Introduction
Salivary agglutinin (SAG) is a high-molecular weight glycoprotein encoded by the gene Deleted in Malignant Brain Tumors 1 (DMBT1) that is identical to gp340. It is a member of the scavenger receptor cysteine-rich (SRCR) family [1] containing 14 SRCR domains, and plays important roles in the innate immune defense against pathogens [2]. gp340 has been identified in a wide variety of secreted and membrane bound forms in lung lavage [3], saliva [4; 5; 6], tears [7], the female reproductive tract [8; 9], and numerous other epithelial sites [10; 11]. Human saliva contains a soluble form of gp340 and was shown by our group to inhibit infectivity of HIV-1 through a specific interaction with HIV-1 gp120 [12; 13].
Salivary gp340 was originally found to aggregate oral bacteria, Streptococcus gordonii and Streptococcus mutans [14] and Helicobacter pylori [15]. Genital tract epithelial cells [16; 17] and macrophages [8] express gp340 is on their surface and this may play a role in enhancing HIV infection in the female reproductive track [18; 19]. There is a clear need for assays that detect and quantify gp340 (DMBT1, SAG) in saliva, CVL, and other body fluids because of its links to salivary gland [20], bladder [21; 22], skin [23], and prostate [24; 25] cancer; its ability to bind and aggregate bacteria [26; 27]; its presence in the oral cavity during oral infection and inflammation; its direct interaction with gastric-cancer causing Helicobacter pylori [26]; its inhibition of HIV-1 infectivity; and the possibility that it may play a role in defense mechanisms against other relevant pathogens. We describe here an assay using an electrochemiluminescent platform (MesoScale Discovery (MSD)) that can accurately measure gp340 levels in saliva and cervical/vaginal lavage using gp340 specific antibodies [4].
Saliva
Whole mouth stimulated saliva (WMSS) was collected on ice, treated with protease inhibitor (Thermo #78415), centrifuged at 2500 rpm, 25 min at 4°C, aliquoted, and stored at −80°C. WMSS is produced by subjects chewing on paraffin wax to stimulate salivary flow, and 10 ml is collected with a Pasteur pipette into an iced 15 mL conical polypropylene tube. gp340 was purified from pooled WMSS as previously described [27; 28]. The purified protein was assessed by Western blot and bacterial aggregation [27]. The first SRCR-1 domain of gp340 was overexpressed in mammalian 293T cells and purified [12]. Preparations retained the ability to aggregate bacteria, bind to gp120 peptide, and inhibit HIV-1 infectivity with both CXCR4 and CCR5 viruses.
Cervical Vaginal Lavage (CVL)
Samples were collected from the same women who provided saliva by inserting a sterile transfer pipette attached to a syringe containing 5 mL of sterile saline into the posterior fornix of the vagina. The vagina was lavaged by flushing 5 times. Fluid was transferred to an iced 10 mL conical polypropylene tube containing 0.5 mL of 10X PBS, centrifuged at 2500 rpm and the supernatant stored at −80°C.
MSD Technology
The MSD electrochemiluminescence detection system (SI2400 image analyzer, MesoScale Discovery) provides a combination of high sensitivity and a 5 log-order dynamic range. Our studies used either a goat anti-rabbit or goat anti-mouse Sulfo-Tagged secondary antibody that is stable, non-radioactive, and emits light when electrochemically stimulated. The inherent noise for this instrumentation is low because the electrochemical stimulation mechanism is isolated from the emitted light signal at 620 nm.
MSD Assay Design
Standard binding MSD plates were coated with 30 μL of 5 μg/mL capture antibody, sealed, rotated at 600 rpm for 20 min at room temperature, and incubated overnight at 4°C. The MSD “High binding” plates are designed for experiments that require the binding of cells and were not found suitable for this assay. Plates were brought to room temperature, the coating solution aspirated, and wells blocked with 150 μL of 3% MSD Blocker A in PBS-T (PBS containing 0.05% Tween 20) for 1 hr followed by the addition of 25 μL of sample diluted in 1% Blocker A-PBS-T, incubated for 1 hr, washed 3X with PBS-T, and the wells incubated with 25 μL of second or detection antibody for 1 hr diluted in 1% Blocker A in PBS, the plate was washed 3X with PBS-T and incubated with the appropriate Sulfo-Tag labeled anti-mouse or anti-rabbit antibody, 1 μg/mL in Blocker A in PBS for 1 hr. Plates were washed 3X with PBS-T and 150 μL of 2X MSD Read Buffer was added and plates were analyzed. The standard curve was generated from a serial dilution of purified gp340 protein, 3–833 ng/mL, in 1% Blocker A in a total volume of 25 μL/well. Twenty-four antibody combinations of Ab17779 (mouse), mAb143 (mouse), mAb116 (mouse), DAPA (mouse), Ab8502 (rabbit), Ab1527 (rabbit), and Ab2 (rabbit) were tested to determine the most sensitive antibody pair. Monoclonal antibody mAb143 detects a linear epitope in the native molecule while mAb116 detects a carbohydrate epitope
Subject Consent
The University of Pennsylvania IRB approved the subject consent form and collection of CVL and WMSS samples.
Results
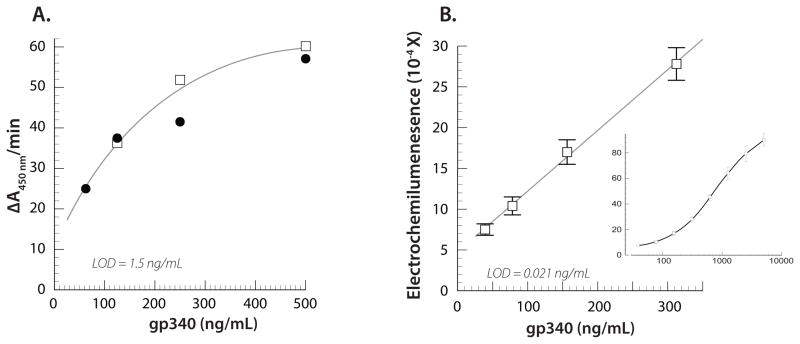
The assay requires that each antibody in the sandwich pair recognize a different gp340 epitope. Antibodies to gp340 were tested in a matrix that compared each as either a detection or capture antibody to determine the optimal pairing. All combinations were detected with the appropriate species-specific MSD Sulfo-Tag anti-IgG antibody. Assays optimal conditions were determined empirically to utilize 1 μg/mL of capture antibody mAb143 and 5 μg/mL of detection antibody Ab1527 polyclonal antibody. Representative kinetic ELISA data are shown in Figure 1A for the detection of gp340 using and capture antibody mAb143 (1 μg/mL). This is compared to results for the MSD assay, which utilizes a smaller sample volume (Figure 1B). Figure 1B insert shows the full dynamic range for the MSD assay. The MSD/chemiluminescence standard curve for gp340 detection has an LOD of 0.02 ng/mL compared to the ELISA with an LOD of 1.5 ng/mL using the same antibody pair. LOD was determined as 3X the standard deviation of the blank for the respective assays. The MSD protocol requires a smaller amount of target than used for the ELISA and has a lower LOD and a broader dynamic assay range.
Figure 1.
A). Kinetic ELISA for gp340 using mAb143 as the capture antibody and Ab1527 as the detection antibody (1μg/mL). gp340 was varied from 61 to 849 ng/mL, but the response saturated by ~500 ngmL. The LOD (3 X the standard deviation of the blank) is 1.5 ng/mL. B) Effect of capture antibody mAb143 concentration on the observed electrochemiluminescence response as a function of gp340 concentration. The detection antibody Ab1527 (1 μg/mL) and gp340 was varied from 39 to 5000 ng/mL (inset). The LOD is 0.021 ng/mL.
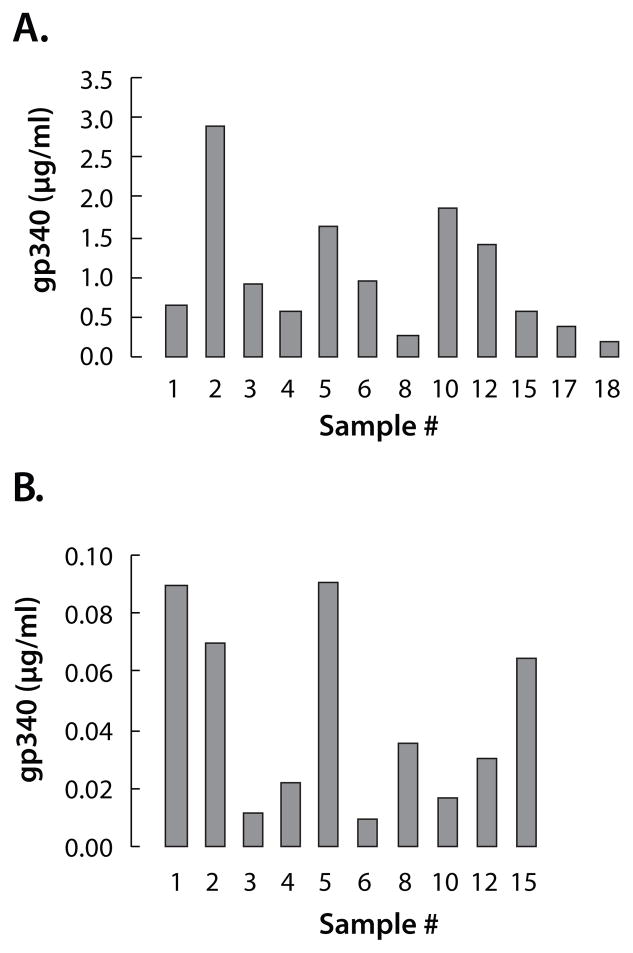
Proof of Principal (Figure 2)
Figure 2.
MSD detection of gp340 in saliva and CVL. Samples with the same # are from the same subject. (A) Of the twelve saliva samples tested, all were detected within the linear range of the MSD assay (B) MSD readily detected 9 ng/mL of gp340 in CVL within the assay’s linear range. Only four samples were within the linear range of the kinetic ELISA for gp340.
To evaluate the system with human samples saliva and CVL were collected from the same individuals. Saliva samples were diluted 1:200 and vaginal lavage samples were diluted 1:5 in buffer. The LOD utilizing MSD was 0.02 ng/mL of gp340, with all the lavage samples in linear range. Most of the CVL samples were undetectable with an ELISA. The levels of gp340 in saliva and CVL are not correlated within each subject (Paired t-Test, for Saliva ≠ CVL, p=0.001).
Discussion
We describe a sensitive assay for gp340 applicable for future studies determining gp340 levels in biological fluids. The MSD platform provides versatility and sensitivity with a 5 log-order range of sensitivity and an LOD for salivary gp340 of 0.02 ng/mL. The MSD protocol allows a smaller sample (25 μL), which is useful for monitoring samples with inherently small volumes: e.g., gingival crevicular fluid; patients suffering from xerostomia due to Sjögren’s syndrome; or currently taking medications that decrease salivary flow.
The MSD assay detected low levels of gp340 present in CVL that were undetectable by ELISA. There was no apparent relationship between the gp340 levels in CVL and the saliva obtained from the same individuals. This is interesting given the apparent dichotomy between HIV inhibition by gp340 in the oral cavity and potential promotion of infection in the genital tract [18; 19]. In addition, gp340 in the oral cavity is secreted, while in the female genital tract gp340 is mainly bound on the cell surface [19]. While it would be expected that constitutive levels of gp340 are genetically controlled, there is evidence that various conditions, e.g. cancer, inflammation, can alter these levels [29]. The expression levels in the oral cavity and the female genital tract are not known at present and are beyond the scope of this report. We conclude that the sandwich MSD assay described is well suited for the detection and quantification of gp340 in saliva and cervical vaginal lavage.
Acknowledgments
The support by the National Institute of Dental and Craniofacial Research (U19DE018385 (DM)) and the National Institute of Allergy and Infectious Disease (AI094599-01 (DM), AI082701 (DW)) is gratefully acknowledged. DM is a NYStar recipient.
Footnotes
Competing Interests: The authors declare no competing interests.
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 2.Madsen J, Mollenhauer J, Holmskov U. Gp-340/DMBT1 in mucosal innate immunity. Innate Immunity. 2010:1–9. doi: 10.1177/1753425910368447. [DOI] [PubMed] [Google Scholar]
- 3.Leito J, Ligtenberg A, Houdt Mv, Berg Tvd, Wouters D. The bacteria binding glycoprotein salivary agglutinin (SAG/gp340) activates complement via the lectin pathway. Molecular Immunology. 2011;49:185–190. doi: 10.1016/j.molimm.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z, Ryk DV, Barber C, Abrams W, Chaiken I, Magnani J, Malamud D. Salivary Agglutinin Inhibits HIV Type 1 Infectivity through Interaction with Viral Glycoprotein 120. AIDS Research and Human Retroviruses. 2003;19:201–209. doi: 10.1089/088922203763315704. [DOI] [PubMed] [Google Scholar]
- 5.Malamud D, Abrams WR, Barber CA, Weissman D, Rehtanz M, Golub E. Antiviral Activities in Human Saliva. Adv Dent Res. 2011;23:34–37. doi: 10.1177/0022034511399282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagashunmugam T, Malamud D, Davis C, Abrams W, Friedman H. Human submandibular saliva inhibits human immunodeficiency virus type 1 infection by displacing envelope glycoprotein gp120 from the virus. Jour Infect Dis. 1998;178(6):1635–41. doi: 10.1086/314511. [DOI] [PubMed] [Google Scholar]
- 7.Schulz BL, Oxley D, Packer NH, Karlsson NG. Identification of two highly sialylated human tear-fluid DMBT1 isoforms: the major high-molecular-mass glycoproteins in human tears. Biochem J. 2002;366:511–20. doi: 10.1042/BJ20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon G, Yi Y, Ni N, Stoddard E, Scales D, Ryk DV, Chaiken I, Malamud D, Weissman D. HIV envelope binding by macrophage-expressed gp340 promotes HIV-1 infection. J Immunol. 2008;181:2065–2070. doi: 10.4049/jimmunol.181.3.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummins JE, Christensen L, Lennox JL, Bush TJ, Wu Z, Malamud D, Evans-Strickfaden T, Siddig A, Caliendo AM, Hart CE, Dezzutti CS. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retroviruses. 2006;22:788–95. doi: 10.1089/aid.2006.22.788. [DOI] [PubMed] [Google Scholar]
- 10.Rosenstiel P, Sina C, End C, Renner M, Lyer S, Till A, Hellmig S, Nikolaus S, Fölsch UR, Helmke B, Autschbach F, Schirmacher P, Kioschis P, Hafner M, Poustka Annemarie, Mollenhauer J, Schreiber S. Regulation of DMBT1 via NOD2 and TLR4 in Intestinal Epithelial Cells Modulates Bacterial Recognition and Invasion. The Journal of Immunology. 2007;178:8203–8211. doi: 10.4049/jimmunol.178.12.8203. [DOI] [PubMed] [Google Scholar]
- 11.Mollenhauer J, Herbertz S, Holmskov U, Tolnay M, Krebs I, Merlo A, Schrøder H, Maier D, Breitling F, Wiemann S, Gröne H, Poustka A. DMBT1 encodes a protein involved in the immune defense and in epithelial differentiation and is highly unstable in cancer. Cancer Research. 2000;60:1704–10. [PubMed] [Google Scholar]
- 12.Wu Z, Lee S, Abrams W, Weissman D, Malamud D. The N-terminal SRCR-SID domain of gp-340 interacts with HIV type 1 gp120 sequences and inhibits viral infection. AIDS Res Hum Retroviruses. 2006;22:508–15. doi: 10.1089/aid.2006.22.508. [DOI] [PubMed] [Google Scholar]
- 13.Wu Z, Golub E, Abrams WR, Malamud D. gp340 (SAG) binds to the V3 sequence of gp120 important for chemokine receptor interaction. AIDS Res Hum Retroviruses. 2004;20:600–7. doi: 10.1089/0889222041217400. [DOI] [PubMed] [Google Scholar]
- 14.Loimaranta V, Jakubovics NS, Hytönen J, Finne J, Jenkinson HF, Strömberg N. Fluid- or Surface-Phase Human Salivary Scavenger Protein gp340 Exposes. Different Bacterial Recognition Properties. Infection and Immunity. 2005;73:2245–2252. doi: 10.1128/IAI.73.4.2245-2252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ligtenberg AJM, Karlsson NG, Veerman ECI. Deleted in Malignant Brain Tumors-1 Protein (DMBT1): A Pattern Recognition Receptor with Multiple Binding Sites. International Journal of Molecular Sciences. 2010;11:5213–5234. doi: 10.3390/ijms1112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoddard E, Ni H, Cannon G, Zhou C, Kallenbach N, Malamud D, Weissman D. gp340 Promotes Transcytosis of Human Immunodeficiency Virus Type 1 in Genital Tract-Derived Cell Lines and Primary Endocervical Tissue. Journal of Virology. 2012;86 doi: 10.1128/JVI.00744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earl Stoddard GC, Ni Houping, Karikó Katalin, Capodici John, Malamud Daniel, Weissman Drew. gp340 Expressed on Human Genital Epithelia Binds HIV-1 Envelope Protein and Facilitates Viral Transmission. The Journal of Immunology. 2007;179:3126–3132. doi: 10.4049/jimmunol.179.5.3126. [DOI] [PubMed] [Google Scholar]
- 18.Stoddard E, Ni H, Cannon G, Zhou C, Kallenbach N, Malamud D, Weissman D. gp340 promotes transcytosis of human immunodeficiency virus type 1 in genital tract-derived cell lines and primary endocervical tissue. J Virol. 2009;83:8596–603. doi: 10.1128/JVI.00744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoddard E, Cannon G, Ni H, Kariko K, Capodici J, Malamud D, Weissman D. gp340 expressed on human genital epithelia binds HIV-1 envelope protein and facilitates viral transmission. J Immunol. 2007;179:3126–32. doi: 10.4049/jimmunol.179.5.3126. [DOI] [PubMed] [Google Scholar]
- 20.Bikker FJ, van der Wal JE, Ligtenberg AJ, Mollenhauer J, de Blieck-Hogervorst JM, van der Waal I, Poustka A, Nieuw Amerongen AV. Salivary agglutinin/DMBT1SAG expression is up-regulated in the presence of salivary gland tumors. J Dent Res. 2004;83:567–71. doi: 10.1177/154405910408300711. [DOI] [PubMed] [Google Scholar]
- 21.Dodurga Y, Avci CB, Satiroglu-Tufan NL, Tataroglu C, Kesen Z, Dogan ZO, Yilmaz S, Gunduz C. Detection of deleted in malignant brain tumors 1 and runt-related transcription factor 3 gene expressions in bladder carcinoma. Mol Biol Rep. 2012;39:4691–5. doi: 10.1007/s11033-011-1261-9. [DOI] [PubMed] [Google Scholar]
- 22.Dodurga Y, Avci CB, Yilmaz S, Dogan ZO, Kesen Z, Tataroglu C, Satiroglu-Tufan NL, Bushra T, Gunduz C. Evaluation of deleted in malignant brain tumors 1 (DMBT1) gene expression in bladder carcinoma cases: preliminary study. Biomarkers. 2011;16:610–5. doi: 10.3109/1354750X.2011.620627. [DOI] [PubMed] [Google Scholar]
- 23.Helmke BM, Renner M, Poustka A, Schirmacher P, Mollenhauer J, Kern MA. DMBT1 expression distinguishes anorectal from cutaneous melanoma. Histopathology. 2009;54:233–40. doi: 10.1111/j.1365-2559.2008.03200.x. [DOI] [PubMed] [Google Scholar]
- 24.Du J, Guan M, Fan J, Jiang H. Loss of DMBT1 expression in human prostate cancer and its correlation with clinical progressive features. Urology. 2011;77:509e9–13. doi: 10.1016/j.urology.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Traka MH, Spinks CA, Doleman JF, Melchini A, Ball RY, Mills RD, Mithen RF. The dietary isothiocyanate sulforaphane modulates gene expression and alternative gene splicing in a PTEN null preclinical murine model of prostate cancer. Mol Cancer. 2010;9:189. doi: 10.1186/1476-4598-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bikker FJ, Ligtenberg AJ, End C, Renner M, Blaich S, Lyer S, Wittig R, Hof Wvt, Veerman E, Nazmi K, Blieck-Hogervorst Jd, Kioschis P, Amerongen AN, Poustka A, Mollenhauer J. Bacteria binding by DMBT1/SAG/gp-340 is confined to the VEVLXXXXW motif in its scavenger receptor cysteine-rich domains. J Biol Chem. 2004;279:47699–703. doi: 10.1074/jbc.M406095200. [DOI] [PubMed] [Google Scholar]
- 27.Golub EE, Thaler M, Davis C, Malamud D. Bacterial aggregating activity in human saliva: simultaneous determination of free and bound cells. Infect Immun. 1979;26:1028–34. doi: 10.1128/iai.26.3.1028-1034.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malamud D, Appelbaum B, Kline R, Golub EE. Bacterial aggregating activity in human saliva: comparisons of bacterial species and strains. Infect Immun. 1981;31:1003–6. doi: 10.1128/iai.31.3.1003-1006.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannon G, Yi Y, Ni H, Stoddard E, Scales DA, Van Ryk DI, Chaiken I, Malamud D, Weissman D. HIV envelope binding by macrophage-expressed gp340 promotes HIV-1 infection. J Immunol. 2008;181:2065–70. doi: 10.4049/jimmunol.181.3.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]