenograft mice (11 total) responded during the first round of sunitinib treatment and 4 mice developed resistance during the second round (Fig. 1A). For SN12C xenografts, 2 mice (out of 10 total) showed phenotypic resistance during the first round of sunitinib treatment and 2 more mice showed resistance during the second round (Fig. 1B). In all, 36% (4 of 11) of treated A-498 xenograft mice and 40% (4 of 10) of treated SN12C xenograft mice showed phenotypic resistance; they either did not respond initially to sunitinib or progressed after an initial response during the second round of treatment. This pattern of response directly mimics the clinical response to sunitinib treatment in patients with ccRCC (3, 4, 10, 11).
Figure 1.
Phenotypic resistance of ccRCC xenografts treated with sunitinib under an intermittent dosing regimen. A, A-498 ccRCC xenografts were treated with 40 mg/kg of sunitinib with a 3-wk-on and 4-wk-off dosing strategy, which mimicked the clinical regimen given to patients (4-wk-on and 2-wk-off) with modification. All A-498 xenograft mice (11 total) responded during the first round of sunitinib treatment and 4 mice developed resistance during the second round of sunitinib treatment (see text for definition of phenotypic resistance). Tumor growth ratio was determined by dividing the tumor volume measured at an indicated time by tumor volume at the start of sunitinib treatment. Tumor growth ratios for each group are presented as mean ± SD (*, P < 0.05). B, SN12C ccRCC xenografts were treated with 80 mg/kg of sunitinib with a 4-wk-on and 4-wk-off dosing strategy. Two mice (out of 10 total) showed phenotypic resistance during the first round of sunitinib treatment and two more mice showed resistance during the second round of sunitinib treatment.
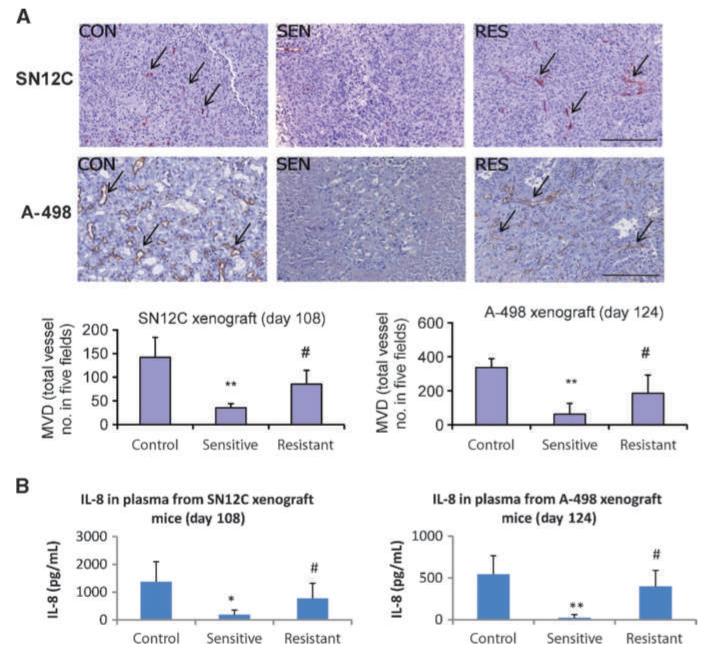
To explore the mechanism underlying this resistance, we first ruled out the possibility of target mutations by sequencing a panel of RTK genes. No mutations in the following RTKs: FLT1 (VEGFR-1), KDR (VEGFR-2), FLT4 (VEGFR-3), PDGFR-α, PDGFR-β, FLT3, c-KIT, or RET, were identified in the resistant, sensitive, or control SN12C xenograft tumors or in the parental cell line. Microarray gene expression analysis also found that the expression levels of these RTKs and their ligands were unchanged in resistant, sensitive, or control SN12C and A-498 xenograft tumors (Supplemental Fig. S2). Because our previous studies have shown that sunitinib exhibits its antitumor effect on ccRCC mainly through the suppression of VEGF/VEGFR-mediated tumor angiogenesis in vivo (12), we evaluated the features of the tumor vasculature both in the sensitive and resistant xenograft tumors by the end of sunitinib treatment. The MVD in the sunitinib-resistant SN12C and A-498 tumors was significantly higher than that found in the sensitive tumors (P < 0.05; Fig. 2A). These results are consistent with the notion that sunitinibinduced growth inhibition occurs mainly through effects on VEGFR-mediated vascularization. Moreover, our results indicate that sunitinib resistance is mediated through an escape from antiangiogenesis in which neovascularization is possibly reactivated through a VEGF/VEGFR-independent mechanism.
Figure 2.
Escape from antiangiogenesis and elevated plasma levels of IL-8 were found in sunitinib-resistant mice treated under an intermittent dosing regimen. A, increased MVD was found in sunitinib-resistant SN12C and A-498 xenograft tumors by the end of the treatment. Tumor sections from mice in Fig. 1 were stained for CD34, a vascular endothelial cell marker, and MVD was quantified using software as indicated in Materials and Methods. Arrows, blood vessels. Bar, 0.20 mm. B, reactivation of tumor angiogenesis was accompanied by a significant increase of IL-8 release in the plasma of resistant SN12C and A-498 xenograft mice, as analyzed by ELISA assay. The data points shown here represent the corresponding time points in Fig. 1. Bars, SD (*, P < 0.05 versus control; **, P < 0.01 versus control; #, P < 0.05 versus sensitive).
We hypothesized that VEGFR-independent vascularization of sunitinib-resistant tumors may be mediated by tumor up-regulation of angiogenic factors other than VEGF. To test this hypothesis, we screened the plasma from SN12C xenograft-bearing mice for changes in secreted angiogenic factors. Antibody arrays were used to screen mouse plasma for the expression of various human cytokines derived from the xenograft tumors. Among 89 factors screened, we found that the plasma levels of human IL-8, a potent proangiogenic chemokine (13–16), were higher in mice with sunitinib-resistant tumors compared with mice with sunitinib-sensitive tumors. In contrast, plasma levels of tumor-derived human VEGF were unchanged and expression of tumor-derived human basic fibroblast growth factor was undetectable (data not shown). The increased secretion of IL-8 from sunitinib-resistant tumors by the end of sunitinib treatment was confirmed by a more specific ELISA assay for both SN12C and A-498 xenografts (P < 0.05; Fig. 2B). We repeated the above experiment with A-498 xenografts and obtained consistent results; more importantly, we also showed that the increase of plasma IL-8 levels in sunitinib-resistant mice was independent of tumor size (Supplemental Fig. S3).
Reacquisition of angiogenesis and elevated plasma IL-8 level is associated with the sunitinib-resistant phenotype in a continuous dosing animal model
In our SN12C and A-498 intermittent dosing models, sunitinib resistance takes ~3 months to develop and is inefficient (only ~40% of tumors develop sunitinib resistance). To develop a more efficient model of sunitinib resistance, we turned to a continuous dosing strategy with a different cell line. We speculated that if IL-8 expression is functionally involved in the development of sunitinib resistance, ccRCC cell lines which express higher basal levels of IL-8 might more efficiently develop sunitinib resistance. Microarray gene expression profiling studies in our lab showed that the 786-O ccRCC cell line expresses high levels of IL-8 (data not shown). We thus decided to try generating sunitinib-resistant xenografts using 786-O under a continuous dosing regimen. Of note, 786-O cells also contained a loss-of-function mutation of VHL. 786-O xenografts were treated with 40 mg/kg of sunitinib continuously. Under this regimen, the majority of 786-O tumors (15 of 18, 83%) developed phenotypic resistance to sunitinib after 34 days of treatment (day 67; Fig. 3A). To verify that sunitinib resistance in the 786-O xenograft model was associated with elevated IL-8 levels, we examined the plasma IL-8 levels from 786-O tumor–bearing mice and normalized to tumor volume. The plasma levels of IL-8 were higher from mice with sunitinib-resistant tumors compared with mice with sunitinib-sensitive tumors independent of tumor size (P < 0.05; Fig. 3B).
Figure 3.
Phenotypic resistance of ccRCC xenografts and elevated plasma levels of IL-8 in sunitinib-resistant mice treated under a continuous dosing regimen. A, 786-O ccRCC xenograft tumors were treated with sunitinib at 40 mg/kg continuously for 34 d (days 33–67). Fifteen out of 18 mice (83%) developed resistance by day 67 (**, P < 0.01). B, plasma levels of human IL-8 were higher in resistant 786-O xenograft–bearing mice compared with sensitive mice. Plasma IL-8 levels were determined by ELISA and are normalized to tumor volume (*, P < 0.05 versus sensitive).
Neutralization of IL-8 activity resensitizes ccRCC tumors to sunitinib treatment
To test the hypothesis that IL-8 is functionally involved in the development of sunitinib resistance, we used a neutralizing antibody (R&D Systems, MAB208, clone 6217.111) to inhibit IL-8 function in xenograft models of sunitinib-resistant ccRCC. This mouse monoclonal anti–human IL-8 antibody has been previously shown to neutralize human IL-8 activity in a mouse xenograft model (14). A-498 tumor–bearing mice were given sunitinib on an intermittent dosing schedule until the development of sunitinib resistance. Mice that developed sunitinib resistance (the same animals as depicted in Supplemental Fig. S3A) were then randomly divided into three groups: one group received sunitinib plus control IgG (n = 4), the second group received IL-8 neutralizing antibody alone (n = 5), and the third group received sunitinib plus IL-8 neutralizing antibody (n = 4) treatment. Sensitive tumors were also treated with sunitinib alone as a control. Resistant tumors started to respond to sunitinib treatment again with the addition of the IL-8 neutralizing antibody (P < 0.05; Fig. 4A). We noted that treatment with IL-8 neutralizing antibody alone (in the absence of concurrent sunitinib treatment) did not reduce tumor growth. Only combination therapy with both IL-8 neutralizing antibody and sunitinib was effective in reducing tumor growth (P < 0.05; Fig. 4A and B). We conclude that inhibition of IL-8 function in sunitinib-resistant A-498 tumors resensitizes the tumors to sunitinib therapy, resulting in decreased tumor growth. To confirm that the effect of IL-8 antibody is through inhibition of tumor angiogenesis, we examined the MVD in sunitinib, sunitinib plus IL-8 antibody, and IL-8 antibody alone–treated tumors (tumor samples from Fig. 4A). We found that the combination of IL-8 neutralizing antibody plus sunitinib resulted in a trend of decreased tumor MVD compared with sunitinib treatment alone (Supplemental Fig. S4). We also noted a large increase of MVD in tumors treated with IL-8 antibody alone, indicating that concurrent sunitinib treatment is required for inhibition of the tumor vasculature.
Figure 4.
Neutralization of IL-8 activity resensitized ccRCC xenografts to sunitinib treatment under an intermittent dosing regimen. A, A-498 xenograft tumors were treated with 40 mg/kg of sunitinib daily with a 3-wk-on and 3-wk-off schedule. Mice that developed phenotypic resistance (the same animals as depicted in Supplemental Fig. S3A) were then randomly divided into three groups. One group of sunitinib-resistant mice was given sunitinib plus IL-8 neutralizing antibody (n = 4), one group was given sunitinib plus control IgG (n = 4), and the third group received IL-8 antibody alone (n = 5). IL-8 neutralizing antibody treatment started on day 98 and stopped on day 112. Tumor growth ratio was determined by dividing the tumor volume measured at an indicated time by tumor volume at the start of IL-8 antibody treatment (day 98) and presented as mean ± SD. Sunitinib plus IL-8 antibody treatment inhibited tumor growth compared with sunitinib treatment plus control IgG or IL-8 antibody treatment alone. B, bar graph of growth ratios of sunitinib-resistant tumors at 98 d (black columns, before IL-8 antibody treatment) and 112 d (white columns, after IL-8 antibody treatment). The means of tumor growth ratios for each treatment group are plotted. Bars, SD (*, P < 0.05; **, P < 0.01).
We next confirmed these findings in our 786-O xenograft model. 786-O xenografts were treated with sunitinib under a continuous dosing regimen until the development of sunitinib resistance. Mice that developed sunitinib resistance (same animals as depicted in Fig. 3A) were randomly divided into two groups: one group received sunitinib plus IL-8 neutralizing antibody (n = 7) and the other group received sunitinib plus control IgG (n = 8). The addition of IL-8 neutralizing antibody to sunitinib treatment resulted in reduced tumor growth (P < 0.01; Fig. 5A and B). As was seen with A-498 xenografts, treatment with IL-8 antibody by itself had no effect on the growth of sunitinib-resistant 786-O xenografts (Supplemental Fig. S5A). The neutralizing activity of the IL-8 antibody was confirmed by measurement of IL-8 plasma levels in antibody-treated mice (Fig. 5C; Supplemental Fig. S5B). We again conclude that inhibition of IL-8 function resensitized ccRCC tumors to sunitinib treatment, resulting in decreased tumor growth. Interestingly, we noted that inhibition of IL-8 activity did not inhibit the growth of sunitinib treatment–naïve ccRCC tumors (Supplemental Fig. S6). This indicates that treatment-naïve tumors do not rely on IL-8 signaling for growth. Our results suggest that ccRCC tumors rely primarily on VEGF/ VEGFR proangiogenic signaling, but that these tumors may switch to IL-8–dependent signaling in the face of a VEGFR blockade.
Figure 5.
Neutralization of IL-8 activity resensitized ccRCC xenografts to sunitinib treatment under a continuous dosing regimen. A, attenuation of sunitinib resistance in the 786-O xenograft model. Starting from day 68, the same sunitinib-resistant animals depicted in Fig. 3A were divided into two groups: one group received sunitinib plus IL-8 neutralizing antibody (SU + IL-8 Ab; n = 7), and the other group received sunitinib plus control IgG (SU + IgG; n = 8). Tumor growth ratio was determined by dividing the tumor volume measured at an indicated time by tumor volume at the start of IL-8 antibody treatment and presented as mean ± SD. B, bar graph of tumor growth ratios plotted before (day 67) and after (day 85) IL-8 antibody treatment. C, the neutralizing activity of IL-8 antibody was confirmed by the detection of plasma levels of IL-8 using ELISA. High plasma levels of IL-8 were detected in sunitinib-resistant mice (SU + IL8 Ab and SU + IgG) compared with sensitive mice (SU-sen) on day 68. By day 76 and day 85, mice treated with neutralizing IL-8 antibody showed reduced levels of plasma IL-8; in contrast, IL-8 plasma levels remained high in the “sunitinib + IgG” group (*, P < 0.05; **, P < 0.01).
IL-8 expression is increased in human ccRCC tumors that are refractory to sunitinib
In clinical practice, it has been observed that a significant number of human ccRCC patients never show positive response to sunitinib treatment; in other words, these patients exhibit intrinsic resistance to sunitinib therapy (4). We hypothesized that high tumor expression levels of IL-8 underlie such intrinsic resistance. To test this hypothesis, we examined IL-8 expression levels in ccRCC tumors from both sunitinib-responsive and sunitinib-nonresponsive patients. ccRCC tumors were resected from untreated ccRCC patients. These patients were subsequently treated with sunitinib and sunitinib response was evaluated by Response Evaluation Criteria In Solid Tumors guidelines. Tumors from patients who progressed while on sunitinib treatment (n = 9) had significantly higher IL-8 expression than tumors from patients that did not progress on sunitinib treatment (n = 11; Fig. 6). These results suggest that IL-8 expression may serve as a useful biomarker to predict clinical response of patients to sunitinib treatment.
Figure 6.
IL-8 expression is increased in human ccRCC tumors with intrinsic resistance to sunitinib treatment. ccRCC tumor samples were collected from patients prior to sunitinib treatment. Patient response to sunitinib treatment was evaluated by Response Evaluation Criteria in Solid Tumors guidelines. Nine patients showed no response to subsequent sunitinib treatment (intrinsic resistance), whereas 11 patients responded to sunitinib. IL-8 expression in ccRCC tumors was evaluated by immunohistochemistry. A, examples of immunohistochemical scoring for IL-8 expression. The cytoplasmic and membranous staining was scored as 0 (negative, left), 1 (weakly positive, middle), and 2 (strongly positive, right). In addition, the percentage of cells with each staining grade was recorded. B, representative IL-8 staining of tumor from a patient who was refractory to sunitinib treatment. Strong IL-8 expression is seen in the primary tumor. C, IL-8 staining from a patient who responded to sunitinib treatment. Only focally weak IL-8 expression is seen in the primary tumor (magnification, ×200). Table summarizes IL-8 staining scores from sunitinib-responsive and -nonresponsive patients.
Discussion
Sunitinib is currently the standard of care for the treatment of advanced ccRCC. However, the development of ccRCC resistance to sunitinib therapy is a major clinical problem. To study this problem, we have established ccRCC xenograft models which mimic the acquired sunitinib resistance seen in the clinical setting. We show that the development of sunitinib resistance was accompanied by evasion of sunitinib’s antiangiogenic effects and by increased expression of tumor-derived IL-8. Notably, three distinct ccRCC cell lines under different sunitinib treatment regimens all showed up-regulation of IL-8 expression upon development of sunitinib resistance. We further showed that inhibition of IL-8 function with a neutralizing antibody attenuated sunitinib resistance in ccRCC. Finally, we show that IL-8 expression was elevated in human ccRCC tumors with intrinsic resistance to sunitinib therapy, indicating that IL-8 levels may serve as a predictive biomarker for clinical response to sunitinib. In summary, our results indicate that IL-8 plays an important role in the resistance of ccRCC to sunitinib, and suggest that IL-8 may potentially serve as both a therapeutic target for the treatment of sunitinib-resistant ccRCC and as a clinical biomarker for both acquired and intrinsic sunitinib resistance.
IL-8 is a member of the CXC family of chemokines and is a potent proangiogenic factor (17, 18). Upregulation of IL-8 in sunitinib-resistant ccRCC may thus activate proangiogenic pathways that allow the tumor to evade the anti-angiogenic effects of sunitinib-mediated VEGFR blockade (Supplemental Fig. S7). Consistent with this idea, Mizukami and colleagues showed that in colon cancer cells, upregulation of IL-8 signaling was able to preserve tumor angiogenesis of xenografts in which VEGF expression had been downregulated (14). Thus, results from both our studies and others suggest that IL-8 angiogenic signaling may functionally compensate for the inhibition of VEGF/VEGFR-mediated angiogenesis.
The adoption of alternative angiogenic signaling pathways to compensate for inhibition of VEGF/VEGFR-mediated signaling may be a common mechanism for the development of cancer resistance to VEGF pathway inhibitors (1, 19–21). Casanovas and colleagues studied tumor evasion of VEGFR-targeted therapy in a mouse model of late-stage pancreatic islet cancer. In this model, acquired tumor resistance to VEGFR-directed antibody treatment was shown to be mediated by upregulation of the proangiogenic factor, basic fibroblast growth factor (22). Interestingly, we were unable to detect any upregulation of basic fibroblast growth factors in our sunitinib-resistant ccRCC models. We speculate that differences in the specific alternate proangiogenic pathways adopted after VEGF/VEGFR blockade may be due to differences in tumor type and the specific nature of the VEGF/VEGFR blockade.
Interestingly, we found that both VHL null (A-498 and 786-O) and VHL wild-type (SN12C) xenografts responded to sunitinib treatment and upregulated IL-8 expression upon the development of sunitinib resistance. The ability of VHL null ccRCC xenografts to respond to sunitinib therapy is consistent with clinical observations. In a retrospective analysis of patients with ccRCC, Choueiri and colleagues found that VHL mutation status had little effect on patient response to sunitinib therapy (23). Our results suggest that the role of IL-8 in sunitinib resistance in ccRCC may similarly hold true across VHL status categories.
We also noted that tumor secretion of IL-8 initially decreases upon sunitinib treatment compared with untreated ccRCC tumors (Figs. 2B and 3B), and that this occurs independent of tumor size (Fig. 3B). Sunitinib treatment has previously been shown to alter the expression of a range of cytokines (24). Previous work has indicated a link between VEGF signaling and IL-8 expression; treatment of cultured cells with VEGF has been shown to induce IL-8 expression (25). We speculate that sunitinib-mediated inhibition of VEGFR activity might thus initially suppress IL-8 expression through the inhibition of VEGF/IL-8 regulatory loops. In the face of a sustained VEGFR blockade, however, tumors may adapt and find a VEGFR-independent mechanism to upregulate IL-8 expression, thus circumventing the antiangiogenic effects of sunitinib.
We noted that inhibition of IL-8 signaling did not reduce the growth of sunitinib treatment–naïve ccRCC (Supplemental Fig. S6). Only after the development of sunitinib resistance did IL-8 inhibition have an effect on tumor growth. These findings suggest that ccRCC tumors initially rely primarily on VEGF/VEGFR proangiogenic signaling, but could become reliant on IL-8–dependent angiogenesis after sustained VEGFR blockade. Notably, inhibition of IL-8 function alone was not sufficient to suppress the growth of tumors that had acquired sunitinib resistance; continuing treatment with sunitinib was also required. This suggests that sunitinib-resistant ccRCCs may rapidly reactivate VEGFR-dependent angiogenesis upon discontinuation of sunitinib treatment. This is consistent with clinical and preclinical observations that sunitinib-inhibited tumors rapidly resume growth when sunitinib treatment is halted (4). In summary, our observations indicate that combination therapy with both sunitinib and IL-8 targeting agents seems valuable in reversing sunitinib resistance once it has occurred. It will now be of critical importance to validate these findings in the clinical setting.
Acknowledgments
We thank Lisa DeCamp, Vivarium Operations, Van Andel Research Institute, for her help with the animal experiments; Bree Berghuis, Eric Hudson, Kristin VandenBeldt, and J.C. Goolsby from the Laboratory of Analytical, Cellular, and Molecular Microscopy, Van Andel Research Institute, and Kelly Simmerman from Cleveland Clinic, for their help in immunohistochemical staining; Eric Kort for the generous sharing of his image analysis software; Vanessa Fogg for scientific editing; and Sabrina Noyes for assistance in preparation and submission of the manuscript.
Grant Support Pfizer Global Pharmaceuticals. We also thank the National Institute of Cancer Research (Singapore) for their funding support. A. Futreal is also supported by Wellcome Trust under grant reference 077012/Z/05/Z.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest B.T. Teh: commercial research grant, Pfizer Global Pharmaceuticals. No other potential conflicts of interest were disclosed.
References
- 1.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–45. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 2.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of antitumour activity. Nat Rev Cancer. 2008;8:579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 3.Reeves DJ, Liu CY. Treatment of metastatic renal cell carcinoma. Cancer Chemother Pharmacol. 2009;64:11–25. doi: 10.1007/s00280-009-0983-z. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Flaherty K. Clinical effect and future considerations for molecularly-targeted therapy in renal cell carcinoma. Urol Oncol. 2008;26:543–9. doi: 10.1016/j.urolonc.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Patel PH, Chadalavada RS, Chaganti RS, Motzer RJ. Targeting von Hippel-Lindau pathway in renal cell carcinoma. Clin Cancer Res. 2006;12:7215–20. doi: 10.1158/1078-0432.CCR-06-2254. [DOI] [PubMed] [Google Scholar]
- 6.Greenman C, Stephens P, Smith R, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang D, Ding Y, Luo WM, et al. Inhibition of MAPK kinase signaling pathways suppressed renal cell carcinoma growth and angiogenesis in vivo. Cancer Res. 2008;68:81–8. doi: 10.1158/0008-5472.CAN-07-5311. [DOI] [PubMed] [Google Scholar]
- 8.Giri D, Ittmann M. Interleukin-8 is a paracrine inducer of fibroblast growth factor 2, a stromal and epithelial growth factor in benign prostatic hyperplasia. Am J Pathol. 2001;159:139–47. doi: 10.1016/S0002-9440(10)61681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Sande WW, Fahal A, Verbrugh H, van Belkum A. Polymorphisms in genes involved in innate immunity predispose toward mycetoma susceptibility. J Immunol. 2007;179:3065–74. doi: 10.4049/jimmunol.179.5.3065. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 11.Reddy K. Phase III study of sunitinib malate (SU11248) versus interferon-α as first-line treatment in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer. 2006;5:23–5. doi: 10.1016/s1558-7673(11)70151-3. [DOI] [PubMed] [Google Scholar]
- 12.Huang D, Ding Y, Li Y, et al. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 2010;70:1053–62. doi: 10.1158/0008-5472.CAN-09-3722. [DOI] [PubMed] [Google Scholar]
- 13.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–33. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizukami Y, Jo WS, Duerr EM, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1α-deficient colon cancer cells. Nat Med. 2005;11:992–7. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 15.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 16.Smith DR, Polverini PJ, Kunkel SL, et al. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med. 1994;179:1409–15. doi: 10.1084/jem.179.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 18.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–91. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 19.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–5. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 21.Kerbel RS, Yu J, Tran J, et al. Possible mechanisms of acquired resistance to anti-angiogenic drugs: implications for the use of combination therapy approaches. Cancer Metastasis Rev. 2001;20:79–86. doi: 10.1023/a:1013172910858. [DOI] [PubMed] [Google Scholar]
- 22.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Choueiri TK, Vaziri SA, Jaeger E, et al. von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol. 2008;180:860–5. doi: 10.1016/j.juro.2008.05.015. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 24.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A. 2007;104:17069–74. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee TH, Avraham H, Lee SH, Avraham S. Vascular endothelial growth factor modulates neutrophil transendothelial migration via up-regulation of interleukin-8 in human brain microvascular endothelial cells. J Biol Chem. 2002;277:10445–51. doi: 10.1074/jbc.M107348200. [DOI] [PubMed] [Google Scholar]