Abstract
Objective
Alteration to blood flow in the maternal-fetal compartment has been proposed as a mechanism underlying maternal psychological effects on pregnancy outcomes. This study characterized the progression of umbilical and uterine blood flow resistance in healthy pregnancies and evaluated concurrent and longitudinal associations with maternal anxiety and other psychological factors.
Methods
The study assessed participants (n = 107) at five visits spanning 24 to 38 weeks gestation. The resistance index (RI) in the uterine and umbilical arteries was measured with Doppler ultrasound. Maternal psychological function was assessed using validated, self-report instruments.
Results
Hierarchical linear modeling revealed that uterine and umbilical RI decreased during the second half of gestation, and that uterine RI was lower in nulliparous women. Few concurrent associations emerged between psychological factors and RI. Longitudinal analyses determined that psychological well-being was associated with decreased left uterine artery RI, and psychological distress was associated with lower right artery RI.
Conclusions
While uterine artery resistance was modestly associated with the maternal psychological milieu during gestation, our findings do not indicate an association between increased maternal distress and decreased RI. Thus, this study fails to affirm a key component of the hypothesized relation of maternal stress to fetal outcomes via vasoconstriction.
Keywords: Doppler, maternal anxiety, resistance index, umbilical artery, uterine artery
Introduction
A woman’s psychological state during pregnancy has long been thought to influence the health and well-being of her offspring. However, empirical findings present a mixed picture of the potential effects of maternal antenatal psychological distress. Maternal antenatal depressive symptoms, anxiety, and perceived stress have been associated with low birthweight and spontaneous preterm birth in some studies [1–5], but others found no adverse effects of antenatal psychological distress on birth weight, gestational age at delivery, obstetric complications, or child development at age 2 [6–10]. Identification of pathophysiology by which maternal psychological factors may influence fetal outcomes is important to understanding such variation in outcomes.
Alteration to blood flow in the maternal-fetal compartment is commonly proposed as a putative mechanism underlying maternal psychological effects on pregnancy outcomes, primarily as a result of vasoconstriction associated with the stress response [2,4,11,12]. Doppler ultrasonography of the uterine and umbilical arteries offers a non-invasive method for studying fetal blood flow. Elevated Dopplers are also associated with maternal complications, including pre-eclampsia, and fetal intrauterine growth restriction [13]. To our knowledge, only four studies have evaluated the relation of maternal psychological factors to maternal blood flow to the fetus, and these have generated mixed results. Teixeira and colleagues reported that both state and trait anxiety scores were associated with increased uterine artery resistance (mean of right and left arteries) in a sample of 100 healthy pregnant women at 32 weeks gestation [14]. However, Kent and colleagues did not find a significant association between self-reported anxiety and uterine artery resistance among 96 healthy pregnant women at 20 weeks gestation [15]. In a study of 37 healthy pregnant women at term, women with high self-reported trait anxiety had higher pulsatility index (PI) values in the umbilical artery than their low trait anxiety counterparts, as well as lower PI values in the fetal middle cerebral artery and lower cerebro-umbilical PI ratios [16]. These studies are limited by cross-sectional designs in which anxiety and blood flow were measured at a single point during the second or third trimester of pregnancy.
More recently, Maina et al. conducted a prospective study that included uterine and umbilical artery Doppler velocimetry measures at 20 and 34 weeks gestation [17]. Three groups of pregnant women were evaluated: women with a diagnosis of depression and/or anxiety disorder (n = 20), women with at least one severe life event during pregnancy or in the year prior (n = 20), and women without psychiatric diagnoses or stressful life events (n = 40). There were no significant differences in uterine or umbilical Dopplers among the three groups. This study has the advantages of a prospective design and inclusion of both uterine and umbilical Doppler measures. However, the study reported only percentages of abnormal Doppler values and did not evaluate relative resistance or pulsatility values, which may have obscured more subtle differences.
The limited literature on maternal psychological state and fetal blood flow also has not addressed two other key aspects of maternal psychological function. The first is the role of pregnancy-specific stressors, which contribute to the total burden of distress and are generally unmeasured [18,19]. Second is the possible salutary influence of positive maternal emotions, which have been associated with positive antenatal health behaviors and more favorable birth outcomes [20].
The present study addressed the limitations of previous studies using a prospective, longitudinal design with repeated assessment of uterine and umbilical Doppler velocimetry collected concurrently with maternal psychological measures, both positive and negative. We hypothesized that indicators of maternal psychological distress would be associated with increased vascular resistance. Based on the evidence cited above for a link between positive maternal emotions and favorable birth outcomes, we also hypothesized that indicators of maternal psychological well-being would be associated with decreased resistance.
Methods
Sample
Participants were 120 normotensive, non-smoking women with normally progressing singleton pregnancies. Participants were self-identified volunteers recruited via local university and hospital-based advertisements. Women with medical conditions that complicate pregnancy (e.g., Type I diabetes) were not eligible to participate. Participants with gestational diabetes (n = 7), current treatment for depression (n = 8), or both (n = 2) were excluded from the analysis resulting in a sample of 107. Accurate dating of the pregnancy, based on early first trimester pregnancy testing or examination and generally confirmed by early ultrasound was required (mean gestational age at pregnancy detection = 4.8 weeks; sd = 1.2). The sample represents a relatively stable population of well-educated (mean years education = 17.2 years, sd = 2.1), mature (mean age = 31.2, sd = 4.6), married (90.7%) women. Most were non-Hispanic white (79.4%); the remainder was African-American (13.1%) and Hispanic or Asian (7.5%). Fifty-six (52.3%) of the fetuses were female, and this was the first pregnancy for 77 (72%) of the participants.
Data assessment and design
In order to fully represent the gestational age span from 24 to 38 weeks gestation, participants were stratified into 3 cohorts with staggered entry into the protocol between 24 and 26 weeks gestation and were tested in 3-week intervals. That is, data collection for the first cohort proceeded at 24, 27, 30, 33, and 36 weeks; the second at 25, 28, 31, 34, and 37 weeks; and the third at 26, 29, 32, 35, and 38 weeks. Data collection took place in a hospital setting. Study visits were scheduled at 13:00 or 15:00 and women were instructed to eat 1.5 hours prior to the visit but not thereafter. Women completed psychosocial questionnaires upon arrival. The local Institutional Review Board approved this research, and women provided informed consent.
Measures
Uterine and umbilical artery blood flow was measured with Doppler ultrasound (Picus, Pie Medical) using a 3.5 MHz transabdominal probe by a single, experienced operator (K.C.) with women in a supine position. Color Doppler imaging was used to locate the main branches of the right and left uterine arteries and the Doppler gate was positioned close to their junction with the internal iliac artery. The angle of insonation was adjusted to maximize the systolic peak with an angle less than 35 degrees. A series of three consecutive waveforms were recorded and traced. A standard clinical indicator of arterial resistance (i.e., resistance index, RI) was calculated using values derived from the single best waveform. The umbilical artery was similarly imaged with color Doppler, and the RI was computed based on sampling at a distance from the placental and umbilical cord insertion site.
The psychosocial questionnaires were administered at each visit and included measures of general anxiety, depressive symptoms, emotional well-being, and pregnancy-specific stress and uplifts. Anxiety symptoms were evaluated by the Spielberger Trait Anxiety Scales (Y-2; STAI) [21], one of the most extensively validated self-administered anxiety measures and the most commonly used measure of anxiety during pregnancy. This questionnaire includes twenty 4-point items; higher scores indicate greater trait anxiety. The STAI has been found to have strong psychometric properties, including a median alpha reliability coefficient of 0.90 [21]. Depressive symptoms were assessed using the Center for Epidemiologic Survey Depression Scale (CES-D) [22]. The CES-D is a widely-used 20-item self-report measure of depressive symptomatology over the past week in which items are rated on a 4-point scale. The CES-D has been used in numerous studies of the perinatal period, and Cronbach α results ranging from 0.83 to 0.88 reflect high internal reliability during pregnancy and postpartum [23–25]. Emotional well-being was assessed using the World Health Organization Five Well-being Index (WHO-5) [26], a five-item self-report measure that has been shown to possess good psychometric properties [27–29]. Pregnancy-specific stress was assessed by a shortened form of the previously validated Pregnancy Experiences Scale (PES) [18]. The PES-Brief includes the 10 most frequently endorsed hassles and uplifts from the full PES, each rated on the original 4-point scale, and averaged. Higher values reflect greater perceived intensity of negative or positive feelings about the pregnancy. Comparable reliability and stability to the original have been demonstrated [30].
Data analysis
Descriptive analyses examining correspondence among Doppler values and with the psychological scales were based on correlation coefficients. Hierarchical linear modeling (HLM) was used to characterize change in RI from 24 to 38 weeks for the umbilical artery and uterine arteries. Restricted maximum likelihood (REML) was used in reporting model parameters when assessing the significance of the random effects; degrees of freedom were estimated using the Satterthwaite method. The 95% confidence interval (CI) for each fixed effect was calculated as its estimate ± 2 standard errors. Random effects specified for cohorts and individuals were assessed (i.e., the covariance of scores at 38 weeks across cohorts and individuals). In the event that the estimate of the variance was very small and not statistically significant, we ignored the effect of between-cohort variance on the total variance and dropped it from the final model, whereas random effects that were statistically significant were retained in the final models. Thus, results reported were based on 2-level HLM models with repeated measurements nested within individuals. A number of covariates at the individual level were evaluated as potential confounders for inclusion into the final HLM models.
Because data were collected at 15 successive gestational weeks, interpreting individual correlations between each psychological measure and the three Dopplers at each gestational week was not feasible. Instead, preliminary analyses were conducted by visit for each of the 3 sequential cohorts. For example, visit 1 includes data collected at 24, 25, or 26 weeks gestation. Because there is conceptual and empirical overlap between psychological constructs measured by these scales, composites were created to reduce the number of final HLM models and increase the stability of the estimates. The STAI, CES-D, and PES-Brief Hassles values were separately standardized using z scores at each time period and then summed to create a maternal distress composite. The same procedures were implemented with the WHO-5 and PES-Brief Uplifts values to create a maternal well-being composite. Composites were entered as predictors in separate HLM models. HLM analyses used the Mixed procedure in SAS version 9.2 (SAS Institute, Cary, NC).
Results
Participation rates were as follows: 102 (95.3%) at visit 1; 95 (88.7%) at visit 2, 90 (84.1%) at visit 3, 97 (90.7%) at visit 4, and 82 (76.6%) at visit 5. At each visit, some missing values were generated by an inability to obtain a sufficiently clear Doppler signal, resulting in additional slight variation in sample sizes over gestation. A single outlier value for right uterine artery RI was removed at visit 2.
Longitudinal progression of blood flow resistance
RI values within each artery displayed low to moderate consistency over the five visits: correlation coefficients ranged from 0.07 to 0.32 with an average correlation of 0.21 for the umbilical artery, from 0.05 to 0.44 for the right uterine artery (average association = 0.26), and from 0.16 to 0.44 for the left uterine artery (average association = 0.29). Intraclass correlation coefficients for measurements of the umbilical, right uterine, and left uterine artery RI values were 0.24, 0.27 and 0.27, respectively.
Fetal sex and maternal characteristics, including parity, mean arterial pressure, maternal age, and maternal weight, were assessed as possible influences on RI. Parity was associated with right (estimate = 0.026, SE = 0.012, t = 2.09, p < 0.05) and left (estimate = 0.26, SE = 0.012, t = 2.3, p < 0.05) uterine RI values such that nulliparous women had lower uterine resistance than parous women. However, nulliparous women were also younger (t = −2.38, p < 0.05) and had higher MAP at 3 of the 5 visits. Umbilical RI was unrelated to these maternal characteristics or fetal sex. Parity (nulliparous versus parous) was controlled in longitudinal models.
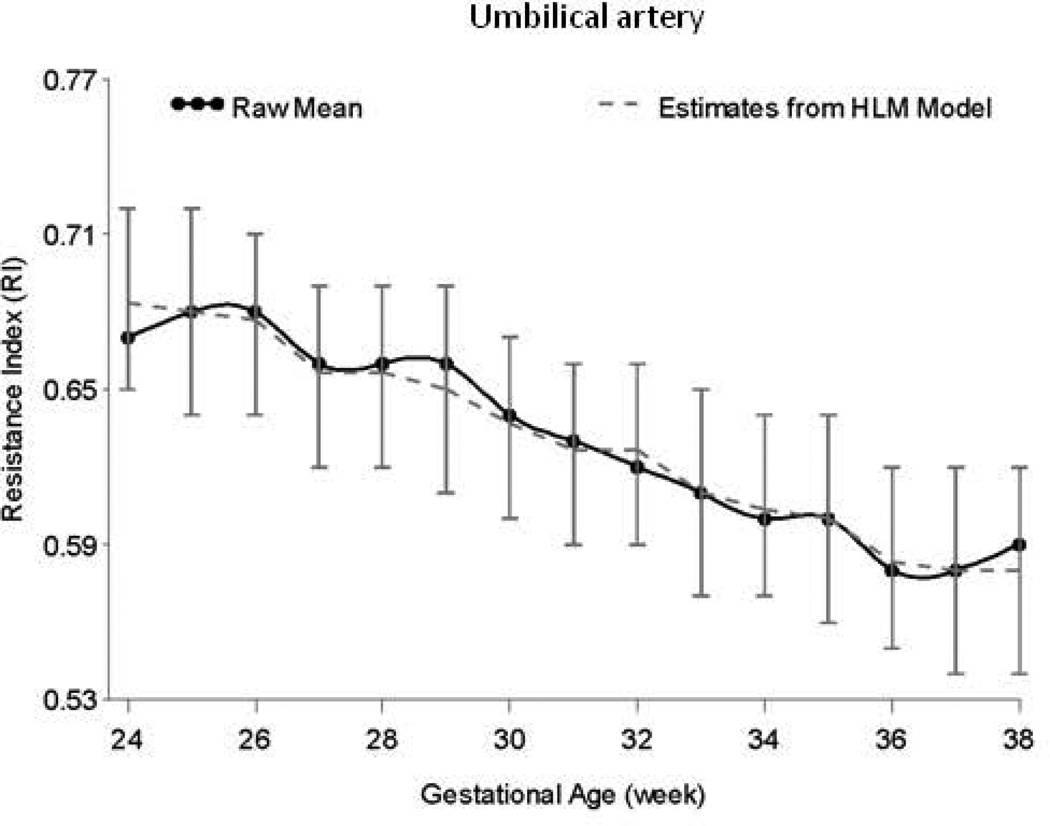
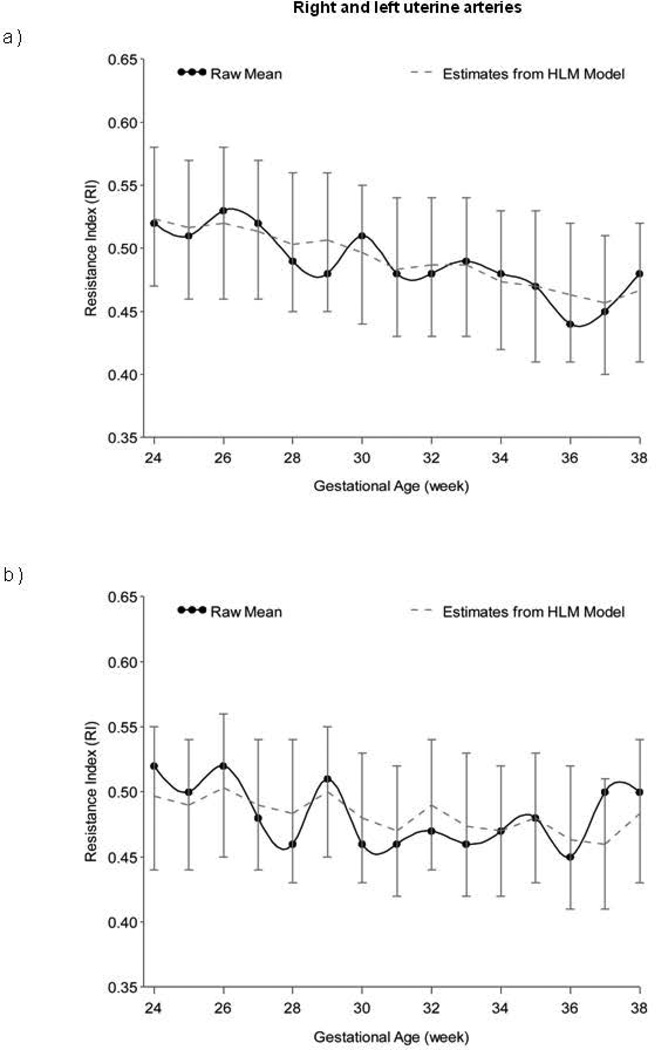
HLM analyses controlling for parity indicated significant decline in RI for the umbilical artery (estimate = −0.008, SE = 0.001, t = −15.08, p < 0.0001), right uterine artery (estimate = −0.005, SE = 0.001, t = −5.59, p < 0.0001), and left uterine artery (estimate = −0.002, SE = 0.001, t = −2.72, p < 0.01) from 24 to 38 weeks. Figures 1 and 2 display RI values for the umbilical and uterine arteries, respectively, over time.
Figure 1.
Longitudinal progression of the resistance index (RI) in the umbilical artery, adjusted for parity, and with confidence intervals at each data point.
Figure 2.
Longitudinal progression of the resistance index (RI) in the a) right and b) left uterine arteries, adjusted for parity, and with confidence intervals at each data point.
Association of maternal psychological factors and blood flow resistance: cross sectional analyses
Associations between right and left uterine RI within women were fairly low (ranging from −0.07 to 0.37 with a mean association of 0.21), and preliminary analyses revealed different patterns of associations with psychosocial values. As a result, analyses evaluating the associations between psychological factors and RI were conducted separately for each side.
STAI, CES-D, WHO-5 and PES-Brief hassle scores were not correlated with umbilical or either uterine artery RI at any visit. PES-Brief uplifts were marginally associated with left uterine artery RI at visits 4 (r = −0.20, p < 0.07) and 5 (r = −0.21, p = 0.08). Correlations were also computed between these measures and averaged right and left uterine RI values to replicate reports from other studies that have used this approach; no significant associations were observed with any psychological factors.
Association of maternal psychological factors and blood flow resistance: longitudinal analyses
Longitudinal analyses were estimated using the two composite scales of maternal distress and well-being. Maternal distress and well-being were negatively correlated at each study visit: r = −0.54 (p < 0.0001) at visit 1, r = −0.49 (p < 0.0001) at visit 2, r = −0.64 (p < 0.0001) at visit 3, r = −0.60 (p < 0.0001) at visit 4, and r = −0.66 (p < 0.0001) at visit 5. Results of the HLM models for maternal distress and well-being are presented in Table 1. Parity was controlled in each model. Maternal well-being was associated with left uterine artery resistance, such that higher levels of well-being were associated with lower RI (estimate = −0.006, SE = 0.003, t = −2.09, p < 0.05). Higher levels of maternal distress were also associated with lower RI, but in the right artery (estimate = −0.005, SE = 0.002, t = −2.35, p < 0.05). Umbilical RI was unrelated to maternal psychological composite scores.
Table 1.
Hierarchical linear models testing the association of maternal psychological distress and well-being with blood flow resistance in the umbilical and uterine arteries.
| Umbilical Artery RI | Right Uterine Artery RI | Left Uterine Artery RI | |||||||
| Fixed Effects | Estimate | SE | t | Estimate | SE | t | Estimate | SE | t |
| Intercept | 0.57 | 0.01 | 95.53*** | 0.45 | 0.01 | 49.08*** | 0.46 | 0.01 | 53.93*** |
| Gestational Age | −0.009 | 0.001 | −15.08*** | −0.005 | 0.001 | −5.60*** | −0.002 | 0.001 | 2.73* |
| Parity | −0.002 | 0.01 | −0.22 | 0.03 | 0.01 | 2.21* | 0.02 | 0.01 | 2.28* |
| Maternal Distress | −0.001 | 0.001 | −0.58 | −0.005 | 0.002 | −2.35* | 0.001 | 0.002 | 0.49 |
| Random Effects | Estimate | SE | Z | Estimate | SE | Z | Estimate | SE | Z |
| Intercept Variance | 0.001 | 0.000 | 3.84*** | 0.002 | 0.001 | 3.88*** | 0.002 | 0.000 | 4.05*** |
| Residual | 0.002 | 0.000 | 12.93*** | 0.005 | 0.000 | 12.47*** | 0.005 | 0.000 | 12.66*** |
| Fixed Effects | Umbilical Artery RI | Right Uterine Artery RI | Left Uterine Artery RI | ||||||
| Intercept | 0.57 | 0.01 | 95.62*** | 0.45 | 0.01 | 48.84*** | 0.46 | 0.01 | 54.60*** |
| Gestational Age | −0.009 | 0.001 | −15.14*** | −0.005 | 0.001 | −5.58*** | −0.002 | 0.001 | 2.79* |
| Parity | −0.003 | 0.01 | −0.39 | 0.03 | 0.01 | 2.25* | 0.02 | 0.01 | 2.09 |
| Maternal Well-being | −0.003 | 0.002 | −1.30 | 0.003 | 0.003 | 0.97 | −0.006 | 0.003 | −2.09* |
| Random Effects | Estimate | SE | Z | Estimate | SE | Z | Estimate | SE | Z |
| Intercept Variance | 0.001 | 0.000 | 3.84*** | 0.002 | 0.001 | 3.88*** | 0.002 | 0.000 | 4.05*** |
| Residual | 0.002 | 0.000 | 12.93*** | 0.005 | 0.000 | 12.47*** | 0.005 | 0.000 | 12.66*** |
p < 0.05
p < 0.01
p < 0.0001
Note Gestational age was centered at 38 weeks. Random effects specified for the models for the covariance of the intercept across individuals were all statistically significant with p < 0.0001.
Discussion
This prospective cohort study of 104 singleton, normally progressing pregnancies assessed the longitudinal progression of blood flow resistance from 24–38 weeks gestation and evaluated the associations of maternal psychological factors with blood flow resistance across that period. Decreases in umbilical and uterine artery resistance were observed with advancing gestation, consistent with a previous report [31]. A progressive decrease in blood flow resistance is consistent with the physiology of a healthy pregnancy: invasion of placental trophoblast cells causes progressive dilation in the maternal spiral arteries, decreasing resistance and facilitating transfer of oxygen and nutrients to the developing fetus [32,33]. Nulliparous women had lower uterine blood flow resistance than parous women, consistent with an earlier result generated between 18 and 23 weeks gestation [34]. The current findings confirm that persistent alterations to maternal vasculature are established in subsequent pregnancies and demonstrate that these remain in place throughout gestation.
Cross sectional analyses did not reveal any significant associations between individual psychological scales and resistance, with the exception of marginal associations between pregnancy-specific uplifts and decreased resistance after 33 weeks. Averaging right and left RI values, as done in some prior studies [14,15,17], also yielded no significant associations at any visit. Our findings are consistent with those of Kent and colleagues [15], in which no association between maternal anxiety and uterine artery resistance at 20 weeks gestation was detected but in contrast to two other studies that reported modest cross sectional associations, i.e., between anxiety at 32 weeks and averaged uterine artery resistance [14] and anxiety at term and umbilical pulsatility [16]. The current results generated from this prospective cohort raise questions about the replicability and generalizability of findings based on single psychological instruments assessed at a single gestational age.
More coherent findings were revealed when the longitudinal study design was used, and composite measures of psychological functioning were formulated. From a conceptual standpoint, analyses based on single instrument assessment of psychological functioning do not adequately capture how various types of emotional experience overlap and cohere. As predicted, maternal psychological well-being was associated with decreased resistance, although only in the left uterine artery. This suggests that a positive outlook during pregnancy confers a benefit to blood flow. This observation is bolstered by a finding that women who slowed their breathing more to an experimental relaxation manipulation had decreased uterine artery resistance following the procedure [35]. Measurement instruments for positive emotions are much less developed than those for negative ones, and better understanding of the complex nature of the interface between maternal emotions and the physiology of pregnancy would be advanced by development of more sophisticated tools.
Maternal distress was also associated with resistance, but only in the right uterine artery, and the direction of association was counter to prediction. An adverse association between distress and uterine artery RI has only been reported in a single study, and that finding was limited to 32 weeks gestation [14]. We have no ready explanation as to why greater maternal distress is associated with decreased resistance. It is possible that greater antepartum emotionality in general, either positive or negative, may confer an advantage to blood flow. We are unable to determine whether the differential findings in the right and left arteries reflect true physiologic differences. Resistance values were generated while women were in a supine, not lateralized position, so it is possible that uterine blood flow characteristics were influenced as well by aortic or IVG compression. We did not document placental lateralization, and since placental location lowers ipsilateral Dopplers, a bias towards right or left implantation in the sample as a whole may have introduced a source of confound.
This study has certain limitations. As we did not recruit women with psychiatric disorders, our findings may not generalize to psychiatric pregnant populations. In this study, psychological factors were assessed using self-report measures and were not evaluated with clinician ratings. Self-report ratings may reflect subjective response biases; however, recall bias is unlikely to have affected results given that participants were asked to report on emotional states over the past 1–2 weeks.
In summary, results suggest that the maternal psychological milieu during pregnancy exerts influence on uterine artery, but not umbilical, blood flow. Study strengths include the prospective longitudinal design with multiple antenatal assessments and measurement of both umbilical and uterine artery resistance as well as multiple maternal psychological indicators. Although the results are statistically significant, they suggest very modest associations. We believe the key implication of these findings is in disputing the assumption that documented associations between maternal distress and poor pregnancy outcomes, including growth restriction, are mediated by changes in blood flow. Findings in the current study illustrate the complexity of the interface between maternal psychological function and the physiology of normal pregnancy, as well as limitations in the current knowledge base.
Current knowledge on this subject.
Alteration in blood flow from mother to fetus has been proposed as a mechanism by which maternal psychological distress may influence pregnancy outcomes.
Only four studies have evaluated the relation of maternal psychological factors to maternal-fetal blood flow, with mixed results.
Previous research in this area has a number of limitations, including cross-sectional designs in most studies.
What this study adds
This study used a prospective longitudinal design with multiple antenatal assessments, and it included measures of both umbilical and uterine artery resistance, as well as multiple maternal psychological indicators.
Our findings suggest uterine artery resistance is modestly associated with the maternal psychological milieu during gestation.
Findings do not indicate an association between increased maternal distress and decreased RI and thus do not support vasoconstriction as a compelling mechanism in translating maternal emotions to fetal growth or other aspects of development.
Acknowledgments
This research was funded by the National Institute for Child Health and Human Development (R01HD27592; P.I.: DiPietro).
Footnotes
Declaration of Interests
The authors report no conflicts of interest.
References
- 1.Austin M, Leader L. Maternal stress and obstetric and infant outcomes: epidemiological findings and neuroendocrine mechanisms. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2000;40:331–337. doi: 10.1111/j.1479-828x.2000.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 2.Copper RL, Goldenberg RL, Das A, Elder N, Swain M, Norman G, Ramsey R, Cotroneo P, Collins BA, Johnson F, Meier AM. The preterm prediction study: Maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks' gestation. American Journal of Obstetrics and Gynecology. 1996;175:1286–1292. doi: 10.1016/s0002-9378(96)70042-x. [DOI] [PubMed] [Google Scholar]
- 3.Glynn L, Schetter C, Hobel C, Sandman C. Pattern of perceived stress and anxiety in pregnant predicts preterm birth. Health Psychology. 2008;27:43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Paarlberg K, Vingerhoets A, Passchier J, Dekker G, Heinen A, vanGeijn H. Psychosocial predictors of low birthweight: a prospective study. British Journal of Obstetrics and Gynaecology. 1999;106:834–841. doi: 10.1111/j.1471-0528.1999.tb08406.x. [DOI] [PubMed] [Google Scholar]
- 5.Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: A prospective investigation. American Journal of Obstetrics and Gynecology. 1993;169:858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- 6.Suri E, Altshuler L, Hellemann G, Burt V, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. American Journal of Psychiatry. 2007;164:1206–1213. doi: 10.1176/appi.ajp.2007.06071172. [DOI] [PubMed] [Google Scholar]
- 7.Littleton H, Breitkopf C, Berenson A. Correlates of anxiety symptoms during pregnancy and association with perinatal outcomes: A meta-analysis. American Journal of Obstetrics and Gynecology. 2007;196:424–432. doi: 10.1016/j.ajog.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Neonatal outcome following maternal antenatal depression and anxiety: a population-based study. American Journal of Epidemiology. 2004;159:872–881. doi: 10.1093/aje/kwh122. [DOI] [PubMed] [Google Scholar]
- 9.DiPietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Development. 2006;77:573–587. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 10.Perkin M, Bland J, Peacock J, Anderson H. The effect of anxiety and depression during pregnancy on obstetric complication. British Journal of Obstetrics and Gynaecology. 1993;100:629–634. doi: 10.1111/j.1471-0528.1993.tb14228.x. [DOI] [PubMed] [Google Scholar]
- 11.Alder J, Fink N, Bitzer J, Hosli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. Journal of Maternal-Fetal & Neonatal Medicine. 2007;20:189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- 12.VandenBergh B, Mulder E, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioral development of the fetus and child: links and possible mechanisms. A review. Neuroscience and Biobehavioral Reviews. 2005;29:237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Cnossen J, Morris R, ter-Riet G, Mol B, VanderPost J, Coomarasamy A, Zwinderman AH, Robson SC, Bindels PJ, Kleijnen J, Khan KS. Use of uterinal artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariate meta-analysis. Canadian Medical Association Journal. 2008;178:701–711. doi: 10.1503/cmaj.070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. British Medical Journal. 1999;318:153–157. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kent A, Hughes P, Ormerod L, Jones G, Thilaganathan B. Uterine artery reistance and anxiety in the second trimester of pregnancy. Ultrasound in Obstetrics and Gynecology. 2002;19:177–179. doi: 10.1046/j.0960-7692.2001.00546.x. [DOI] [PubMed] [Google Scholar]
- 16.Sjostrom K, Valentin L, Thelin T, Marsal K. Maternal anxiety in late pregnancy and fetal hemodynamics. European Journal of Obstretics & Gynecology. 1997;74:149–155. doi: 10.1016/s0301-2115(97)00100-0. [DOI] [PubMed] [Google Scholar]
- 17.Maina G, Saracco P, Giolito M, Danelon D, Bogetto F, Todros T. Impact of maternal psychological distress on fetal weight, prematurity and intrauterine growth retardation. Journal of Affective Disorders. 2008;111:214–220. doi: 10.1016/j.jad.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 18.DiPietro JA, Ghera MM, Costigan KA, Hawkins M. Measuring the ups and downs of pregnancy. Journal of Psychosomatic Obstetrics and Gynaecology. 2004;25:189–201. doi: 10.1080/01674820400017830. [DOI] [PubMed] [Google Scholar]
- 19.Lobel M, Cannella D, Graham J, DeVincent C, Schneider J, Meyer B. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychology. 2008;27:604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 20.Lobel M, DeVincent C, Kaminer A, Meyer B. The impact of prenatal maternal stress and optimistic disposition on birth outcomes in medically high-risk women. Health Psychology. 2000;19:544–553. doi: 10.1037//0278-6133.19.6.544. [DOI] [PubMed] [Google Scholar]
- 21.Spielberger C. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Mind Garden, Inc; 1983. [Google Scholar]
- 22.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 23.Mercer R, Ferketich S. Stress and social support as predictors of anxiety and depression during pregnancy. ANS. Advances in Nursing Science. 1988;10:36–39. doi: 10.1097/00012272-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Mosack V, Shore E. Screening for depression among pregnant and postpartum women. Journal of Community Health Nursing. 2006;23:37–47. doi: 10.1207/s15327655jchn2301_4. [DOI] [PubMed] [Google Scholar]
- 25.Troy N. A comparison of fatigue and energy levels at 6 weeks and 14 to 19 months postpartum. Clinical Nursing Research. 1999;8:135–152. doi: 10.1177/10547739922158205. [DOI] [PubMed] [Google Scholar]
- 26.Regional Office for Europe; World Health Organization (WHO) Stockholm, Sweden: 2008. Well-being measures in primary health care: The DepCare Project. [Google Scholar]
- 27.Heun R, Burkat M, Maier W, Bech P. Internal and external validity of the WHO Well-Being Scale in the elderly general population. Acta Psychiatrica Scandinavica. 1999;99:171–178. doi: 10.1111/j.1600-0447.1999.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 28.deWit M, Pouwer F, Gemke R, Waal HD-vd, Snoek F. Validation of the WHO-5 Well-Being Index in adolescents with type I diabetes. Diabetes Care. 2007;30:2003–2006. doi: 10.2337/dc07-0447. [DOI] [PubMed] [Google Scholar]
- 29.Lowe B, Spitzer R, Grafe K, Kroenke K, Quenter A, Zipfel S, al e. Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians' diagnoses. Journal of Affective Disorders. 2004;78:131–140. doi: 10.1016/s0165-0327(02)00237-9. [DOI] [PubMed] [Google Scholar]
- 30.DiPietro JA, Christensen A, Costigan KA. The Pregnancy Experience Scale - Brief version. Journal of Psychosomatic Obstetrics & Gynaecology. 2008;29:262–267. doi: 10.1080/01674820802546220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurmanavicius J, Florio I, Wisser J, Hebisch G, Zimmerman R, Müller R, Huch R, Huch A. Reference resistance indices of the umbilical, fetal middle cerebral and uterine arteries at 24–42 weeks of gestation. Ultrasound in Obstetrics and Gynecology. 1997;10:112–120. doi: 10.1046/j.1469-0705.1997.10020112.x. [DOI] [PubMed] [Google Scholar]
- 32.Hollis B, Prefumo F, Bhide A, Rao S, Thilanganathan B. First-trimester uterine artery blood flow and birth weight. Ultrasound in Obstetrics and Gynecology. 2003;22:373–376. doi: 10.1002/uog.231. [DOI] [PubMed] [Google Scholar]
- 33.Sciscione A, Hayes E. Uterine artery Doppler flow studies in obstetric practice. American Journal of Obstetrics and Gynecology. 2009;201:121–126. doi: 10.1016/j.ajog.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Prefumo F, Bhide A, Sairam S, Penna L, Hollis B, Thilaganathan B. Effect of parity on second-trimester uterine artery Doppler flow velocity and waveforms. Ultrasound in Obstetrics and Gynecology. 2004;23:46–49. doi: 10.1002/uog.908. [DOI] [PubMed] [Google Scholar]
- 35.DiPietro J, Costigan K, Nelson P, Gurewitsch E, Laudenslager M. Maternal and fetal responses to induced relaxation during pregnancy. Biological Psychology. 2008;77:11–19. doi: 10.1016/j.biopsycho.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]