Abstract
It is known that bone morphogenetic proteins (BMPs) regulate gonadotropin transcription and production by pituitary gonadotrope cells. However, the role of BMPs in gonadotropin-releasing hormone (GnRH)-induced FSH production remains uncertain. Here, we describe a functional link between BMP-6 and BMP-7 signals and FSH transcriptional activity induced by GnRH using mouse gonadotrope LβT2 cells. In LβT2 cells, BMP-6 and BMP-7 increased mouse FSHβ-promoter activity in a concentration-dependent manner. The induction by BMP-6 and BMP-7 was inhibited by treatment with extracellular domains of ActRII but not BMPRII. These findings suggest that the type II receptor ActRII participates in BMP-induced FSHβ transcription regulation. Notably, BMP-6, but not BMP-7, enhanced GnRH-induced FSHβ-promoter activity in LβT2 cells. Since GnRH stimulated MAPK phosphorylation in LβT2 cells, a functional link between MAPK and FSHβ transcription was examined. Inhibition of the ERK pathway, but not that of p38 or SAPK/JNK signaling, suppressed GnRH-induced FSHβ transcription, suggesting that ERK is functionally involved in GnRH-induced FSHβ transcription. Co-treatment with BMP-7, but not with BMP-6, suppressed GnRH-induced MAPK phosphorylation in LβT2 cells. Thus, the difference between BMP-6 and BMP-7 in enhancing GnRH-induced FSHβ transcription may be due to the differential effects of BMP ligands on GnRH-induced ERK signaling. On the other hand, GnRH reduced Smad1/5/8 phosphorylation but increased Smad6/7 expression. These findings imply the presence of a functional link between GnRH action, MAPK signaling and the BMP system in pituitary gonadotropes for fine-tuning of FSH gene expression.
Keywords: Bone morphogenetic protein, Follicle-stimulating hormone (FSH), Gonadotrope, Gonadotropin-releasing hormone (GnRH), LβT2 cells
1. Introduction
Reproduction in mammals is centrally controlled by the physiologic coordination of episodic release of gonadotropin-releasing hormone (GnRH) and concomitant secretion of pituitary gonadotropins. GnRH is a highly conserved decapeptide that is secreted by a diffused population of cells located in the hypothalamus. The pattern of GnRH secretion is regulated by intrinsic oscillatory activity of GnRH neurons and the integration of presynaptic inputs of various neurotransmitters. Furthermore, GnRH synthesis and release are tightly regulated by gonadal steroids, which maintain control through a negative feedback system (Petersen et al., 2003).
It is known that the bone morphogenetic protein (BMP) system in the ovary including BMP-15 plays a key role in the regulation of gonadotropin-induced steroidogenesis as well as mitosis of granulosa cells (Shimasaki et al., 2004; Otsuka et al., 2011). On the basis of extensive studies using rat and mouse ovaries (Otsuka et al., 2000, 2001; McMahon et al., 2008), BMP-15 is most likely to regulate follicle-stimulating hormone (FSH) receptor signaling leading to normal follicular development in the ovary with prevention of immature ovulation, although there may be species differences in the effects of BMP-15 on the control of FSH receptor signaling (McNatty et al., 2009).
Recent studies have shown that BMPs are directly involved in the regulation of gonadotrope function. Huang and colleagues first reported that BMP-6 and BMP-7 stimulate FSH synthesis and secretion in the mouse gonadotrope cell line LβT2 (Huang et al., 2001). Subsequently, it was also reported that BMP-15 selectively stimulates FSH biosynthesis and secretion by rat primary pituitary cells without affecting luteinizing hormone (LH) secretion or GnRH receptor (GnRH-R) transcription (Otsuka and Shimasaki, 2002). In gonadotropes, GnRH induces an antiproliferative response that is further modified by activin, suggesting that transforming growth factor (TGF)-β signaling cooperates with GnRH to provide the appropriate differentiated phenotype (Zhang et al., 2006). There is also increasing evidence that locally produced BMPs play a critical role in the pathogenesis of human pituitary tumors (Labeur et al., 2010). In this regard, we previously reported the characteristics of human FSH-producing adenomas compared with those of nonfunctioning adenomas and showed that the expression of follistatin, which neutralizes BMP/activin actions by binding, was decreased in human FSH-producing adenomas (Takeda et al., 2003). Thus, the pituitary BMP system is likely to act as a major functional regulator for pituitary cells in a cell-specific manner.
It is notable that the pituitary BMP system is substantially involved in FSH production by gonadotropes (Huang et al., 2001; Otsuka and Shimasaki, 2002; Faure et al., 2005; Lee et al., 2007; Nicol et al., 2008; Young et al., 2008), albeit there are species differences in BMP actions on FSH secretory responses. BMPs play a crucial role in female reproduction not only by regulating ovarian FSH responsiveness but also by activating pituitary gonadotropin secretion in an autocrine/paracrine manner (Shimasaki et al., 2004; Otsuka, 2010). Therefore, communication between the ovary and the central nervous system including the hypothalamus–pituitary axis ensures that neural signaling for ovulation occurs when ovarian follicles are fully matured. However, the mechanism of BMP action in GnRH-induced FSH production remains uncertain.
Here we examined the functional link between BMP signals and FSH transcriptional activity induced by GnRH using mouse gonadotrope LβT2 cells. Our results demonstrate the presence of a functional link between GnRH action and MAPK and BMP signaling in pituitary gonadotropes that may allow fine-tuning of FSH gene expression and production.
2. Materials and methods
2.1. Reagents and supplies
Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin–streptomycin solution, dimethylsulfoxide (DMSO) and luteinizing hormone releasing hormone (LHRH/GnRH) human acetate salt were purchased from Sigma-Aldrich Co. Ltd. (St. Louis, MO). Recombinant human BMP-6 and BMP-7 and extracellular domains (ECDs) that lack transmembrane and intracellular domains of human ActRII and BMPRII (Moore et al., 2003; Inagaki et al., 2006) were purchased from R&D Systems, Inc. (Minneapolis, MN). The ERK inhibitor U0126 and the p38-MAPK inhibitor SB203580 were purchased from Promega Corp. (Madison, WI), and the SAPK/JNK inhibitor SP600125 was from Biomol Lab. Inc. (Plymouth Meeting, PA). LβT2 cells were provided by Dr. Pamela L. Mellon, University of California, San Diego. A promoter region (−1526 bp) of the mouse FSHβ gene (mFSHβ) was cloned into pGL3-Basic luciferase reporter plasmid at the KpnI and HindIII sites (Promega Corp., Madison, WI), which was named mFSHβ-Luc (Thackray et al., 2006).
2.2. Cell culture, transient transfection and luciferase assay
LβT2 cells were grown in DMEM containing 10% fetal calf serum (FCS) supplemented with penicillin-streptomycin at 37 °C in an atmosphere of 5% CO2 in air. LβT2 cells maintained in 10-cm plates until they became confluent and then transferred into 24-well plates. After 24-h preculture, the cells (1.5 × 105 viable cells/cm2) were transiently transfected with 500 ng of mFSHβ-Luc plasmid (Thackray et al., 2006) and 50 ng of cytomegalovirus-β-galactosidase plasmid (pCMV-β-gal) (Sosnowski et al., 2000) using FuGENE six transfection reagent (Roche Molecular Biochemicals, Indianapolis, IN) for 24 h. The cells were treated with BMPs, BMP receptor-ECDs and MAPK inhibitors in the presence or absence of GnRH in fresh DMEM containing 1% FCS for 24 h and then washed with PBS and lysed with Cell Culture Lysis Reagent (Toyobo, Osaka, Japan). Luciferase activity and β-galactosidase (β-gal) activity of the cell lysate were measured by a luminometer. The data are shown as the ratio of luciferase to β-gal activity.
2.3. RNA extraction, RT-PCR, and quantitative real-time PCR analysis
To prepare total cellular RNA, LβT2 cells (3 × 105 viable cells/cm2) were cultured and treated with indicated concentrations of GnRH in fresh DMEM containing 1% FCS. After 24-h culture, the medium was removed, and total cellular RNA was extracted using TRIzol® (Invitrogen Corp., Carlsbad, CA), quantified by measuring absorbance at 260 nm, and stored at −80 °C until assay. Expression of BMPs, BMP receptors and Smad mRNAs was detected by RT-PCR analysis. The extracted RNA (1.0 µg) was subjected to an RT reaction using the First-Strand cDNA synthesis system® (Invitrogen Corp.) with random hexamer (2 ng/µl), reverse transcriptase (200 U), and deoxynucleotide triphosphate (0.5 mM) at 42 °C for 50 min and 70 °C for 10 min. Subsequently, hot-start PCR was performed using MgCl2 (1.5 mM), deoxynucleotide triphosphate (0.2 mM), and 2.5 U of Taq DNA polymerase (Invitrogen Corp.). Oligonucleotides used for PCR were custom-ordered from Invitrogen Corp. Primer pairs were selected from different exons of the corresponding genes. Primer pairs and the optimal PCR conditions for BMP-6, -7, ALK-2, -3, ActRII, BMPRII, Smad6/7 and RPL19 were selected as reported previously (Takeda et al., 2007; Miyoshi et al., 2008). For the quantification of indicated mRNA levels, real-time PCR was performed using the LightCycler-FastStart DNA Master SYBR Green I system® (Roche Diagnostic Co., Tokyo, Japan) under conditions of annealing at 60 °C with 4 mM MgCl2, following the manufacturer’s protocol. Accumulated levels of fluorescence for each product were analyzed by the second derivative method after melting-curve analysis (Roche Diagnostic Co.), and then, following assay validation by calculating each amplification efficiency, the expression levels of target genes were quantified on the basis of standard curve analysis for each product. For each transcript, all treatment groups were quantified simultaneously in a single LightCycler run. To correct for differences in RNA quality and quantity between samples, the expression levels of target gene mRNA were normalized by dividing the quantity of the target gene by the quantity of RPL19 mRNA in each sample. The raw data for each target mRNA level (/RPL19) were statistically analyzed as indicated and are shown as fold changes in the figures.
2.4. Western immunoblot analysis
Cells (3 × 105 viable cells/cm2) were precultured in 12-well plates in DMEM containing 10% FCS for 24 h. After preculture, the medium was replaced with serum-free fresh DMEM, and then indicated concentrations of BMPs and GnRH were added to the culture medium. After stimulation with growth factors for 1 h, cells were solubilized in 100 µl RIPA lysis buffer (Upstate Biotechnology, Inc., Lake Placid, NY) containing 1 mM Na3VO4, 1 mM sodium fluoride, 2% sodium dodecyl sulfate, and 4% β-mercaptoethanol. The cell lysates were then subjected to SDS–PAGE/immunoblotting analysis (Nupage blot system, Invitrogen Corp.) using 1:1000 dilution of each antibody including anti-phospho-Smad1/5/8 (pSmad1/5/8) antibody, anti-phospho- and anti-total-extracellular signal-regulated kinase (ERK) 1/2 MAPK antibody, anti-phospho- and anti-total-p38-MAPK antibody, anti-phospho- and anti-total-stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK) MAPK antibody (Cell Signaling Technology, Inc., Beverly, MA), and anti-actin antibody (Sigma-Aldrich Co. Ltd.) as we previously reported (Inagaki et al., 2006; Otani et al., 2007). The relative integrated density of each protein band was digitized by NIH image J 1.34s and ratios of the band intensities of phosphorylated proteins/total proteins were calculated for evaluating the protein phosphorylation.
2.5. Statistical analysis
All results are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. The data were subjected to one-way or two way ANOVA to determine differences and synergy (StatView 5.0 software, Abacus Concepts, Inc., Berkeley, CA or JMP 7, SAS Inc., Carey, NC). If differences were detected, Fisher’s protected least significant difference (PLSD) test (StatView 5.0 software) or Tukey’s HSD test (JMP) was used to determine group differences (Figs. 1A and B, 2B and D, and 3A and B). The data in Fig. 2A and E were subjected to an unpaired t-test to determine differences (StatView 5.0 software). P values <0.05 were accepted as statistically significant.
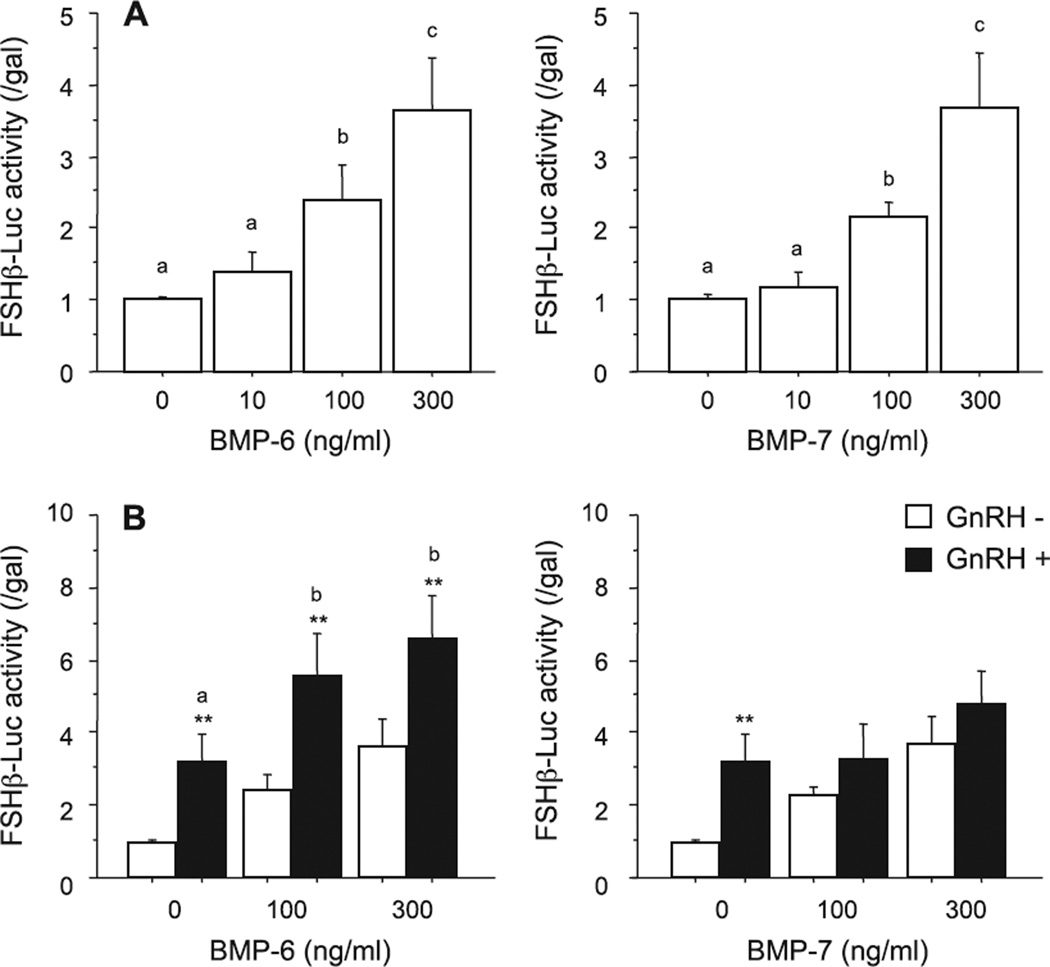
Fig. 1.
Effects of BMPs and GnRH on FSH-induced transcriptional activity in LβT2 cells. After preculture, cells (1.5 × 105 viable cells/cm2) were transiently transfected with 500 ng of FSHβ-Luc and 50 ng of cytomegalovirus-β-galactosidase plasmid (pCMV-β-gal). The cells were then treated with indicated concentrations of BMP-6 and BMP-7 in the (A) absence or (B) presence of GnRH (10 nM) for 24 h. Cells were washed with PBS and lysed, and the luciferase activity and β-galactosidase (β-gal) activity were measured by a luminometer. Results are shown as the ratio of luciferase to β-gal activity and graphed as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. For each result within panels (A) and (B), values with different superscript letters are significantly different at P ≤ 0.05, and for each result within panel B), **P ≤ 0.01 vs. GnRH (−) group.
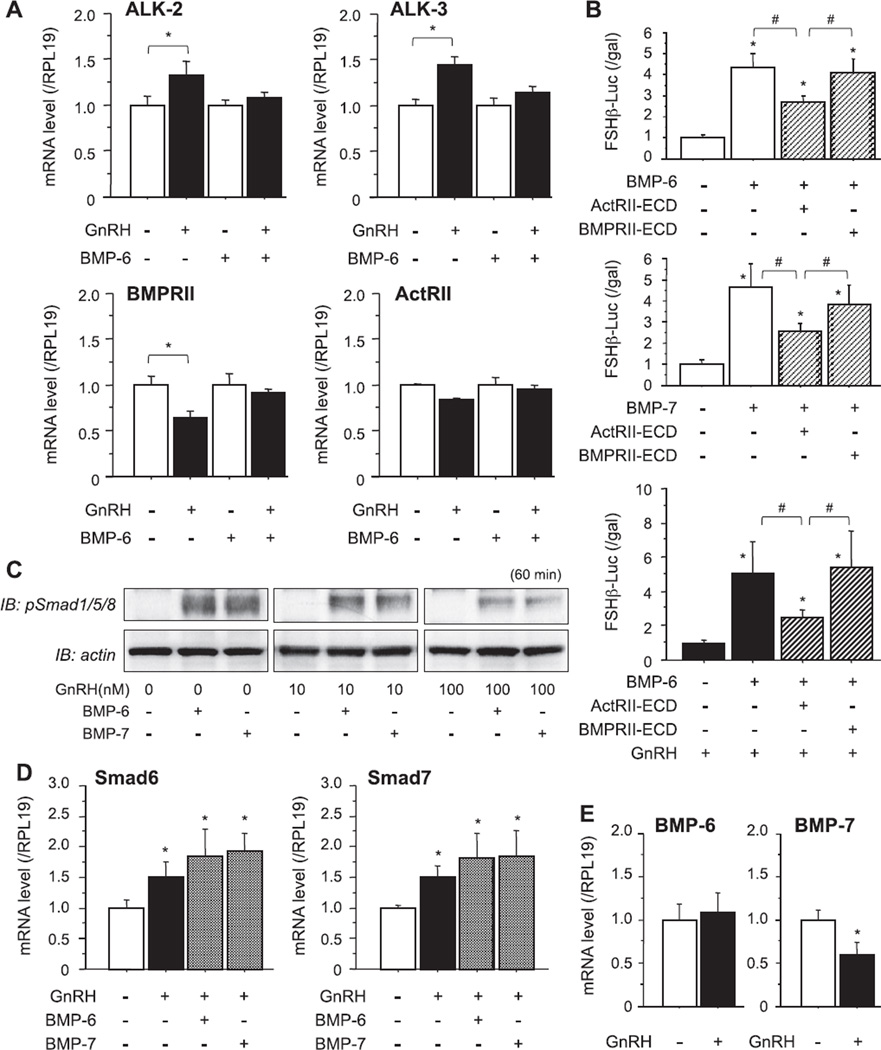
Fig. 2.
Effects of GnRH on BMP receptor signaling in LβT2 cells. (A) After preculture, cells (3 × 105 viable cells/cm2) were treated with GnRH (100 nM) in combination with BMP-6 (100 ng/ml), and total cellular RNAs were extracted and subjected to RT reaction. Levels of steady-state mRNAs of ALK-2, -3, BMPRII and ActRII were analyzed by quantitative real-time PCR and standardized by the level of RPL19 in each sample. Changes in mRNA levels by treatments were graphed. Results are shown as means ± SEM of data obtained from at least three separate experiments, each performed with triplicate samples; *P < 0.05 vs. indicated GnRH (−) group. (B) After preculture, cells (1.5 × 105 viable cells/cm2) were transiently transfected with 500 ng of FSHβ-Luc and 50 ng of cytomegalovirus-β-galactosidase plasmid (pCMV-β-gal). The cells were then treated with indicated concentrations of BMP-6, BMP-7 (100 ng/ml) and GnRH (10 nM) in the presence of extracellular domain (ECD) proteins of either ActRII or BMPRII (1 µg/ml) for 24 h. The cells were washed with PBS and lysed, and the luciferase activity and β-galactosidase (β-gal) activity were measured by a luminometer. Results are shown as the ratio of luciferase to β-gal activity and graphed as means ± SEM of data from at least three separate experiments, each performed with triplicate samples; *P ≤ 0.05 vs. control group and #P ≤ 0.05 between the indicated groups. (C) After 24-h preculture, cells (3 × 105 viable cells/cm2) were treated with BMP-6 and BMP-7 (100 ng/ml) in combination with GnRH (10 and 100 nM) in serum-free conditions. Following 1-h stimulation with growth factors, cells lysates were obtained and subjected to SDS–PAGE/immunoblotting (IB) analysis using anti-phospho-Smad1/5/8 (pSmad1/5/8) antibody and anti-actin antibody. (D and E) Cells (3 × 105 viable cells/cm2) were treated with GnRH (100 nM) in combination with BMP-6 or BMP-7 (100 ng/ml), and total cellular RNAs were extracted and subjected to RT reaction. Levels of steady-state mRNAs of (D) Smad6 and Smad7, and (E) BMP-6 and BMP-7 were analyzed by quantitative real-time PCR and standardized by the level of RPL19 in each sample. Changes in mRNA levels by treatments were graphed. Results are shown as means ± SEM of data obtained from at least three separate experiments, each performed with triplicate samples; *P < 0.05 vs. control group.
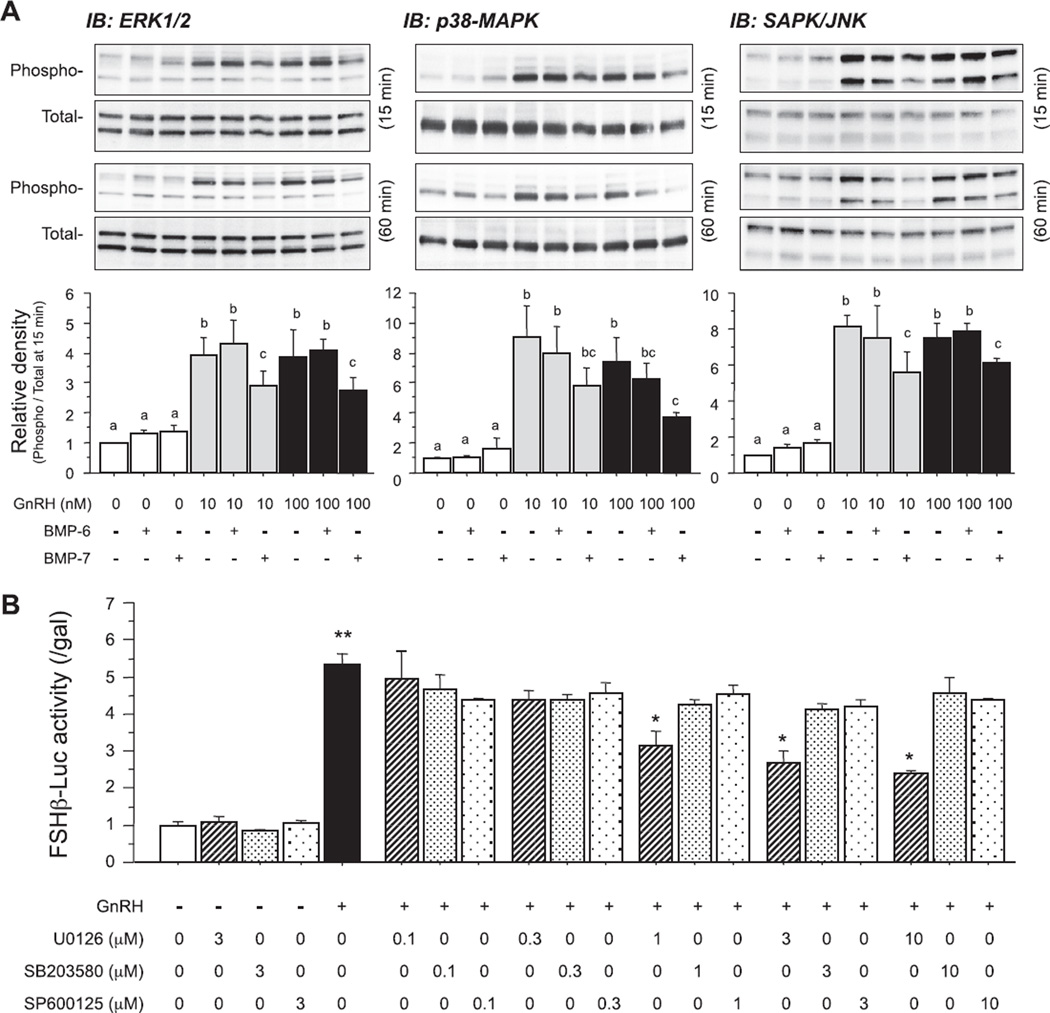
Fig. 3.
Effects of BMPs on GnRH-induced MAPK pathways in LβT2 cells. (A) Cells (3 × 105 viable cells/cm2) were precultured for 24 h and treated with BMP-6 and BMP-7 (100 ng/ml) in the absence or presence of GnRH (10 and 100 nM). After 15- and 60-min culture, cells were lysed and subjected to SDS–PAGE/immunoblotting (IB) analysis using anti-phospho-ERK1/2 and anti-total-ERK1/2, anti-phospho-p38 and anti-total-p38, and anti-phospho-SAPK/JNK and anti-total-SAPK/JNK antibodies. The results shown are representative of those obtained from three independent experiments. The relative integrated density of each protein band at 15-min stimulation was digitized by NIH image J 1.34s and shown as phospho-/total-protein levels in each panel. Results are shown as means ± SEM of data from at least three separate experiments, each performed with triplicate samples. For each result within panel (A), the values with different superscript letters are significantly different at P < 0.05. B) After preculture, cells (1.5 × 105 viable cells/cm2) were transiently transfected with 500 ng of FSHβ-Luc and 50 ng of cytomegalovirus-β-galactosidase plasmid (pCMV-β-gal). The cells were then treated with indicated concentrations (µM) of MAPK inhibitors including U0126, SB203580 and SP600125 in the presence of GnRH (10 nM) for 24 h. The cells were washed with PBS and lysed, and the luciferase activity and β-galactosidase (β-gal) activity were measured by a luminometer. Results are shown as the ratio of luciferase to β-gal activity and graphed as means ± SEM of data from at least three separate experiments, each performed with triplicate samples; *P < 0.05 and **P < 0.01 in each set of the bar graphs within panel (B).
3. Results
In the present study, we examined the functional link between BMP signals and FSHβ transcriptional activity induced by GnRH using mouse gonadotrope LβT2 cells. It has been reported that mRNAs of BMP-6 and BMP-7 and their receptors including type I (ALK-2 and -3) and type II (ActRII and BMPRII) subtypes were expressed in mouse gonadotrope and LβT2 cells and were regulated by GnRH (Huang et al., 2001; Otsuka and Shimasaki, 2002; Zhang et al., 2006). BMP-6 and BMP-7 (10 to 300 ng/ml) individually enhanced mouse FSHβ-promoter (−1.5 kb) activity in a concentration-dependent manner (Fig. 1A). Notably, BMP-6 (100–300 ng/ml) significantly enhanced the GnRH (10 nM)-induced FSHβ-promoter activity (Fig. 1B) and the difference was confirmed by two-way factorial ANOVA. In contrast to the effect of BMP-6 on FSHβ transcriptional activation, BMP-7 had no effect on GnRH-induced FSHβ-promoter activity in LβT2 cells (Fig. 1B).
Next, the effects of GnRH on the expression levels of BMP receptors were examined. We have previously investigated the expression pattern of BMP receptor subtype mRNA in LβT2 cells, finding that LβT2 cells hardly express ALK-6 among BMP type-I receptors (Otsuka and Shimasaki, 2002; Takeda et al., 2007). Therefore, we focused on changes in the expression levels of ALK-2 and -3 in GnRH-treated LβT2 cells. As shown in Fig. 2A, the expression levels of ALK-2 and -3 were increased by GnRH (100 nM) treatment. In contrast, GnRH significantly reduced BMPRII expression, while ActRII expression was preserved in GnRH-treated LβT2 cells. Significant reduction (by 53.6%) of BMPRII transcription was confirmed by a reporter assay using a construct of the BMPRII-promoter (−523 bp) region (data not shown). In the presence of BMP-6 (100 ng/ml), the increasing effects of GnRH on the expression levels of ALK-2 and -3 and the decreasing effects of GnRH on BMPRII expression were found to be impaired. The extracellular domains (ECDs) of BMP receptor constructs are proteins that lack transmembrane and intracellular regions of BMP receptors, which extracellularly act as dominant negatives for the corresponding receptors as we reported previously (Moore et al., 2003; Inagaki et al., 2006). The FSHβ-promoter activity induced by BMP-6 and BMP-7 was significantly inhibited by treatment with ECDs (1 µg/ml) of ActRII (ActRII-ECD) but not by treatment with BMPRII (BMPRII-ECD) as shown in Fig. 2B. In the presence of GnRH (10 nM), FSHβ-promoter activity induced by BMP-6 (100 ng/ml) was also preferentially blocked by treatment with ActRII-ECD (Fig. 2B, lower panel). These findings suggest that ActRII is a functional type II receptor for FSHβ transcription stimulated by BMP-6 and BMP-7 and that ActRII is a key component for the combined effects of BMP-6 and GnRH on the enhancement of FSHβ transcription. Involvement of GnRH action in BMP-induced Smad1/5/8 signaling was next examined by Western immunoblot analysis. As shown in Fig. 2C, Smad1/5/8 phosphorylation induced by BMP-6 and BMP-7 was inhibited by the addition of 100 nM of GnRH and, to a lesser degree, by 10 nM of GnRH. Since GnRH treatment increased Smad6/7 mRNA levels in LβT2 cells regardless of the presence of BMP-6 and BMP-7 (Fig. 2D), the upregulation of inhibitory Smads is likely to be linked to the impairment of BMP-Smad1/5/8 signaling caused by GnRH. Furthermore, the expression level of BMP-7 mRNA was concomitantly reduced by GnRH (100 nM) in LβT2 cells, while BMP-6 expression was not affected by GnRH treatment (Fig. 2E).
Since GnRH stimulates MAPK phosphorylation in LβT2 cells, a functional link between MAPK and FSHβ transcription was next examined. As shown in Fig. 3A, 10 and 100 nM of GnRH readily activated phosphorylation of ERK1/2, p38-MAPK and SAPK/JNK pathways. BMP-6 and BMP-7 alone (100 ng/ml) did not directly activate the MAPK pathways; however, it was of interest that co-treatment with BMP-7, but not with BMP-6, suppressed GnRH-induced MAPK phosphorylation in LβT2 cells (Fig. 3A). Namely, GnRH (10 and 100 nM)-induced ERK1/2 activation was inhibited by BMP-7 (100 ng/ml) but not by BMP-6. Activation of p38-MAPK and SAPK/JNK induced by GnRH (10 and 100 nM) was also inhibited by BMP-7 (100 ng/ml). Furthermore, as shown in Fig. 3B, inhibition of the ERK pathway by U0126 (0.1–10 µM) significantly suppressed GnRH (10 nM)-induced FSHβ transcription. On the other hand, inhibition of the p38 pathway by SB203580 (0.1–10 µM) or the SAPK/JNK pathway by SP600125 (0.1–10 µM) failed to inhibit GnRH (10 nM)-induced FSHβ transcription, suggesting that ERK signaling is functionally involved in GnRH-induced FSHβ transcription.
4. Discussion
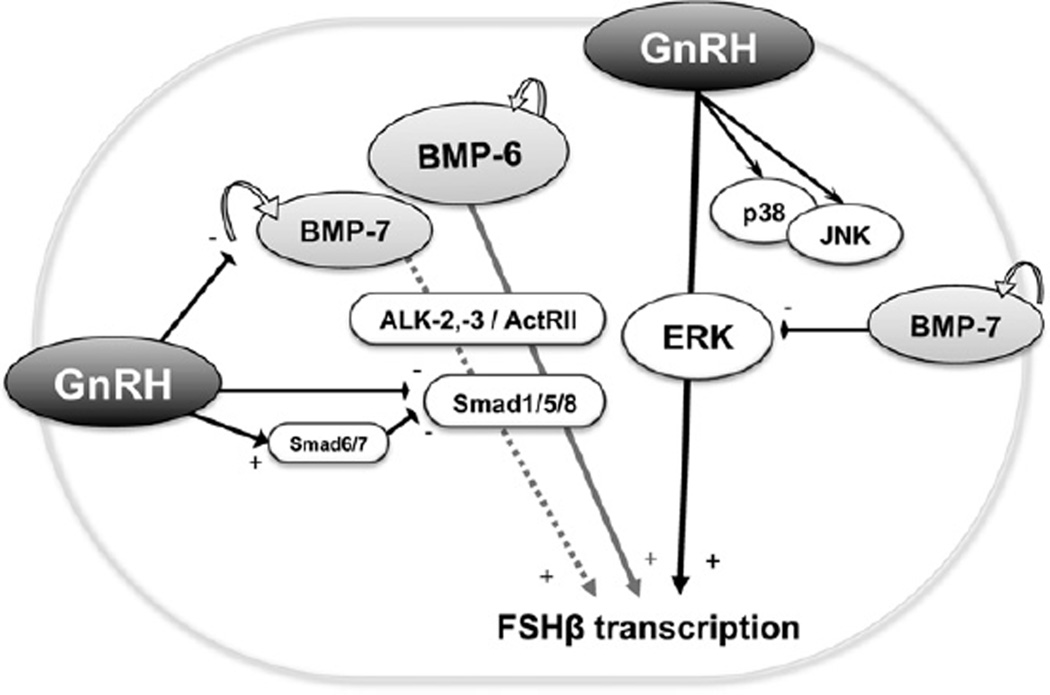
In the present study, it was revealed that BMP-6 and BMP-7 increased FSHβ transcription in LβT2 cells through ActRII action (Fig. 4). Of note, BMP-6, but not BMP-7, enhanced GnRH-induced FSHβ-promoter activity in LβT2 cells. The difference between BMP-6 and BMP-7 in enhancing GnRH-induced FSHβ transcription was found to be possibly due to the diverged effects of BMP ligands on GnRH-induced ERK signaling. Furthermore, it was found that GnRH inactivated BMP-induced Smad1/5/8 phosphorylation, in which downregulation of BMP-7 and BMPRII and upregulation of inhibitory Smad6/7 might be functionally involved (Fig. 4).
Fig. 4.
A functional link between the BMP system and GnRH-induced FSH transcription. BMP-6 and GnRH mutually augment FSHβ transcription in LβT2 cells. GnRH activates FSHβ transcription by activating the ERK pathway, which is negatively regulated by BMP-7. GnRH also provides a feedback inhibition of BMP-Smad signaling by suppressing Smad1/5/8, in part via increasing Smad6/7 expression, and reducing endogenous BMP-7 expression. There is a functional link between GnRH action and the BMP system for regulating FSHβ transcription in gonadotrope cells.
There is increasing evidence that locally produced BMPs play critical roles in regulating pituitary functions. Earlier studies have identified BMPs as modulators in the regulation of FSH synthesis and release. In rodent primary pituitary cells and the mouse gonadotrope cell line LβT2, different BMPs including BMP-6, BMP-7 and BMP-15, at high doses, exerted stimulatory effects on FSHβ transcription (Huang et al., 2001; Otsuka and Shimasaki, 2002). It has also been reported that BMP-4 alone had no effect on FSHβ mRNA and FSH release in LβT2 mouse gonadotrope cells (Nicol et al., 2008). Nevertheless, BMP-4 increased both FSHβ mRNA and FSH secretion when combined with activin A and GnRH in LβT2 cells (Nicol et al., 2008). Recent studies have also shown synergistic effects on FSHβ transcription between BMP-2 and activin A in LβT2 cells (Lee et al., 2007). On the other hand, BMP-4 and BMP-6 decreased both basal and activin-stimulated FSHβ mRNA levels and FSH release in ewe pituitary cells (Faure et al., 2005). This discrepancy of BMP responsiveness could be due to species differences, different genetic background (Young et al., 2008) or to estrous cycle status (Sallon et al., 2010). The differential expression patterns of BMP receptors between mixed primary pituitary cell cultures in ewe and mouse LβT2 cells may also be involved in the activation of diverged signaling pathways and/or intracellular components (Nicol et al., 2008).
Lopez et al. reported that Differential screening-selected gene Aberrative in Neuroblastoma (DAN), a secreted BMP antagonist, was controlled by GnRH in LβT2 cells (Lopez de Maturana et al., 2007). GnRH induces a rapid expression of DAN and co-expression of DAN inhibits the synergistic effect of GnRH and activin on GnRH-R expression, suggesting an auto-regulatory role of DAN in inhibiting GnRH-induced transcription of its own receptor. Considering that DAN family has binding affinity to BMP ligands including BMP-6 and BMP-7, this machinery may play a physiological role in the termination of GnRH effects after LH surge (Lopez de Maturana et al., 2007).
There is accumulating evidence that BMPs operate through Smad-independent pathways such as MAPK signaling molecules. It has been reported that GnRH activated the phosphorylation of ERK1/2 in mouse gonadotrope-derived αT3-1 (Roberson et al., 1995) and LβT2 cells (Kanasaki et al., 2005). It was also reported that GnRH-activated FSHβ transcription was blocked by inhibition of ERK1/2 pathway (Kanasaki et al., 2005). Nicol et al. reported that ERK1/2 response to GnRH was not functionally modified by activin or BMP-4 in LβT2 cells (Nicol et al., 2008). However, the level of GnRH-enhanced p38 activation was increased by activin, in which p38 activation in response to GnRH plus activin was reduced by BMP-4 (Nicol et al., 2008), contrary to the increase in FSHβ mRNA and FSH secretion. Based on these findings, the activin-Smad2/3 and GnRH-MAPK pathways are not directly modified by BMP-4 actions. In this regard, Lee et al. also reported that BMP-2 stimulates FSHβ subunit transcription and increases FSHβ mRNA levels in LβT2 cells by synergizing with activin A to upregulate FSHβ-promoter activities (Lee et al., 2007). The mechanisms of BMP-2 action have yet to be elucidated; however, BMP-2 appears to signal through complexes of BMPRII and ALK-2 to activate the Smad8/4-dependent signaling cascade (Lee et al., 2007). This receptor complex is different from the observed effects of BMP-6 and BMP-7 signaling through a complex including ActRII. The convergent effects of independent signaling pathways elicited by BMP/activin and GnRH are likely to be linked to the combined response leading to the effective amplification of FSHβ transcription. Since it has not been determined whether BMPs exert their effect directly on FSHβ promoter activity, further analysis is necessary to identify the site of BMP actions.
In the present study, BMP-6 and BMP-7 individually stimulated FSHβ transcription, which was further enhanced by a combination of BMP-6 and GnRH in LβT2 cells. Namely, in addition to the main cascade stimulated by GnRH, the BMP system including BMP-6 and BMP-7 upregulates FSHβ transcription in gonadotrope cells. Interestingly, the combined effects of BMP-6 and GnRH enhanced transcriptional activity of FSHβ, while GnRH inactivated BMP-Smad1/5/8 signaling activity. Since the expression levels of BMP receptors were differentially modified by GnRH treatment, these discrepancies might indicate the existence of an autoregulatory system to maintain FSHβ transcriptional activity in gonadotrope cells. Interestingly, GnRH treatment increased the expression levels of inhibitory Smad6 and Smad7 regardless of the presence of BMP-6 and BMP-7. Since Smad6/7 is directly involved in suppression of BMP receptor-mediated phosphorylation of Smad1/5/8, it is thought that GnRH functionally inhibits Smad1/5/8 phosphorylation induced by BMP-6 and BMP-7. In addition, the expression of key BMP receptors for FSHβ transcription such as ALK-2/3 and ActRII was preserved in LβT2 cells treated with GnRH and BMP-6. Further studies are required to clarify the physiological interaction between GnRH regulation of the pituitary BMP system and BMP action on GnRH-induced FSHβ transcription.
Recent studies have shown that BMPs regulate the entire hypothalamo–pituitary–ovarian (HPO) axis (Otsuka, 2010). Understanding the neural mechanisms through which estradiol regulates GnRH neurons is a key for elucidating reproductive control of the HPO axis. In females, estrogens act directly or indirectly on the GnRH neuronal network to modulate the final output of GnRH into the median eminence (Herbison, 1998; Herbison and Pape, 2001). We recently reported the effects of BMPs on GnRH regulation controlled by estrogen in hypothalamic neuron cells (Otani et al., 2009). In particular, BMP-2 and BMP-4 attenuated the suppressive effects of estrogen on GnRH production by limiting estrogen-ERK signaling and ER expression. On the other hand, BMP-6 and BMP-7 contributed to increased GnRH secretion, implying that a new interaction of BMPs and ER is involved in controlling hypothalamic GnRH production and secretion by an autocrine/paracrine mechanism (Otani et al., 2009).
Collectively, the results indicate that the BMP system may play a key role not only in regulation of ovarian folliculogenesis by controlling gonadotropin sensitivity but also in modulation of hypothalamic GnRH secretion leading to fine-tuning of gonadotropin secretion from pituitary gonadotropes. Furthermore, BMP-6 and GnRH mutually augment FSHβ transcription in LβT2 cells, in which GnRH provides a feedback inhibition of BMP-Smad signaling. On the contrary, BMP-7 may act independently or redundantly with GnRH. The presence of a functional link between GnRH action and the BMP system in pituitary gonadotropes is likely to play a key role for fine-tuning of FSH secretion (Fig. 4).
Acknowledgements
This work was supported by the Eunice Kennedy Shriver NIH/NICHD Grants R01 HD037568 to M.A.L. and through U54 HD012303 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Abbreviations
- ALK
activin receptor-like kinase
- ActRII
activin type II receptor
- BMP
bone morphogenetic protein
- BMPRII
BMP type II receptor
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinase
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- MAPK
mitogen-activated protein kinase
- SAPK/JNK
stress-activated protein kinase/c-Jun NH2-terminal kinase
- TGFβ
transforming growth factor-β
Footnotes
Disclosure statement
None declared.
References
- Faure MO, Nicol L, Fabre S, Fontaine J, Mohoric N, McNeilly A, Taragnat C. BMP-4 inhibits follicle-stimulating hormone secretion in ewe pituitary. J. Endocrinol. 2005;186:109–121. doi: 10.1677/joe.1.05988. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr. Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front. Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Huang H-J, Wu JC, Su P, Zhirnov O, Miller WL. A novel role for bone morphogenetic proteins in the synthesis of follicle-stimulating hormone. Endocrinology. 2001;142:2275–2283. doi: 10.1210/endo.142.6.8159. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Otsuka F, Suzuki J, Kano Y, Takeda M, Miyoshi T, Otani H, Mimura Y, Ogura T, Makino H. Involvement of bone morphogenetic protein-6 in differential regulation of aldosterone production by angiotensin II and potassium in human adrenocortical cells. Endocrinology. 2006;147:2681–2689. doi: 10.1210/en.2005-1250. [DOI] [PubMed] [Google Scholar]
- Kanasaki H, Bedecarrats GY, Kam KY, Xu S, Kaiser UB. Gonadotropin-releasing hormone pulse frequency-dependent activation of extracellular signal-regulated kinase pathways in perifused LbetaT2 cells. Endocrinology. 2005;146:5503–5513. doi: 10.1210/en.2004-1317. [DOI] [PubMed] [Google Scholar]
- Labeur M, Paez-Pereda M, Haedo M, Arzt E, Stalla GK. Pituitary tumors: cell type-specific roles for BMP-4. Mol. Cell. Endocrinol. 2010;326:85–88. doi: 10.1016/j.mce.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Lee KB, Khivansara V, Santos MM, Lamba P, Yuen T, Sealfon SC, Bernard DJ. Bone morphogenetic protein 2 and activin A synergistically stimulate follicle-stimulating hormone beta subunit transcription. J. Mol. Endocrinol. 2007;38:315–330. doi: 10.1677/jme.1.02196. [DOI] [PubMed] [Google Scholar]
- Lopez de Maturana R, Martin B, Millar RP, Brown P, Davidson L, Pawson AJ, Nicol MR, Mason JI, Barran P, Naor Z, Maudsley S. GnRH-mediated DAN production regulates the transcription of the GnRH receptor in gonadotrope cells. NeuroMol. Med. 2007;9:230–248. doi: 10.1007/s12017-007-8004-z. [DOI] [PubMed] [Google Scholar]
- McMahon HE, Hashimoto O, Mellon PL, Shimasaki S. Oocyte-specific overexpression of mouse bone morphogenetic protein-15 leads to accelerated folliculogenesis and an early onset of acyclicity in transgenic mice. Endocrinology. 2008;149:2807–2815. doi: 10.1210/en.2007-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNatty KP, Heath DA, Hudson NL, Lun S, Juengel JL, Moore LG. Gonadotrophin-responsiveness of granulosa cells from bone morphogenetic protein 15 heterozygous mutant sheep. Reproduction. 2009;138:545–551. doi: 10.1530/REP-09-0154. [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Otsuka F, Otani H, Inagaki K, Goto J, Yamashita M, Ogura T, Iwasaki Y, Makino H. Involvement of bone morphogenetic protein-4 in GH regulation by octreotide and bromocriptine in rat pituitary GH3 cells. J. Endocrinol. 2008;197:159–169. doi: 10.1677/JOE-07-0549. [DOI] [PubMed] [Google Scholar]
- Moore RK, Otsuka F, Shimasaki S. Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J. Biol. Chem. 2003;278:304–310. doi: 10.1074/jbc.M207362200. [DOI] [PubMed] [Google Scholar]
- Nicol L, Faure MO, McNeilly JR, Fontaine J, Taragnat C, McNeilly AS. Bone morphogenetic protein-4 interacts with activin and GnRH to modulate gonadotrophin secretion in LbetaT2 gonadotrophs. J. Endocrinol. 2008;196:497–507. doi: 10.1677/JOE-07-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H, Otsuka F, Inagaki K, Takeda M, Miyoshi T, Suzuki J, Mukai T, Ogura T, Makino H. Antagonistic effects of bone morphogenetic protein-4 and -7 on renal mesangial cell proliferation induced by aldosterone through MAPK activation. Am. J. Physiol. Renal. Physiol. 2007;292:F1513–F1525. doi: 10.1152/ajprenal.00402.2006. [DOI] [PubMed] [Google Scholar]
- Otani H, Otsuka F, Takeda M, Mukai T, Terasaka T, Miyoshi T, Inagaki K, Suzuki J, Ogura T, Lawson MA, Makino H. Regulation of GNRH production by estrogen and bone morphogenetic proteins in GT1-7 hypothalamic cells. J. Endocrinol. 2009;203:87–97. doi: 10.1677/JOE-09-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F. Multiple endocrine regulation by bone morphogenetic protein system. Endocr. J. 2010;57:3–14. doi: 10.1507/endocrj.k09e-310. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Shimasaki S. A novel function of bone morphogenetic protein-15 in the pituitary: selective synthesis and secretion of FSH by gonadotropes. Endocrinology. 2002;143:4938–4941. doi: 10.1210/en.2002-220929. [DOI] [PubMed] [Google Scholar]
- Otsuka F, McTavish K, Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011;78:9–21. doi: 10.1002/mrd.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J. Biol. Chem. 2001;276:11387–11392. doi: 10.1074/jbc.M010043200. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Yao Z, Lee TH, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15: identification of target cells and biological functions. J. Biol. Chem. 2000;275:39523–39528. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol. Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- Roberson MS, Misra-Press A, Laurance ME, Stork PJ, Maurer RA. A role for mitogen-activated protein kinase in mediating activation of the glycoprotein hormone alpha-subunit promoter by gonadotropin-releasing hormone. Mol. Cell. Biol. 1995;15:3531–3539. doi: 10.1128/mcb.15.7.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallon C, Faure MO, Fontaine J, Taragnat C. Dynamic regulation of pituitary mRNAs for bone morphogenetic protein (BMP) 4, BMP receptors, and activin/inhibin subunits in the ewe during the estrous cycle and in cultured pituitary cells. J. Endocrinol. 2010;207:55–65. doi: 10.1677/JOE-10-0035. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- Sosnowski R, Mellon PL, Lawson MA. Activation of translation in pituitary gonadotrope cells by gonadotropin-releasing hormone. Mol. Endocrinol. 2000;14:1811–1819. doi: 10.1210/mend.14.11.0550. [DOI] [PubMed] [Google Scholar]
- Takeda M, Otsuka F, Suzuki J, Kishida M, Ogura T, Tamiya T, Makino H. Involvement of activin/BMP system in development of human pituitary gonadotropinomas and nonfunctioning adenomas. Biochem. Biophys. Res. Commun. 2003;306:812–818. doi: 10.1016/s0006-291x(03)01052-0. [DOI] [PubMed] [Google Scholar]
- Takeda M, Otsuka F, Otani H, Inagaki K, Miyoshi T, Suzuki J, Mimura Y, Ogura T, Makino H. Effects of peroxisome proliferator-activated receptor activation on gonadotropin transcription and cell mitosis induced by bone morphogenetic proteins in mouse gonadotrope LbT2 cells. J. Endocrinol. 2007;194:87–99. doi: 10.1677/JOE-07-0138. [DOI] [PubMed] [Google Scholar]
- Thackray VG, McGillivray SM, Mellon PL. Androgens, progestins, and glucocorticoids induce follicle-stimulating hormone beta-subunit gene expression at the level of the gonadotrope. Mol. Endocrinol. 2006;20:2062–2079. doi: 10.1210/me.2005-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM, Juengel JL, Dodds KG, Laird M, Dearden PK, McNeilly AS, McNatty KP, Wilson T. The activin receptor-like kinase 6 Booroola mutation enhances suppressive effects of bone morphogenetic protein 2 (BMP2), BMP4, BMP6 and growth and differentiation factor-9 on FSH release from ovine primary pituitary cell cultures. J. Endocrinol. 2008;196:251–261. doi: 10.1677/JOE-07-0148. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bailey JS, Coss D, Lin B, Tsutsumi R, Lawson MA, Mellon PL, Webster NJ. Activin modulates the transcriptional response of LbetaT2 cells to gonadotropin-releasing hormone and alters cellular proliferation. Mol. Endocrinol. 2006;20:2909–2930. doi: 10.1210/me.2006-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]