Abstract
Breast and ovarian cancer are two of the leading causes of cancer deaths among women in the United States. Overexpression of the HER2/neu oncoprotein has been reported in patients affected with breast and ovarian cancers, and is associated with poor prognosis. To develop a novel targeted therapy for HER2/neu expressing tumors, we have constructed a fully human IgE with the variable regions of the scFv C6MH3-B1 specific for HER2/neu. This antibody was expressed in murine myeloma cells and was properly assembled and secreted. The Fc region of this antibody triggers in vitro degranulation of rat basophilic cells expressing human FcεRI (RBL SX-38) in the presence of murine mammary carcinoma cells that express human HER2/neu (D2F2/E2), but not the shed (soluble) antigen (ECDHER2) alone. This IgE is also capable of inducing passive cutaneous anaphylaxis in a human FcεRIα transgenic mouse model, in the presence of a cross-linking antibody, but not in the presence of soluble ECDHER2. Additionally, IgE enhances antigen presentation in human dendritic cells and facilitates cross-priming, suggesting that the antibody is able to stimulate a secondary T-cell anti-tumor response. Furthermore, we show that this IgE significantly prolongs survival of human FcεRIα transgenic mice bearing D2F2/E2 tumors. We also report that the anti-HER2/neu IgE is well tolerated in a preliminary study conducted in Macaca fascicularis (cynomolgus) monkeys. In summary, our results suggest that this IgE should be further explored as a potential therapeutic against HER2/neu overexpressing tumors, such as breast and ovarian cancers.
Keywords: HER2/neu, IgE, Immunotherapy, Cancer, AllergoOncology
Introduction
Breast cancer is one of the leading causes of cancer deaths, second only to lung cancer, among women in the United States [1]. Ovarian cancer is the leading cause of death from all gynecological malignancies and is the fifth overall cause of cancer deaths among women in the United States [1]. Additionally, advanced disease remains generally incurable for both malignancies, and the 5-year survival rate for these patients ranges from 23 to 28% [1]. Approximately 25% of breast cancers demonstrate amplification of the oncogene HER2/neu, which is associated with more aggressive disease and poor prognosis [2, 3]. Overexpression of HER2/neu has also been described in 9–32% (depending on the study) ovarian cancer tissue [3, 4]. As is the case for breast cancer, HER2/neu overexpression in ovarian cancer is associated with poor prognosis [5, 6]. HER2/neu is a member of the epidermal growth factor receptor (EGFR) family that have intrinsic tyrosine kinase activity that leads to the activation of downstream signaling pathways of cell proliferation and survival [7].
The humanized monoclonal IgG1 antibody trastuzumab (Herceptin®) that binds to the extracellular domain of HER2/neu was initially approved in 1998 by the Food and Drug Administration for the treatment of HER2/neu overexpressing advanced breast cancer. Since then trastuzumab has shown efficacy against breast cancer both as an adjuvant therapy and as a treatment of metastatic disease (reviewed in [8]). However, the majority of patients with advanced breast cancer that are treated with trastuzumab alone or combined with chemotherapeutic agents eventually relapse and the median time to progression is less than 1 year [8, 9]. Additionally, a significant number of breast cancer patients do not respond to trastuzumab-based therapies despite the high level of HER2/neu expression [8–10]. Furthermore, in a Phase II clinical trial in patients with HER2/neu overexpressing recurrent or refractory ovarian or primary peritoneal carcinoma that were treated with trastuzumab alone, a low rate of objective response (7.3%) was observed [11]. While trastuzumab has shown efficacy in a subset of patients with either breast or ovarian cancer, additional strategies to target HER2/neu overexpressing tumors are still needed.
Like trastuzumab, the majority of antibody therapies for the treatment of cancer utilize antibodies that are of the IgG class. However, antibodies of the IgE class may also be potential cancer therapeutics since they have several potential advantages over their IgG counterparts. IgE mediates allergic reactions, which is due to the presence of effector cells in the tissue that are sensitized by IgE bound to Fc epsilon receptor I (FcεRI). These effector cells are degranulated after crosslinking of the IgE that is triggered by a multi-epitope antigen interaction. IgE can also mediate antigen presentation via the interaction with FcεRs expressed on antigen-presenting cells (APC) such as dendritic cells (DC) [12–14]. IgE has been suggested to provide protection against parasitic infections [15], although this function is controversial [16, 17]. Research on cancer and IgE belongs to the new field of “AllergoOncology” [12]. This field has two aims: (1) to reveal the function of IgE-mediated immune responses against cancer cells in order to elucidate the understanding of its biology and (2) to develop novel IgE-based treatment options against malignant diseases [18]. A key advantage associated with IgE is its exceptionally high affinity for the FcεRs. There are two FcεRs, the FcεRI which binds IgE with high affinity (Ka = 1010 M−1) and is expressed on human monocytes, macrophages, eosinophils, basophils, mast cells, Langerhans cells, and DC, and the FcεRII (CD23) which binds IgE with lower affinity (Ka = 108 M−1) and is expressed on human eosinophils, monocytes, macrophages, and DC [12–14, 19]. Thus, the affinity of IgE for FcεRI is at least two orders of magnitude higher than that of IgG for the FcγRs (FcγRI-III) and in the case of FcεRII is as high as that of IgG for its high-affinity receptor FcγRI (CD64). Another advantage of the IgE molecule is the low endogenous serum concentration in humans, which is only 0.02% of total circulating immunoglobulins, whereas IgG is the most abundant at 85% [20]. Thus, the competition for FcR occupancy is much lower for IgE. Another potential advantage is that there is no known inhibitory FcεR as there is for FcγR. In order to develop a new therapy for HER2/neu expressing malignancies, we now describe the development and characterization of a novel fully human anti-HER2/neu IgE and evaluate its potential as a cancer therapeutic.
Materials and methods
Cell lines
The human breast cancer cell line SK-BR-3 was obtained from ATCC (American Type Culture Collection, Manassas, VA) and cultured in RPMI 1640 (Invitrogen Corporation, Carlsbad, CA). D2F2/E2 murine mammary cells that express human HER2/neu and the parental cell line D2F2 (syngeneic to BALB/c mice) were kind gifts from Dr. Wei-Zen Wei (Wayne State University, Detroit, MI) [21] and were grown in IMDM medium (Invitrogen Corporation). The Chinese Hamster Ovary cell line (CHO 3D10) expressing the human FcεRIα subunit [22] and the RBL SX-38 rat basophil leukemic cell line expressing the full human receptor (FcεRI) [23] were kindly provided by Dr. Jean-Pierre Kinet (Beth Israel Deaconess Medical Center, Boston, MA) and grown in IMDM. Both RPMI and IMDM media for all cell lines were supplemented with 100 U/ml penicillin, 10 μg/ml streptomycin, and 10% (v/v) heat-inactivated fetal bovine serum (Atlanta Biologicals, Atlanta, GA). Growth medium for D2F2/E2 and RBL SX-38 cells was additionally supplemented with 1 mg/ml G418 (Invitrogen Corporation).
Production of recombinant antibodies
The fully human anti-HER2/neu IgE was constructed using the DNA encoding the variable regions of the single-chain Fv (scFv) C6MH3-B1 that was isolated from a human phage library and affinity matured in vitro by sequential mutation of the third complimentary determining region (CDR3) in both the variable heavy chain (VH) and the variable light chain (VL) regions [24, 25]. The affinity-matured VL and VH regions were cloned into either the human κ light chain or the human ε heavy chain (the classic secreted isoform) expression vectors, respectively, both of which were obtained as kind gifts from Dr. Sherrie L. Morrison (UCLA, Los Angeles, CA) [26]. The antibody was expressed in the murine myeloma cell line P3X63Ag8.653 and the transfectomas were grown in rollerbottles for antibody production as previously described [27]. The IgE was then purified from cell culture supernatants on an immunoaffinity column prepared by coupling an anti-human IgE (omalizumab, Xolair®, Genentech, Inc. San Fransisco, CA, USA) to cyanogen bromide-activated Sepharose (GE Healthcare, Piscataway, NJ), as recommended by the manufacturer. A non-HER2/neu-specific IgE (NS IgE) with human constant regions was produced in the same manner alongside the anti-HER2/neu IgE and was used as a negative control. The anti-HER2/neu IgG3 that contains the C6MH3-B1 scFv variable regions has been previously described [28]. Rituximab (anti-CD20 IgG1; NS IgG) and trastuzumab (anti-HER2/neu IgG1) were obtained from Genentech. The extracellular domain of HER2/neu (ECDHER2) was produced as previously described [29]. All proteins were quantified using the BCA Protein Assay (ThermoFisher Scientific, Walnut, CA).
Flow cytometry (binding analysis)
Cells were detached from tissue culture dishes using 0.5 mM EDTA and incubated with 1 μg of the various antibodies for 2 h on ice. Cells were then washed in buffer (2 mM EDTA, 0.5% BSA in PBS) and binding was detected using an anti-human κ fluorescein isothiocyanate (FITC)-conjugated secondary antibody (BD Biosciences, San Jose, CA). Cells were also stained with an anti-FcεRI phycoerythrin (PE)-conjugate (eBioscience, San Diego, CA) to ensure expression of the receptor on the surface of the cells. All cells were washed, fixed with 2% paraformaldehyde in PBS, and analyzed on a Becton–Dickinson FACScan Analytic Flow Cytometer in the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility. Histograms were created using the FCS Express V3 software.
In vitro degranulation assay
RBL SX-38 cells were seeded in a 48-well plate at a density of 105 cells/well. After adherence to the plates, these cells were sensitized with 1 μg of the anti-HER2/neu IgE, anti-HER2/neu IgG3, or the NS IgE for 2 h. Sensitized RBL SX-38 cells were then incubated for an additional 2 h with either buffer alone, D2F2/E2 (50,000 cells), D2F2 (50,000 cells), or soluble ECDHER2 (1 or 5 μg). Cell supernatants (50 μl) were transferred to a fresh 96-well plate, and the amount of β-hexosaminidase released into the medium was determined using 100 μl of 2.5 mM p-nitrophenyl-N-acetyl-β-D-glucosamine (Sigma Aldrich, St. Louis, MO) in 50 mM citrate buffer (50 mM citric acid, 50 mM tribasic sodium citrate, pH 4.5). Reactions were quenched by the addition of 100 μl sodium carbonate buffer (50 mM sodium carbonate, 50 mM sodium bicarbonate, pH 10). Absorbance at 405 nm was determined using a DTX880 Multimode detector (Beckman Coulter, Fullerton, CA). β-hexosaminidase release in experimental samples is expressed as a percent of total content within the basophils as determined by separate treatment with 1% Triton X-100 in PBS. The percent degranulation was determined by the following equation [(experimental release–spontaneous release)/(maximum release–spontaneous release)] × 100.
In vivo passive cutaneous anaphylaxis assay
Since human IgE does not interact with murine FcεRI [14], BALB/c human FcεRIα transgenic mice (a kind gift from Dr. Jean-Pierre Kinet, Beth Israel Deaconess Medical Center, Boston, MA) were used for this assay [30]. In this transgenic mouse model, the murine FcεRIα is knocked out and replaced by its human couterpart. The human FcεRIα associates with the murine FcεRIλ and FcεRIβ chains resulting in a chimeric FcεRI that can be activated by human IgE [30–32]. The expression of the human FcεRIα in these animals mimics the expression pattern found in humans. It is important to note that these animals are not transgenic for human CD23 and thus, only express murine CD23 (which does not bind human IgE [33]). The assay was performed as previously described [34]. Specifically, the animals were intradermically injected with 1 µg of antibody or buffer (PBS) control. After a 4-h incubation, the mice were injected intravenously (i.v.) with 25 µg of anti-human κ antibody (used to artificially cross-link the human IgE) or 2 µg ECDHER2 in 1% Evan’s Blue tetrasodium salt (Tocris Bioscience, Ellisville, MO) in PBS. After 10 min, mice were euthanized and the skin removed. Leakage of the blue dye into the skin due to a local IgE-induced inflammatory response was assessed using the NIH ImageJ software. Color intensity is reported as the mean signal per pixel.
Antigen presentation assay
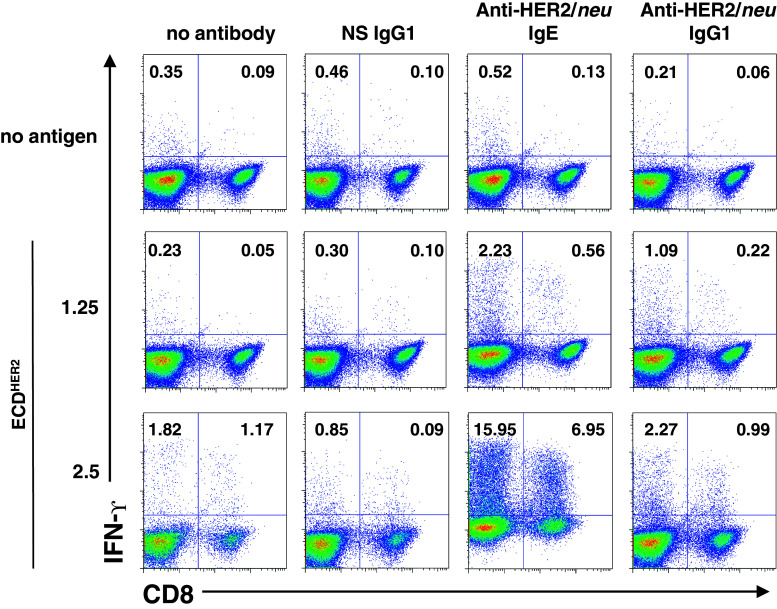
Human monocytes were isolated from peripheral blood mononuclear cells (PBMC) using the EasySep Human Monocyte Enrichment Negative Selection Kit (StemCell Technologies, British Columbia, Canada). Monocytes were cultured for 7 days in 4 ng/ml IL-4 and 0.1 ng/ml GM-CSF to differentiate them to DC, which are then loaded with 1.25 or 2.5 μg/ml ECDHER2 complexed to equimolar amounts of either a NS IgG1 (2.5 μg/ml), anti-HER2/neu IgE (2.0 μg/ml), or anti-HER2/neu IgG1 (2.5 μg/ml). After loading for 4 h, the DC were matured with 0.18 ng/ml IFN-α and 10 ng/ml TNF-α. Autologous T cells are added on day 8. Cells were incubated for 7 days and restimulated with freshly pulsed DC. The cells were stimulated for a total of three rounds. During the last stimulation round, cells were treated with Brefeldin A to block cytokine secretion, and T cells were harvested and analyzed for activation of CD4 (CD3+/CD8−) and CD8 (CD3+/CD8+) IFN-γ producing T cells by intracellular cytokine staining and flow cytometry.
Anti-tumor activity in FcεRIα transgenic mice
Female BALB/c human FcεRIα transgenic mice 6-12 weeks old were challenged intraperitoneally (i.p.) with 2 × 105 D2F2/E2 cells. Two days after tumor challenge, age-matched mice were treated i.p. with 100 μg of the anti-HER2neu IgE or Hank’s Balanced Salt Solution (HBSS) alone (5-6 animals per group per experiment). Mice were treated i.p. for a second time on day 4 with 100 μg of the same treatments and survival was recorded. Kaplan–Meier plots were generated using GraphPad Prism 4. Statistical analysis was performed using this software and the Log Rank Test.
Pilot study in M. fascicularis (cynomolgus) monkeys
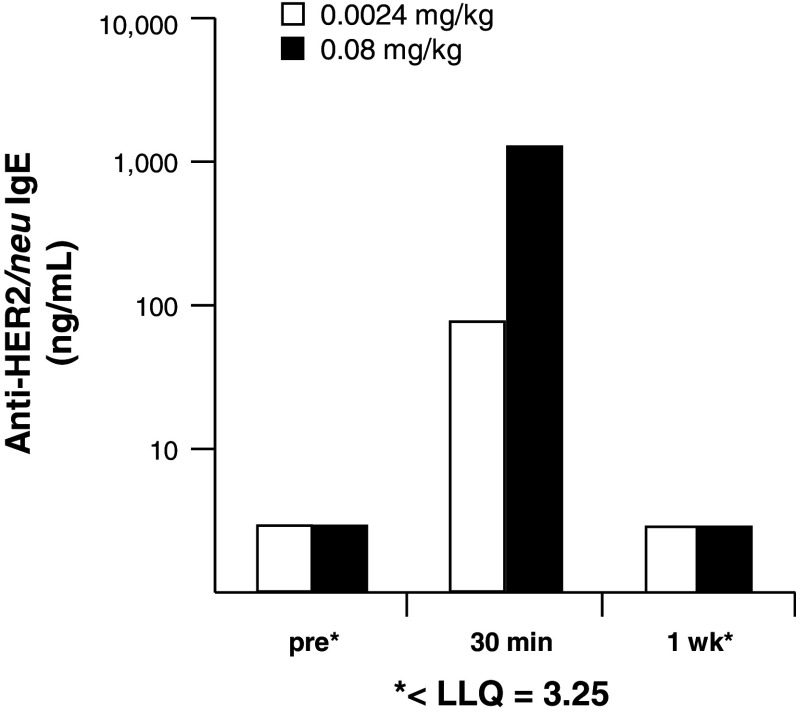
This study was conducted at WuXi AppTec (XiShan Site, Suzhou, Jiangsu Province, China) under the approval of that facility's IACUC. Two male monkeys with a body weight of 4.74 and 6.57 kg were used in the study. One animal received a low-dose i.v. infusion of 0.0024 mg/kg of the anti-HER2/neu IgE (10 ml over 20 min of infusion) and the other animal received 0.08 mg/kg, (10 ml over 20 min). Blood was collected from the animals at pre-dose, 30 min, and 1 week post-dose. Serum concentration of anti-HER2/neu IgE was determined using ELISA (Immunology Consultants Laboratory, Inc., Newberg, OR). Animals were monitored closely by a veterinarian for signs of distress for 1 h following the infusion and monitored for change in food consumption and general health for one additional week following dosing.
Results
Binding to antigen and FcεRI
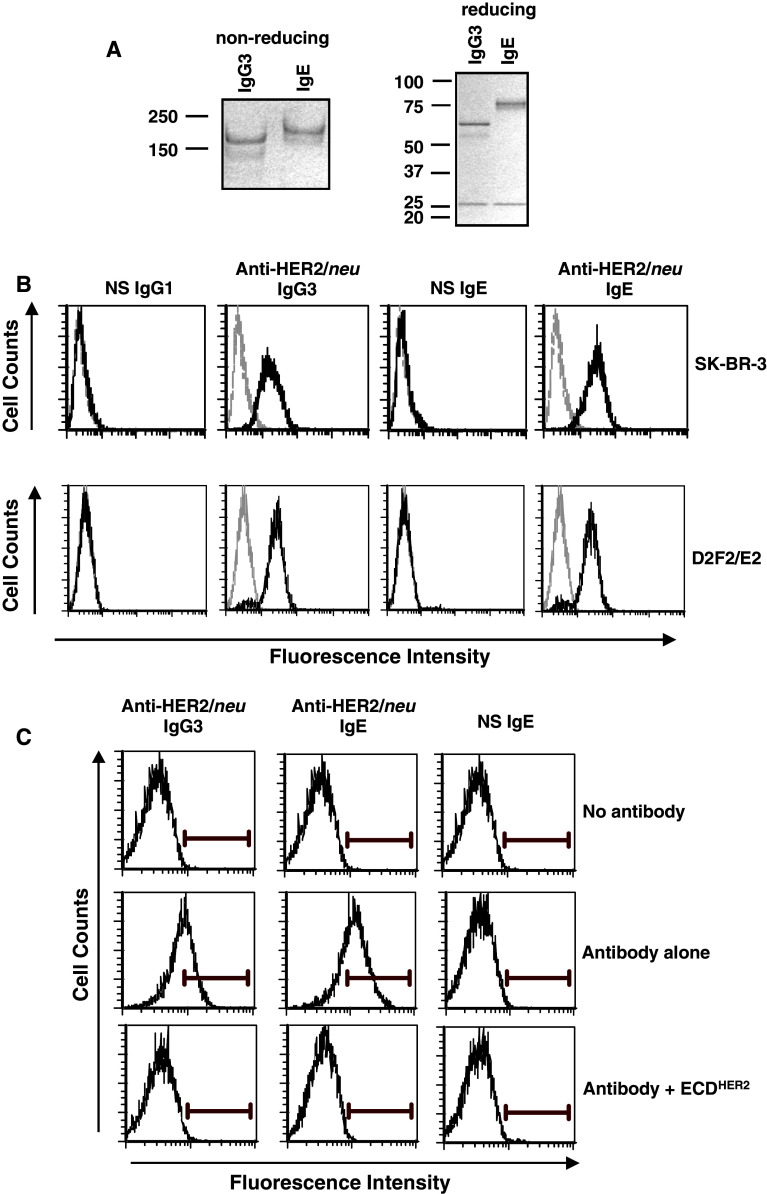
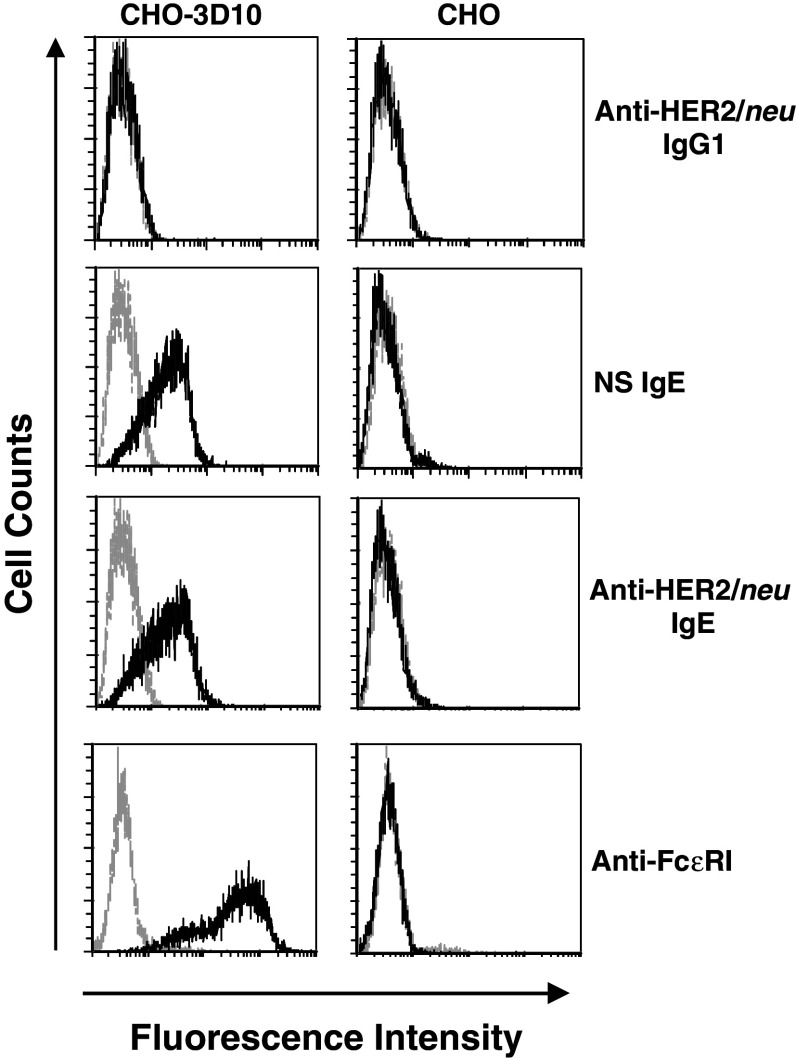
The fully human anti-HER2/neu IgE, expressed in murine myeloma cells, was properly assembled and secreted and showed the expected molecular weight (190 kDa) as verified using SDS-PAGE analysis (Fig. 1a). Binding of both the anti-HER2/neu IgG3 and anti-HER2/neu IgE to the surface of the human breast cancer cell line SK-BR-3 was detected by flow cytometry (Fig. 1b). Binding of both antibodies was also detected at similar levels on the surface of D2F2/E2 murine mammary carcinoma cells that express human HER2/neu (Fig. 1b). Importantly, binding to cell surface HER/neu could be blocked by the addition of soluble antigen (ECDHER2; Fig. 1c). Thus, the anti-HER2/neu IgE is able to bind the soluble antigen in solution as well as cell surface antigen. Since binding of IgE to FcεRI occurs through the α subunit, we used CHO cells that express the human FcεRIα subunit on the cell surface (CHO-3D10 cells) to detect this interaction. Both the anti-HER2/neu IgE and the NS IgE bound to the surface of CHO-3D10 cells (Fig. 2) as expected since they contain the same Fc region. These studies confirm that the fully human IgE is able to bind antigen (both soluble as well as cell-surface bound) and the FcεRI.
Fig. 1.
Binding to HER2/neu on the surface of cancer cells. a SDS–PAGE analysis of the anti-HER2/neu IgE under both reducing and non-reducing conditions as compared to the anti-HER2/neu IgG3. b SK-BR-3 (top panel) and D2F2/E2 (bottom panel) cells were incubated with 1 μg of a non-specific (NS) IgG1, anti-HER2/neu IgG3, anti-HER2/neu IgE, or a NS IgE. Binding was detected using an anti-human κ-FITC antibody. Samples were analyzed by flow cytometry. Each histogram shows cells incubated with labelled secondary antibody alone (gray) and experimental antibody (black). Data are representative of three independent experiments. c D2F2/E2 cells were incubated with 0.1 μg of the anti-HER2/neu IgG3, anti-HER2/neu IgE, or a NS IgE with or without tenfold excess (1 μg) soluble ECDHER2. Binding was detected using an anti-human κ-FITC antibody. Samples were analyzed by flow cytometry. Data are representative of three independent experiments
Fig. 2.
Binding to the human FcεRIα subunit. CHO-3D10 or the parental CHO (not expressing human FcεRIα) cells were treated with 1 μg of anti-HER2/neu IgG1 (trastuzumab), NS IgE, or anti-HER2/neu IgE. Binding was detected with an anti-human κ-FITC antibody. Cells were also incubated with an anti-FcεRIα PE-conjugated antibody to verify receptor surface expression. All samples were analyzed by flow cytometry. Each histogram shows cells incubated with labelled secondary antibody alone (gray) and experimental antibody (black). Data are representative of three independent experiments
IgE-mediated in vitro degranulation
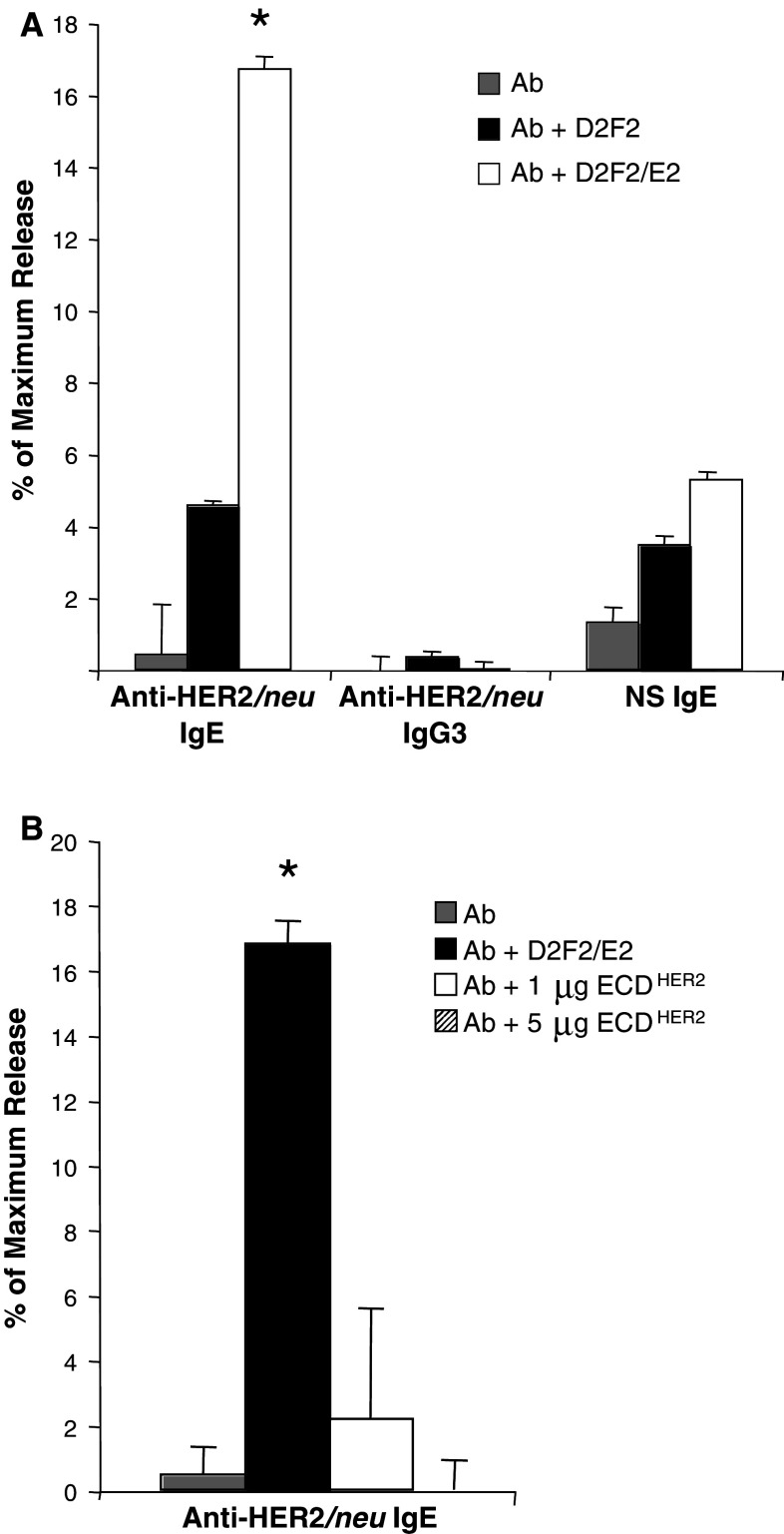
When FcεRI is crosslinked due to the binding of antigens (most commonly known as allergens) to multiple IgE on the surface of an effector cell (either mast cells or basophils), these cells rapidly degranulate, releasing a variety of factors that trigger the acute IgE-mediated type I immediate hypersensitivity (anaphylactic-type) response [15]. We explored the capability of the anti-HER2/neu IgE to induce degranulation of such effector cells in vitro. For this assay, RBL SX-38 rat basophil leukemic cells expressing the entire human FcεRI α, β, and γ subunits) were used [23]. These cells are also appropriate to use in this assay since they are a natural effector cell for this type of reaction. β-hexosaminidase is an enzyme that is contained within the secretory granules of basophils and mast cells and was used to measure degranulation, as reported previously [23, 35]. Allergens are multi-epitopic and induce crosslinking and degranulation of sensitized effector cells. Our IgE is not expected to induce cross-linking of the FcεRI when complexed with soluble antigen, consistent with the interaction of ECDHER2 with the anti-HER2/neu IgE, which is expected to be mono-epitopic in nature, as well as the fact that ECDHER2 does not form homodimers in solution [36]. Our data show that degranulation of RBL SX-38 cells was only observed in the presence of D2F2/E2 cells, which contain multiple copies of the receptor on the cell surface, and not with the D2F2 parental cell line that lacks expression of the antigen (Fig. 3a). Additionally, degranulation was not observed in the presence of the IgE and soluble ECDHER2 (Fig. 3b). These studies were conducted under established conditions in which IgE bound to a multi-epitopic antigen results in extensive basophilic degranulation. Taken together, these studies show that the anti-HER2/neu IgE is functional in vitro.
Fig. 3.
IgE-mediated in vitro degranulation. RBL SX-38 cells were sensitized by a 2-h incubation with either anti-HER2/neu IgE, anti-HER2/neu IgG3, or the NS IgE. The release of β-hexosaminidase into cell supernatants was monitored in the presence of a D2F2/E2 (HER2/neu + cells), D2F2 (HER2/neu − cells), or buffer alone, or b D2F2/E2 cells, soluble ECDHER2, or buffer alone. Data are representative of three separate experiments. Error bars represent SD *P < 0.05 (Student’s t test) compared with either component alone
In vivo passive cutaneous anaphylaxis assay
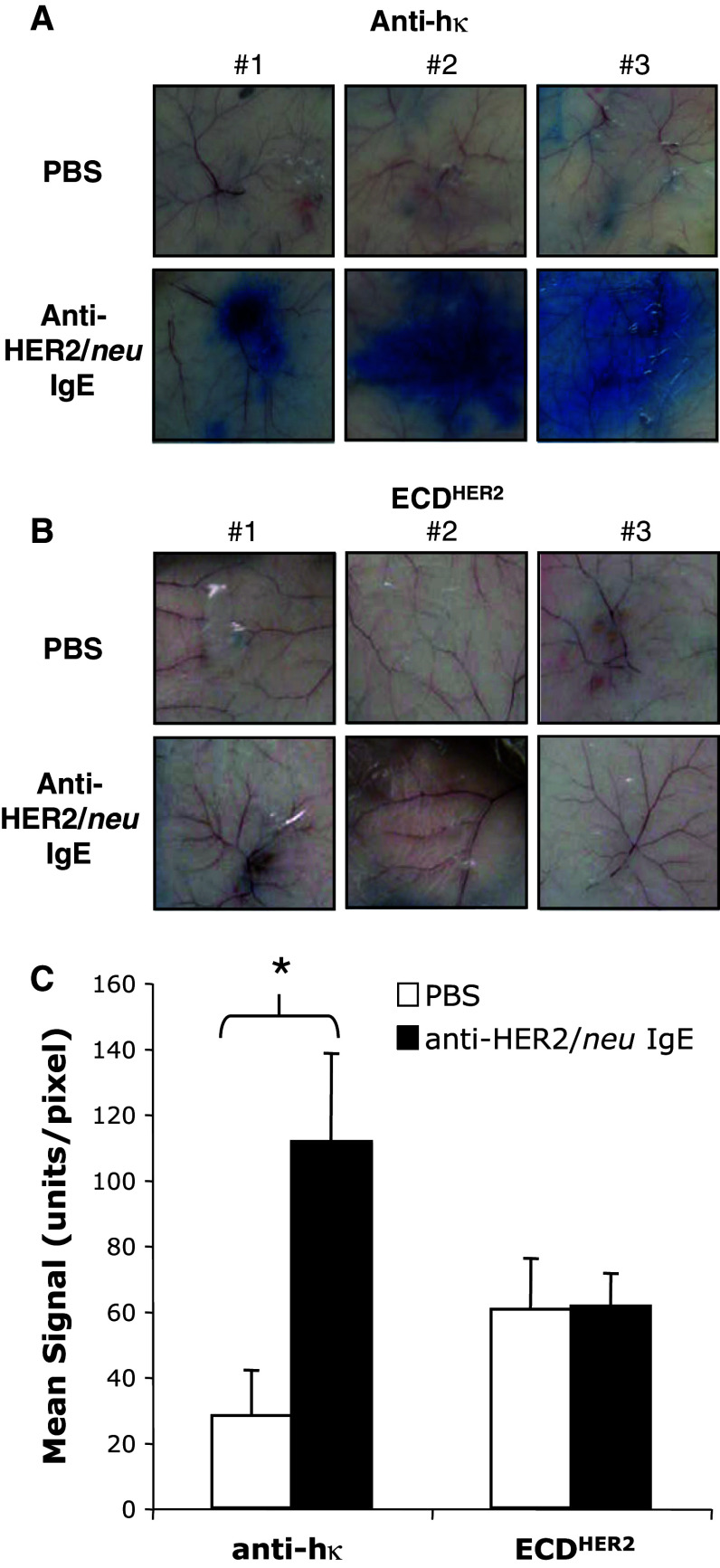
Since human IgE does not interact with murine FcεRI [14], the ability of the anti-HER2/neu IgE to induce a local hypersensitivity type I (anaphylactic) response in vivo was evaluated using human FcεRIα transgenic animals. Sensitization of effector cells in the skin of these animals with anti-HER2/neu IgE lead to a local hypersensitivity reaction after artificial cross-linking by systemic injection of an anti-human κ antibody (Fig. 4a). This IgE-mediated inflammatory reaction is evidenced by the leakage of the Evan’s blue dye into the skin due to vasodilation. This reaction was not observed when ECDHER2 was injected systemically (Fig. 4b), which is consistent with our in vitro data. Leakage of the dye into the skin was quantified and is shown in Fig. 4c. Taken together, these studies indicate that the anti-HER2/neu IgE is capable of inducing a local anaphylactic reaction when adequately crosslinked and further suggest that soluble ECDHER2 will not induce a hypersensitivity reaction.
Fig. 4.
IgE-mediated passive cutaneous anaphylaxis in human FcεRIα transgenic animals. Images of the skin of from mice injected intradermically with PBS or 1 μg anti-HER2/neu IgE in a volume of 50 μl. Panels 1, 2, and 3 show images from three different animals per treatment. After 4 h, a 25 μg of anti-human kappa (hκ) or b 2 μg ECDHER2 was injected in 1% Evan’s Blue in PBS. The mice were euthanized after 10 min. c Leakage of the blue dye into the skin due to a local IgE-induced inflammatory response was assessed using the NIH ImageJ Software (graph corresponds to the images shown). The color intensity is reported as the mean signal per pixel and is the average of three different animals corresponding to the images shown in (A) and (B). *P < 0.01 Student's t test
IgE enhanced in vitro antigen presentation
In humans, in addition to mast cells and basophils, FcεRI is expressed on monocytes, macrophages, and DC [13, 14]. DC are the most potent APC and can process antigens via cross-presentation, allowing the induction of a cell-mediated immune response [37, 38]. Human DC express both FcεRI and FcεRII (CD23) [14, 19] and are capable of interacting with human IgE bound to antigen. IgE-facilitated antigen presentation can occur through binding of human IgE to either CD23 [39] or FcεRI [40]. It has been shown that processing and presentation of the antigen is required for T-cell activation [39]. The ability of cultured human DC isolated from human healthy donors to induce T-cell activation was studied in vitro. Human DC stimulated with the ECDHER2 complexed to the anti-HER2/neu IgG1 slightly increased the activation of IFN-γ producing autologous T cells (Fig. 5). Complexes consisting of ECDHER2 and anti-HER2/neu IgE increased T-cell activation, both for CD4 and CD8 cells (Fig. 5). The activation and expansion of CD8 cells is indicative of cross presentation. These data show that the anti-HER2/neu IgE enhances antigen presentation in vitro and suggest that the antibody is capable of stimulating a secondary T-cell-mediated response that would enhance the in vivo anti-tumor immune response.
Fig. 5.
IgE enhanced antigen presentation and activation of CD8+ and CD4+ T cells. In vitro T-cell activation by human monocyte-derived DC. CD4+ and CD8+ T-cell responses generated by DC armed with ECDHER2 alone or combined with anti-HER2/neu IgG1, anti-HER2/neu IgE, or a NS IgG1. After three rounds of weekly stimulation, responses were measured as numbers of CD4+ and CD8+ T cells producing intracellular IFN-γ. Activated CD8 cells (CD8+/IFN-γ+) are shown in the upper right quadrants and activated CD4 cells (CD8−/IFN-γ+) are shown in the upper left quadrants. Data are representative of two independent experiments
Anti-tumor activity in FcεRIα transgenic mice
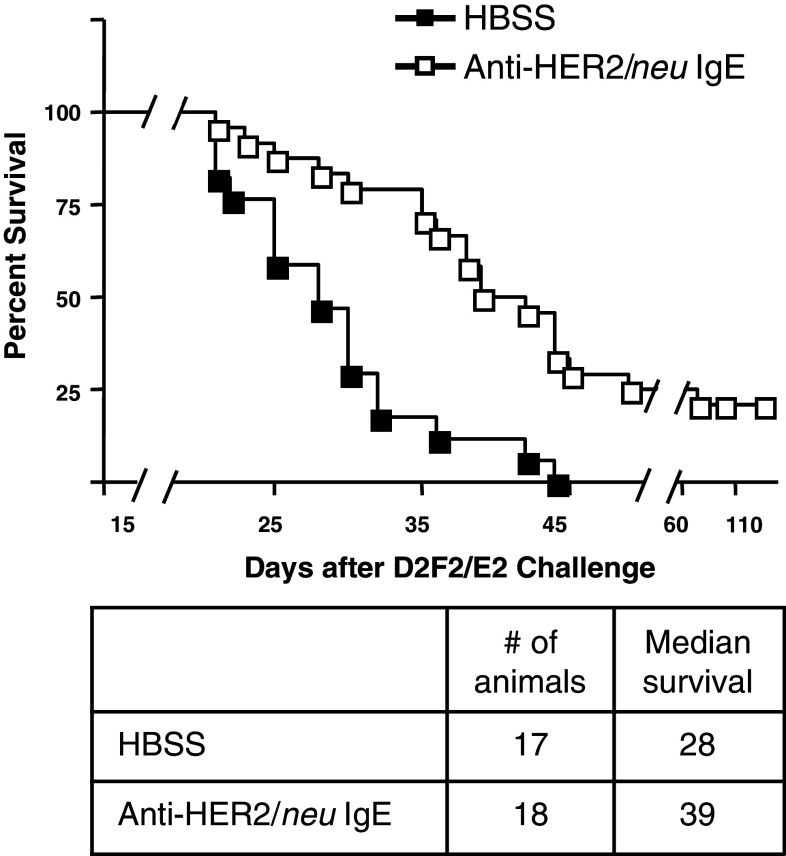
Transgenic mice expressing human FcεRIα were used to study the in vivo anti-cancer activity of the anti-HER2/neu IgE. Two treatments of 100 μg anti-HER2/neu IgE on days 2 and 4 after tumor challenge significantly prolonged the survival (P < 0.001) of mice bearing D2F2/E2 murine mammary carcinoma cells (syngeneic to BALB/c) expressing human HER2/neu compared with buffer alone (Fig. 6). The median survival time was increased from 28 to 39 days. This included 5 long-term survivors that survived beyond 98 days.
Fig. 6.
In vivo anti-cancer activity in human FcεRIα transgenic mice. Survival plot showing survival of animals challenged i.p. with D2F2/E2 mammary carcinoma cells expressing human HER2/neu on day 0. Animals were treated i.p. with 100 μg anti-HER2/neu IgE on day 2 and again on day 4. Data shown are the combined results from three independent experiments
Pilot study in cynomolgus monkeys
Cynomolgus monkeys (M. fascicularis) were chosen to evaluate the overall safety of the anti-HER/neu IgE since human IgE has been shown to mediate anaphylaxis in these animals [41]. Additionally, due to the high sequence homology (about 99%) between human and cynomolgus ECDHER2, this species has been used previously for studies on other HER2/neu-targeted antibodies, including trastuzumab [42–44]. Two doses were chosen for this initial study: a low dose (0.0024 mg/kg) and a high dose (0.08 mg/kg). Serum from the two animals was collected before dosing, as well as 30 min and 1 week after dosing. Serum concentrations 30 min after dosing showed the presence of circulating human anti-HER2/neu IgE (Fig. 7). One week later, no human IgE was detected in the serum of the animals. This decrease is not surprising due to the serum half-life of IgE (2 days), which is short compared with IgG (21 days) [45]. No changes in eating habits or general health were observed for 1 week after dosing. This preliminary study shows that the anti-HER2/neu IgE can be safely infused into cynomolgus monkeys and that the antibody redistributes out of the circulation, presumably to the tissues where it is bound to the FcεRs or HER2/neu.
Fig. 7.
Pilot study in M. fascicularis (cynomolgus) monkeys. Single intravenous infusion of the anti-HER2/neu IgE at 0.0024 mg/kg and at 0.08 mg/kg over 20 min. Anti-HER2/neu IgE serum levels predose, 30-min post-infusion, and at 1 week post infusion are shown. LLQ lowest limit of quantification
Discussion
Most therapeutic antibodies used in the clinic for cancer therapy are of the IgG isotype [46]. However, antibodies of the IgE isotype also have the potential to be particularly useful for this purpose. Evaluation of the anti-cancer efficacy of IgE molecules was pioneered by Nagy et al. [47] who developed a murine monoclonal IgE specific for the major envelope glycoprotein (gp36) of mouse mammary tumor virus (MMTV) and demonstrated its in vivo efficacy. Since then, other anti-cancer IgE molecules have been developed. Kershaw et al. [48] developed a murine monoclonal IgE (30.6) specific for an antigenic determinant expressed on colorectal adenocarcinoma cells. Mouse IgE 30.6 transiently inhibited the growth of established human colorectal carcinoma COLO 205 cells growing subcutaneously in severe combined immune deficient (SCID) mice. By contrast, a mouse IgG 30.6 and a mouse/human chimeric IgE 30.6 did not show anti-tumor effect. The lack of effect exhibited by the mouse/human chimeric IgE 30.6 is expected since mouse FcεRI does not bind human IgE [14, 31, 32]. Gould et al. [49–51] developed a mouse/human chimeric IgE (MOv18-IgE) and IgG MOv18 (IgG1) specific for the ovarian cancer antigen folate binding protein. In a SCID mouse xenograft model of human ovarian carcinoma, mice were reconstituted with human PBMC to provide effector cells capable of binding human IgE. The beneficial effects of MOv18-IgE were greater and of longer duration than those of MOv18-IgG1. More recently, a humanized anti-HER2/neu IgE with the variable regions of trastuzumab (Herceptin®) was developed and produced transiently in human embryonic kidney (HEK293) cells. This IgE was capable of triggering basophilic cell degranulation and antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro in the presence of human HER2/neu expressing cells [35].
We now report the development of a new fully human anti-HER2/neu IgE, expressed in murine myeloma cells, that does not compete with trastuzumab for binding to HER2/neu [52]. A murine myeloma cell-based expression system was used since it is a reliable and permanent source of antibody. Additionally, several FDA-approved therapeutic antibodies have been expressed using murine myeloma cells [53]. Obviously, this does not preclude the use of other expression systems in the future if deemed necessary. We have found that this IgE binds antigen, binds effector cells, and that the Fc region is active since it can induce degranulation of FcεRI bearing cells both in vitro and in vivo. This suggests that the IgE is capable of mediating other Fc effector functions such as ADCC and ADCP (antibody-dependent cell-mediated phagocytosis) as has been described for other IgE [35, 54]. Mast cells often infiltrate tumors and in some cases support angiogenesis and tumor growth [55]. However, it has also been shown that mast cells can modulate regulatory T cell and DC function leading to a potent T-cell-mediated immune response [56, 57]. Our data suggests that in the tumor microenvironment, the anti-HER2/neu IgE may induce an acute inflammatory response leading to an anti-tumor effect. Even binding of the IgE to dead cells or their fragments in the tumor microenvironment may contribute to such an acute inflammatory response, which through the bystander effect may damage live tumor cells and their supportive stromal cells. Further studies are needed to fully examine the effect of the IgE and FcεR-bearing cells including mast cells in the complex tumor microenvironment.
A major concern of the systemic use of an IgE molecule that targets a self-protein for therapeutic purposes is the possible induction of a systemic hypersensitivity (anaphylactic) reaction. This is a particular concern since patients with breast and ovarian cancer that overexpress HER2/neu often have elevated levels of circulating ECDHER2 in the blood [58–61]. However, consistent with a mono-epitopic interaction with the antigen, we did not observe in vitro or in vivo degranulation in the presence of ECDHER2. A serum concentration of ECDHER2 above 15 ng/ml is considered to be elevated in cancer patients, with highly elevated levels being above 100 ng/ml [58, 62]. The average concentration in the serum of patients with advanced disease is 35–40 ng/ml [58, 59]. Even at the high concentration of ECDHER2 (which is equivalent to 1–2 μg/ml based on the blood volume of a mouse) used in our in vivo studies, soluble ECDHER2 did not induce a local hypersensitivity reaction. Additionally, we have also observed in preliminary studies that systemic injection of complexes of ECDHER2 and anti-HER2/neu IgE (with fourfold molar excess of ECDHER2) or systemic injection of 100 μg of the anti-HER2/neu IgE followed 4 h later by systemic injection of 38 μg of ECDHER2 did not induce systemic anaphylactic shock and were well tolerated in human FcεRIα transgenic mice. Furthermore, no systemic reactions or other unexpected adverse events were evident in cynomolgus monkeys injected with up to 0.08 mg/kg of the anti-HER2/neu IgE despite the fact that HER2/neu is expressed in many normal tissues and a basal level of ECDHER2 can be found in the blood of healthy individuals [7, 63]. Although we used relatively low doses for the monkey study, they were based on previous studies in humans using different immune stimulating therapeutic antibodies [64–66]. Additionally, it is the first study carried out in this animal model, and it serves as a starting point for further toxicity, pharmacokinetic, and biodistribution studies. Our data suggest that a systemic anaphylactic reaction may not be induced by circulating soluble antigen, even in cancer patients with high serum levels of ECDHER2. We have demonstrated for the first time that systemic infusion of IgE targeting a tumor-associated antigen is feasible in non-human primates. Although additional cautious studies are required, our initial results suggest that the anti-HER2/neu IgE has a potential manageable safety profile allowing the antibody to mobilize novel immune effector mechanisms to fight cancer.
IgE, through the interaction with FcεRI, has been shown to act as an adjuvant in a tumor vaccination setting [67]. This study showed that vaccination with IgE-coated tumor cells protected animals against subsequent tumor challenge. ECDHER2 alone is known to be poorly immunogenic in patients [68]. Our results show that the anti-HER2/neu IgE, when complexed with soluble ECDHER2, stimulates more IFN-γ production than IgG complexed with that antigen. This suggests that it is more effective in antigen presentation (and the subsequent activation of T cells) when compared with the IgG molecule under these conditions. However, further studies are needed to confirm these results. Thus, our studies support the adjuvant effect of IgE and suggest that the interaction of anti-HER2/neu IgE with circulating ECDHER2 in cancer patients may elicit an anti-HER2/neu T-cell response through increased antigen presentation and ultimately anti-cancer immunity. It is also expected that binding of the IgE to HER2/neu on cancer cells, apoptotic cells, and even membrane fragments resulting from tumor cell lysis can facilitate their uptake by APC, thereby enhancing antigen presentation and the secondary immune response. It has been previously shown that IgE complexed with a mono-epitopic antigen through its interaction with CD23/FcεRII is capable of mediating antigen presentation in the absence of receptor cross-linking [69]. Additionally, both mast cells [70] and basophils [71] have been shown to act as APC of antigen bound to IgE. Further studies are needed to determine the potential involvement of APC and their FcεRs in the anti-cancer effect of our anti-HER2/neu IgE.
In the current study, an in vivo anti-cancer effect was observed in a syngeneic model of intraperitoneal tumors in human FcεRIα transgenic mice. Breast cancer patients may present with peritoneal carcinomatosis as part of the metastasis process [72, 73]. Most ovarian cancers are epithelial tumors that can slough off the ovary and enter the peritoneal cavity [74]. We observed significant protection in the peritoneal cancer model, suggesting that the anti-HER2/neu IgE may be potentially useful for the treatment of peritoneal cancers. Since our IgE showed no direct in vitro cytotoxicity against D2F2/E2 cells (Daniels et al., unpublished data), our data suggest that the anti-cancer effect observed in vivo is due to antibody Fc effector functions. It is important to note that the animals are only transgenic for human FcεRIα. Since human IgE does not interact with mouse CD23/FcεRII [33], the role of this receptor in the anti-tumor effects mediated by the anti-HER2/neu IgE could not be evaluated. Thus, the overall effects of the IgE may be underestimated. However, additional studies are needed to understand the mechanism of the observed in vivo anti-cancer activity and to compare this efficacy with that of anti-HER/neu IgG.
In summary, our studies describe a totally human anti-HER2/neu IgE molecule that demonstrates in vitro activity and anti-tumor protection in mice, while being well tolerated in animal models. Although further evaluation of the anti-cancer effect and safety profile of this molecule is warranted, our results suggest the potential use of this novel molecule as a therapy of HER2/neu overexpressing cancers, such as breast and ovarian cancer. Importantly, this IgE can potentially be used in combination treatment strategies with various other anti-cancer agents in order to achieve a maximal therapeutic effect.
Acknowledgments
We thank Lin Wang, Julie Lucas, and Sara Buczynski (AIT, Inc.) for their assistance with this project. We also thank Dr. Andrew Saxon, Dr. Ke Zhang, Dr. Sherie L. Morrison, Ryan K.Trinh, and Letitia A. Wims (UCLA) for their support to this project and Dr. Gang Li and Xuyang Lu (UCLA) for statistical consultation. This work was funded in part by NIH/NCI R41CA137881, R01CA136841, R01CA121195, K01CA138559, R01CA57152, ANPCyT-FONARSEC PICT-PRH 2008-00315, the Susan G. Komen Breast Cancer Foundation Basic, Clinical and Translational Research Grant BCTR0706771, and by Advanced Immune Therapeutics, Inc. The UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility is supported by the NIH Awards CA16042 and AI28697, the Jonsson Cancer Center, the UCLA AIDS Institute, and the UCLA School of Medicine. Gustavo Helguera is member of the National Council for Scientific and Technological Research (CONICET), Argentina.
Conflict of interest
CFN and BCS are advisors to and own shares in Advanced Immune Therapeutics, Inc. All other authors have no conflicts to disclose.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Releas. 2010;146:264–275. doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 5.Meden H, Marx D, Rath W, Kron M, Fattahi-Meibodi A, Hinney B, Kuhn W, Schauer A. Overexpression of the oncogene c-erb B2 in primary ovarian cancer: evaluation of the prognostic value in a Cox proportional hazards multiple regression. Int J Gynecol Pathol. 1994;13:45–53. doi: 10.1097/00004347-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, Soper JT, Dodge R, Clarke-Pearson DL, Marks P, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50:4087–4091. [PubMed] [Google Scholar]
- 7.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 8.Ahn ER, Vogel CL (2011) Dual HER2-targeted approaches in HER2-positive breast cancer. Breast Cancer Res Treat [Epub ahead of print] [DOI] [PubMed]
- 9.Nahta R, Shabaya S, Ozbay T, Rowe DL. Personalizing HER2-targeted therapy in metastatic breast cancer beyond HER2 status: what we have learned from clinical specimens. Curr Pharmacogenomics Pers Med. 2009;7:263–274. doi: 10.2174/187569209790112337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez Y, Cueva J, Palomo AG, Ramos M, de Juan A, Calvo L, Garcia-Mata J, Garcia-Teijido P, Pelaez I, Garcia-Estevez L. Novel therapeutic approaches to the treatment of metastatic breast cancer. Cancer Treat Rev. 2010;36:33–42. doi: 10.1016/j.ctrv.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the gynecologic oncology group. J Clin Oncol. 2003;21:283–290. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 12.Jensen-Jarolim E, Achatz G, Turner MC, Karagiannis S, Legrand F, Capron M, Penichet ML, Rodriguez JA, Siccardi AG, Vangelista L, Riemer AB, Gould H. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63:1255–1266. doi: 10.1111/j.1398-9995.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniels TR, Rodriguez JA, Ortiz-Sanchez, Helguera G, Penichet ML (2010) The IgE antibody and its use in cancer immunotherapy. In: Penichet ML, Jensen-Jarolim E (eds) Cancer and IgE: introducing the concept of allergooncology. New York, Springer, pp 159–184
- 14.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 15.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe N, Bruschi F, Korenaga M. IgE: a question of protective immunity in Trichinella spiralis infection. Trends Parasitol. 2005;21:175–178. doi: 10.1016/j.pt.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Cooper PJ, Ayre G, Martin C, Rizzo JA, Ponte EV, Cruz AA. Geohelminth infections: a review of the role of IgE and assessment of potential risks of anti-IgE treatment. Allergy. 2008;63:409–417. doi: 10.1111/j.1398-9995.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 18.Penichet ML, Jensen-Jarolim E. Cancer and IgE: Introducing the Concept of AllergoOncology. New York: Springer; 2010. [Google Scholar]
- 19.Conrad DH. Fc epsilon RII/CD23: the low affinity receptor for IgE. Annu Rev Immunol. 1990;8:623–645. doi: 10.1146/annurev.iy.08.040190.003203. [DOI] [PubMed] [Google Scholar]
- 20.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 21.Wei WZ, Shi WP, Galy A, Lichlyter D, Hernandez S, Groner B, Heilbrun L, Jones RF. Protection against mammary tumor growth by vaccination with full-length, modified human ErbB-2 DNA. Int J Cancer. 1999;81:748–754. doi: 10.1002/(SICI)1097-0215(19990531)81:5<748::AID-IJC14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Hakimi J, Seals C, Kondas JA, Pettine L, Danho W, Kochan J. The alpha subunit of the human IgE receptor (FcERI) is sufficient for high affinity IgE binding. J Biol Chem. 1990;265:22079–22081. [PubMed] [Google Scholar]
- 23.Wiegand TW, Williams PB, Dreskin SC, Jouvin MH, Kinet JP, Tasset D. High-affinity oligonucleotide ligands to human IgE inhibit binding to Fc epsilon receptor I. J Immunol. 1996;157:221–230. [PubMed] [Google Scholar]
- 24.Schier R, McCall A, Adams GP, Marshall KW, Merritt H, Yim M, Crawford RS, Weiner LM, Marks C, Marks JD. Isolation of picomolar affinity anti-c-erbB-2 single-chain Fv by molecular evolution of the complementarity determining regions in the center of the antibody binding site. J Mol Biol. 1996;263:551–567. doi: 10.1006/jmbi.1996.0598. [DOI] [PubMed] [Google Scholar]
- 25.Schier R, Marks JD, Wolf EJ, Apell G, Wong C, McCartney JE, Bookman MA, Huston JS, Houston LL, Weiner LM, Adams GP. In vitro and in vivo characterization of a human anti-c-erbB-2 single-chain Fv isolated from a filamentous phage antibody library. Immunotechnology. 1995;1:73–81. doi: 10.1016/1380-2933(95)00007-0. [DOI] [PubMed] [Google Scholar]
- 26.Lyczak JB, Zhang K, Saxon A, Morrison SL. Expression of novel secreted isoforms of human immunoglobulin E proteins. J Biol Chem. 1996;271:3428–3436. doi: 10.1074/jbc.271.7.3428. [DOI] [PubMed] [Google Scholar]
- 27.Helguera G, Penichet ML. Antibody-cytokine fusion proteins for the therapy of cancer. Methods Mol Med. 2005;109:347–374. doi: 10.1385/1-59259-862-5:347. [DOI] [PubMed] [Google Scholar]
- 28.Huang TH, Morrison SL. A trimeric anti-HER2/neu ScFv and tumor necrosis factor-alpha fusion protein induces HER2/neu signaling and facilitates repair of injured epithelia. J Pharmacol Exp Ther. 2006;316:983–991. doi: 10.1124/jpet.105.095513. [DOI] [PubMed] [Google Scholar]
- 29.Dela Cruz JS, Lau SY, Ramirez EM, De Giovanni C, Forni G, Morrison SL, Penichet ML. Protein vaccination with the HER2/neu extracellular domain plus anti-HER2/neu antibody-cytokine fusion proteins induces a protective anti-HER2/neu immune response in mice. Vaccine. 2003;21:1317–1326. doi: 10.1016/S0264-410X(02)00741-7. [DOI] [PubMed] [Google Scholar]
- 30.Dombrowicz D, Lin S, Flamand V, Brini AT, Koller BH, Kinet JP. Allergy-associated FcRbeta is a molecular amplifier of IgE- and IgG-mediated in vivo responses. Immunity. 1998;8:517–529. doi: 10.1016/S1074-7613(00)80556-7. [DOI] [PubMed] [Google Scholar]
- 31.Fung-Leung WP, De Sousa-Hitzler J, Ishaque A, Zhou L, Pang J, Ngo K, Panakos JA, Chourmouzis E, Liu FT, Lau CY. Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor alpha chain respond to human IgE in mast cell degranulation and in allergic reactions. J Exp Med. 1996;183:49–56. doi: 10.1084/jem.183.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dombrowicz D, Brini AT, Flamand V, Hicks E, Snouwaert JN, Kinet JP, Koller BH. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157:1645–1651. [PubMed] [Google Scholar]
- 33.Bettler B, Hofstetter H, Rao M, Yokoyama WM, Kilchherr F, Conrad DH. Molecular structure and expression of the murine lymphocyte low-affinity receptor for IgE (Fc epsilon RII) Proc Natl Acad Sci USA. 1989;86:7566–7570. doi: 10.1073/pnas.86.19.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat Med. 2002;8:518–521. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karagiannis P, Singer J, Hunt J, Gan SK, Rudman SM, Mechtcheriakova D, Knittelfelder R, Daniels TR, Hobson PS, Beavil AJ, Spicer J, Nestle FO, Penichet ML, Gould HJ, Jensen-Jarolim E, Karagiannis SN. Characterisation of an engineered trastuzumab IgE antibody and effector cell mechanisms targeting HER2/neu-positive tumour cells. Cancer Immunol Immunother. 2009;58:915–930. doi: 10.1007/s00262-008-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson KM, Darling PJ, Mohan MJ, Macatee TL, Lemmon MA. Extracellular domains drive homo- but not hetero-dimerization of erbB receptors. EMBO J. 2000;19:4632–4643. doi: 10.1093/emboj/19.17.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. J Clin Invest. 2002;110:71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–368. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 39.van der Heijden FL, Joost van Neerven RJ, van Katwijk M, Bos JD, Kapsenberg ML. Serum-IgE-facilitated allergen presentation in atopic disease. J Immunol. 1993;150:3643–3650. [PubMed] [Google Scholar]
- 40.Maurer D, Ebner C, Reininger B, Petzelbauer P, Fiebiger E, Stingl G. Mechanisms of Fc epsilon RI-IgE-facilitated allergen presentation by dendritic cells. Adv Exp Med Biol. 1997;417:175–178. [PubMed] [Google Scholar]
- 41.Weichman BM, Hostelley LS, Bostick SP, Muccitelli RM, Krell RD, Gleason JG. Regulation of the synthesis and release of slow-reacting substance of anaphylaxis from sensitized monkey lung. J Pharmacol Exp Ther. 1982;221:295–302. [PubMed] [Google Scholar]
- 42.Adams CW, Allison DE, Flagella K, Presta L, Clarke J, Dybdal N, McKeever K, Sliwkowski MX. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother. 2006;55:717–727. doi: 10.1007/s00262-005-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Junutula JR, Flagella KM, Graham RA, Parsons KL, Ha E, Raab H, Bhakta S, Nguyen T, Dugger DL, Li G, Mai E, Lewis Phillips GD, Hiraragi H, Fuji RN, Tibbitts J, Vandlen R, Spencer SD, Scheller RH, Polakis P, Sliwkowski MX. Engineered thio-trastuzumab-DM1 conjugate with an improved therapeutic index to target human epidermal growth factor receptor 2-positive breast cancer. Clin Cancer Res. 2010;16:4769–4778. doi: 10.1158/1078-0432.CCR-10-0987. [DOI] [PubMed] [Google Scholar]
- 44.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blattler WA, Lambert JM, Chari RV, Lutz RJ, Wong WL, Jacobson FS, Koeppen H, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 45.Janeway CA, Travers P, Walport M, Shlomchik M. The humoral immune response. In Immunobiology: the immune system in health and disease. New York: Garland Science Publishing; 2005. pp. 367–406. [Google Scholar]
- 46.Helguera G, Daniels TR, Rodriguez JA., Penichet ML (2010) Monoclonal antibodies, Human engineered. In: Flickinger M (ed) Encyclopedia of industrial biotechnology: bioprocess, bioseparation, and cell technology. New York, John Wiley & Sons, Inc
- 47.Nagy E, Berczi I, Sehon AH. Growth inhibition of murine mammary carcinoma by monoclonal IgE antibodies specific for the mammary tumor virus. Cancer Immunol Immunother. 1991;34:63–69. doi: 10.1007/BF01741326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kershaw MH, Darcy PK, Trapani JA, MacGregor D, Smyth MJ. Tumor-specific IgE-mediated inhibition of human colorectal carcinoma xenograft growth. Oncol Res. 1998;10:133–142. [PubMed] [Google Scholar]
- 49.Gould HJ, Mackay GA, Karagiannis SN, O’Toole CM, Marsh PJ, Daniel BE, Coney LR, Zurawski VR, Jr, Joseph M, Capron M, Gilbert M, Murphy GF, Korngold R. Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur J Immunol. 1999;29:3527–3537. doi: 10.1002/(SICI)1521-4141(199911)29:11<3527::AID-IMMU3527>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 50.Karagiannis SN, Bracher MG, Hunt J, McCloskey N, Beavil RL, Beavil AJ, Fear DJ, Thompson RG, East N, Burke F, Moore RJ, Dombrowicz DD, Balkwill FR, Gould HJ. IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J Immunol. 2007;179:2832–2843. doi: 10.4049/jimmunol.179.5.2832. [DOI] [PubMed] [Google Scholar]
- 51.Karagiannis SN, Wang Q, East N, Burke F, Riffard S, Bracher MG, Thompson RG, Durham SR, Schwartz LB, Balkwill FR, Gould HJ. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur J Immunol. 2003;33:1030–1040. doi: 10.1002/eji.200323185. [DOI] [PubMed] [Google Scholar]
- 52.Tang Y, Lou J, Alpaugh RK, Robinson MK, Marks JD, Weiner LM. Regulation of antibody-dependent cellular cytotoxicity by IgG intrinsic and apparent affinity for target antigen. J Immunol. 2007;179:2815–2823. doi: 10.4049/jimmunol.179.5.2815. [DOI] [PubMed] [Google Scholar]
- 53.Yoo EM, Chintalacharuvu KR, Penichet ML, Morrison SL. Myeloma expression systems. J Immunol Methods. 2002;261:1–20. doi: 10.1016/S0022-1759(01)00559-2. [DOI] [PubMed] [Google Scholar]
- 54.Karagiannis SN, Bracher MG, Beavil RL, Beavil AJ, Hunt J, McCloskey N, Thompson RG, East N, Burke F, Sutton BJ, Dombrowicz D, Balkwill FR, Gould HJ. Role of IgE receptors in IgE antibody-dependent cytotoxicity and phagocytosis of ovarian tumor cells by human monocytic cells. Cancer Immunol Immunother. 2008;57:247–263. doi: 10.1007/s00262-007-0371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Vries VC, Wasiuk A, Bennett KA, Benson MJ, Elgueta R, Waldschmidt TJ, Noelle RJ. Mast cell degranulation breaks peripheral tolerance. Am J Transplant. 2009;9:2270–2280. doi: 10.1111/j.1600-6143.2009.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wasiuk A, de Vries VC, Nowak EC.,Noelle RJ (2010) Mast cells in allergy and tumor disease. In: Penichet ML, Jensen-Jarolim E (eds) Cancer and IgE: introducing the concept of AllergoOncology. New York, Springer, pp 137–158
- 58.Lennon S, Barton C, Banken L, Gianni L, Marty M, Baselga J, Leyland-Jones B. Utility of serum HER2 extracellular domain assessment in clinical decision making: pooled analysis of four trials of trastuzumab in metastatic breast cancer. J Clin Oncol. 2009;27:1685–1693. doi: 10.1200/JCO.2008.16.8351. [DOI] [PubMed] [Google Scholar]
- 59.Garoufali A, Kyriakou F, Kountourakis P, Yioti I, Malliou S, Nikaki A, Kardara E, Frangos I, Koumna S, Baziotis N, Scorilas A, Ardavanis A. Extracellular domain of HER2: a useful marker for the initial workup and follow-up of HER2-positive breast cancer. J Buon. 2008;13:409–413. [PubMed] [Google Scholar]
- 60.Cheung TH, Wong YF, Chung TK, Maimonis P, Chang AM. Clinical use of serum c-erbB-2 in patients with ovarian masses. Gynecol Obstet Invest. 1999;48:133–137. doi: 10.1159/000010155. [DOI] [PubMed] [Google Scholar]
- 61.Meden H, Marx D, Fattahi A, Rath W, Kron M, Wuttke W, Schauer A, Kuhn W. Elevated serum levels of a c-erbB-2 oncogene product in ovarian cancer patients and in pregnancy. J Cancer Res Clin Oncol. 1994;120:378–381. doi: 10.1007/BF01247465. [DOI] [PubMed] [Google Scholar]
- 62.Hoopmann M, Sachse K, Valter MM, Becker M, Neumann R, Ortmann M, Gohring UJ, Thomas A, Mallmann P, Schondorf T. Serological and immunohistochemical HER-2/neu statuses do not correlate and lack prognostic value for ovarian cancer patients. Eur J Cancer Care (Engl) 2010;19:809–815. doi: 10.1111/j.1365-2354.2009.01112.x. [DOI] [PubMed] [Google Scholar]
- 63.Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta. 2008;1785:232–265. doi: 10.1016/j.bbcan.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol. 2009;27:418–425. doi: 10.1200/JCO.2008.17.8400. [DOI] [PubMed] [Google Scholar]
- 65.King DM, Albertini MR, Schalch H, Hank JA, Gan J, Surfus J, Mahvi D, Schiller JH, Warner T, Kim K, Eickhoff J, Kendra K, Reisfeld R, Gillies SD, Sondel P. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol. 2004;22:4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braly P, Nicodemus CF, Chu C, Collins Y, Edwards R, Gordon A, McGuire W, Schoonmaker C, Whiteside T, Smith LM, Method M. The Immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J Immunother. 2009;32:54–65. doi: 10.1097/CJI.0b013e31818b3dad. [DOI] [PubMed] [Google Scholar]
- 67.Nigro EA, Brini AT, Soprana E, Ambrosi A, Dombrowicz D, Siccardi AG, Vangelista L. Antitumor IgE adjuvanticity: key role of Fc epsilon RI. J Immunol. 2009;183:4530–4536. doi: 10.4049/jimmunol.0900842. [DOI] [PubMed] [Google Scholar]
- 68.Disis ML, Gralow JR, Bernhard H, Hand SL, Rubin WD, Cheever MA. Peptide-based, but not whole protein, vaccines elicit immunity to HER-2/neu, oncogenic self-protein. J Immunol. 1996;156:3151–3158. [PubMed] [Google Scholar]
- 69.Bheekha Escura R, Wasserbauer E, Hammerschmid F, Pearce A, Kidd P, Mudde GC. Regulation and targeting of T-cell immune responses by IgE and IgG antibodies. Immunology. 1995;86:343–350. [PMC free article] [PubMed] [Google Scholar]
- 70.Gong J, Yang NS, Croft M, Weng IC, Sun L, Liu FT, Chen SS. The antigen presentation function of bone marrow-derived mast cells is spatiotemporally restricted to a subset expressing high levels of cell surface FcepsilonRI and MHC II. BMC Immunol. 2010;11:34. doi: 10.1186/1471-2172-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 72.Satoh H, Ishikawa H, Yamashita YT, Kurishima K, Ohtsuka M, Sekizawa K. Peritoneal carcinomatosis in lung cancer patients. Oncol Rep. 2001;8:1305–1307. doi: 10.3892/or.8.6.1305. [DOI] [PubMed] [Google Scholar]
- 73.Hanbidge AE, Lynch D, Wilson SR. US of the peritoneum. Radiographics. 2003;23:663–684. doi: 10.1148/rg.233025712. [DOI] [PubMed] [Google Scholar]
- 74.Ozols RF, Schwartz PE, Eifel PJ (2001) Ovarian cancer, fallopian tube carcinoma, and peritoneal carcinoma. In: DeVita VT, Hellman S, Rosenberg SA (eds) Cancer: principles and practice of oncology. Philadelphia, PA, Lippincott Williams & Wilkins, pp 1597–1632