Abstract
Purpose.
The goal of this study was to functionally compare prostaglandin E2 (PGE2)-sensitive receptors in human primary cells involved in conventional outflow.
Methods.
The expression profile of prostaglandin (PG) receptors in primary cultures of human trabecular meshwork (TM) and Schlemm's canal (SC) cells were determined by quantitative-PCR. The functional activities of endogenous PGE2-sensitive receptors were evaluated using subtype-selective agonists and antagonists with cell impedance technology.
Results.
Agonist-sensitive EP1, EP2, and EP4 receptors were present in TM cells, all increasing cell stiffness (or contractility) in a dose-dependent manner. Rank order of efficacy (Emax) for agonists in TM cells were EP1 greater than EP2 greater than EP4 with EC50 1.1 μM, 0.56 μM, and 0.1 μM, respectively, and no functional EP3 receptors were found. Of the four EP receptor subtypes active in SC cells, EP1 and EP3 receptor activation increased cell stiffness, while EP2 and EP4 agonists dose-dependently decreased cell stiffness 47% and 23% with EC50 values of 170 nM and 69 nM, respectively. Consistent with these observations, the Rho kinase inhibitor Y-27632 decreased cell impedance (stiffness) of TM and SC cells (∼60%), while Rho GTPase activator thrombin caused cell impedance to increase in both cell types (168%–190%).
Conclusions.
Cell impedance positively correlates with cellular stiffness/contractility. Because EP2/4 receptors caused decreased cell stiffness in SC, but not in TM cells, both receptors appear to mediate IOP lowering via changes in SC cell stiffness in the conventional outflow pathway.
Keywords: cell impedance, conventional outflow, prostaglandin E2 receptors, Schlemm's canal, trabecular meshwork
Cell impedance positively correlated with cellular stiffness/contractility. Prostaglandin E2 (PGE2)-sensitive receptors EP2 and EP4 appear to mediate IOP lowering via changes in Schlemm's canal (SC) cell stiffness in the conventional outflow pathway.
Introduction
There are two pathways for aqueous humor drainage in human eyes. One pathway is uveoscleral outflow, which decreases with age and is responsible for a minor fraction (10% ∼ 30%) of the total aqueous flow in the adult human eye.1 The other pathway is the pressure-dependent, conventional outflow pathway comprised of the trabecular meshwork (TM) and Schlemm's canal (SC), which facilitates the majority of aqueous humor outflow. Together, these two tissues regulate aqueous outflow resistance and, thus, IOP.2 A malfunction of TM and/or SC cells sequentially causes increased outflow resistance, ocular hypertension, glaucoma, and eventual vision loss.
Prostaglandins (PGs) lower IOP in man, in animal models, and in perfused human eyes.3 Of these, the PGF2α mimetics are the only class of PGs on the market and the most effective topical medication for glaucoma patients currently.4 About a decade ago, the uveoscleral outflow pathway was thought to be the only site of action for this class of ocular antihypertensive agents. The involvement of PGF2α mimetics in enhanced conventional outflow facility was first uncovered in glaucoma patients undergoing Bimatoprost or Travoprost treatment,5,6 then confirmed in studies testing Bimatoprost and Latanoprost in perfused human anterior chamber segments.7,8 Other PGs, like the EP4 agonist 3,7-dithia PGE1, also impact conventional outflow function, reducing IOP in cynomolgus monkeys by 40% to approximately 50% without effecting uveoscleral outflow.9 Effects of this EP4 agonist on conventional outflow were validated in perfused human and mouse eyes where outflow facility increased by 69% and 106%, respectively.3,10 Of the four EP receptor subtypes (EP1–4), EP2 and EP4 are known to mediate IOP lowering more profoundly than prostaglandin F2α receptor (FP), however, the mechanism of action of any of the PGE2-sensitive receptors on conventional outflow function are unknown.9,11 We hypothesized that activation of PGE2-sensitive receptors in the conventional tract impacts the contractility state of human outflow cells. Cell contraction and relaxation of TM and SC cells are thought to modulate conventional outflow function. TM and SC cell contraction is associated with increased cell stiffness, cell–cell attachment, and outflow resistance, whereas cell relaxation is doing the exact opposite.12,13 For example, the Rho kinase inhibitor Y-27632 increased outflow facility in eyes of living animals,12,14–16 deceased actin stress fiber, and cell–cell attachment, caused cell retraction/relaxation in TM and SC cells, as well as increased monolayer permeability of SC cells.12 On the other hand, the Rho GTPase activator thrombin has been reported to induce endothelial cell contraction, decrease outflow facility in porcine eyes, increase actin stress fiber, and decease monolayer permeability in cultured primary SC cells.17–21 Moreover, stiffness of SC cells has been suggested to correlate better with decreased conventional outflow facility.13,18 To investigate the IOP-lowering mechanism of action and to extend our previous study on FP, prostaglandin I2 receptor (IP), and thromboxane A2 receptor (TP) in human TM and SC cells, the functional activities of PGE2-sensitive receptors were examined using cell impedance technology, and PG receptor gene expression profile was determined using RT–quantitative PCR (qPCR). To understand the relationship between cell impedance and cell stiffness, the current study compared PG results with two well characterized agents, Rho kinase inhibitor Y-27632, and the Rho GTPase activator thrombin.
Materials and Methods
Cell Culture
SC and TM cells were isolated and cultured from human eyes as previously described.4,22,23 Three cell strains of each cell type (isolated from six different donors) were examined in the present study. Cell strain number and age of donor at time of death are indicated: TM86 (3 months), TM93 (35 years), and TM96 (28 years), and SC58 (34 years), SC67 (44 years), and SC75 (10 years).
Reagents
The EP1/3 agonist Sulprostone, the EP2 agonist Butaprost, and the FP agonist 17-phenyl PGF2α, and the TP agonist U46619 were purchased from Cayman (Ann Arbor, MI). The following compounds were synthesized at Target Molecules (Southampton, England), of which the selective activities have been published in the cited references: EP1 antagonist SC-51322,24 EP2 antagonist PF-04418948,25 EP3 antagonist compound 3ap,26 EP4 agonist L-902688,27 and antagonist GW627368X,28 and IP agonist Cicaprost.24 Thrombin from human plasma and Y-27632 were purchased from Sigma (St. Louis, MO). All reagents were stored as 10 mM stock solution in dimethyl sulfoxide (DMSO), except for thrombin, which was solubilized in serum free low glucose Dulbecco's modified Eagles medium (DMEM; Invitrogen, Carlsbad, CA) medium at 1000 National Institutes of Health (NIH) U/ml.
PG Receptor RNA Expression by RT-qPCR
Total RNA was isolated from 1.25 × 106 to 2.9 × 106 SC or TM cells from each donor using RNeasy Plus Mini Kit (Qiagen, Valencia, CA). The procedures for two-step RT-qPCR and primers for PG receptors EP1-4, FP, and TP were previously described.29 Primers for IP receptor: forward primer 5′-GTG TGC TCC CTG CCT CTC-3′, reverse primer 5′-AGG AGG TCC CCC ATC TCA-3′. All PG receptor primers were validated using complementary DNAs (cDNAs) from recombinant human cells that are known to express a specific target gene, and the negative control cDNA. Total RNA of 200 ng was used per 20 μL RT reaction to generate the first-strain cDNA. Then 1 μL first-strain cDNA was used per 20 μL qPCR reaction.
Cell Impedance Assay
The cell impedance assay, also described as cellular dielectric spectroscopy in our previously published article,4 was performed using the xCelligence RTCA System (Roche, Indianapolis, IN). This is a real-time, label-free cell analysis, which allows measurement of impedance-based cell index on native G-protein coupled receptors in primary cells. All conditions were tested in duplicate on SC (passage 5–7) and TM (passage 3–5) cells from three cell strains.
The 96-well E-Plates (Roche, Indianapolis, IN) containing 50 μL of seeding medium (low glucose DMEM media with 10% fetal bovine serum, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 0.29 mg/mL glutamine) were scanned by the xCelligence system for baseline calibration. Then, human SC or TM cells were seeded at 10,000 cells/well onto the 96-well E-Plates, incubated in 37°C with 5% CO2, and monitored for cell index changes overnight. The next morning, the seeding medium was changed to warm DMEM assay medium. Cells were placed back to the incubator and monitored until cell index was stabilized before dosing. The standard agonist dose curves were generated by a 6-point, 10-fold serial dilution at 10−5 to approximately 10−10 M final concentrations. Antagonists were tested in a single dose in comparison to the DMSO vehicle control against the appropriate agonist curve. Basal activity of the antagonists was tested without any agonists. The antagonists were dosed 30 minutes prior to agonist addition, with final concentration of 0.5 μM SC-51322 for blocking the Sulprostone signal on EP1, 0.5 μM compound 3ap for blocking the Sulprostone signal on EP3, 0.5 μM SC-51322 and 0.5 μM compound 3ap for blocking EP1 and EP3 signaling of Sulprostone, 1 μM PF-04418948 for EP2 selective agonist Butaprost antagonism, and 0.3 μM GW627368X for EP4 selective agonist L-902688 antagonism.
The effects of Y-27632 and thrombin on cell impedance were tested in agonist mode with 17-phenyl-PGF2α as the reference compound. Y-27632 was tested in 6-point, 10-fold serial dilution at 10−5 to approximately 10−10 M final concentrations. Thrombin was tested in 5-point, 2-fold serial dilution at 10, 5, 2.5, 1.25, and 0.625 U/mL final concentrations.
Cell impedance or cell index (CI) was recorded in real time throughout the experiment until at least 2 hours after the agonist addition. For data analysis, CI was normalized to 1 at time 0 of agonist addition (T0). The normalized maximum CI (CImax) within 30 minutes after agonist addition (T ≤ 30min) versus concentration were exported to Prism 4 (GraphPad, La Jolla, CA) to calculate the percentage activity of each data point relative to the positive control (the selective FP receptor agonist 17-phenyl-PGF2α at 10−6 M). Average effect of a standard agonist versus vehicle (EC50) or versus an antagonist (IC50) was obtained from nonlinear regression curve fit. The antagonism was expressed as the blocking constant Kb which equals [Antagonist Concentration]/(IC50/EC50-1). Because of the high sensitivity of the assay, when no antagonism was detected or when Kb was greater than or equal to 1000 nM, the antagonist was defined as not active (NA). Each graph represents the mean normalized maximum CI ± SEM at T less than or equal to 30min after agonist addition from cells of three different cell strains.
Cell Morphology and Viability Evaluation
TM or SC cells were seeded at 10,000 cells/well onto 96-well cell culture plates in the seeding medium mentioned as above, incubated in 37°C with 5% CO2 overnight. The next morning, the seeding medium was changed to warm DMEM medium and incubated for 2 hours before dosing with 1, 5, or 10 μM of Rho kinase inhibitor Y-27632, FP agonist 17-phenyl-PGF2α, EP2 agonist Butaprost, EP1/3 agonist Sulprostone, EP4 L-902688, IP agonist Cicaprost or TP agonist U46619, with 0.1% DMSO as the vehicle control. Thrombin was dosed at 10, 5, or 2.5 U/mL with DMEM medium as control. Cell morphology was examined at 2 and 4 hours, and overnight after dosing under a microscope with ×40 magnification. Cell viability was evaluated after overnight compound treatment by adding 10 μL Alamar Blue dye (Invitrogen) to the 100 μL dosing medium. After incubation at 37°C for 4 hours, cell plates were scanned for Alamar Blue intensity using a SpectraMAX GeminiEM fluorescent plate reader (Molecular Devices, Sunnyvale, CA) at excitation 530 and emission 590 nm.
Results
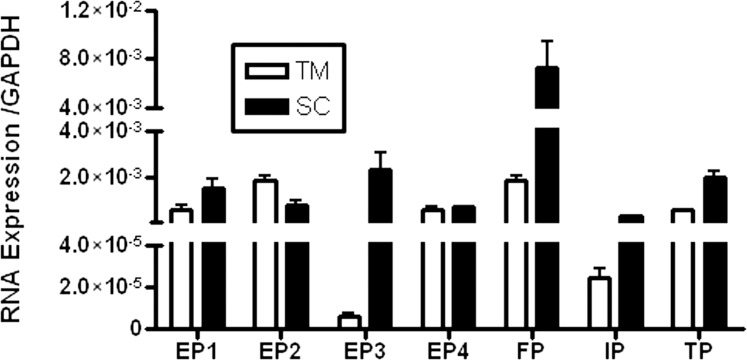
Combined data from three cell strains for each cell type show that TM cells express about 2-fold more total RNA than SC, as evidenced by the crossing point (Cp) value of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (17.3 in TM and 18.7 in SC) and the yield of total RNA per million of each cell type (data not shown). However, SC cells express more PG receptors, especially EP3, IP, and FP, than TM cells (Table 1). EP4 receptor expression is about equal in both cell types, and EP2 expression is less in SC cells. FP is the most abundant PG receptor expressed in both outflow cell types. Of the EP receptor subtypes, EP2 is most abundant in TM, while EP3 is most abundant in SC. The rank order of PG receptor RNA expression is FP equals EP2 greater than EP4 greater than or equal to EP1 greater than or equal to TP greater than IP greater than EP3 with relative copy number in the range of 1.9 × 10−3 to 6.3 × 10−6 in TM, and FP greater than EP3 greater than or equal to TP greater than or equal to EP1 greater than EP2 greater than EP4 greater than IP with relative copy number in the range of 7.3 × 10−3 to 3.6 × 10−4 in SC (Table 1 and Fig. 1).
Table 1.
PG Receptor RNA Expression Profile for Primary Cultures of Human TM and SC Cells by RT-qPCR (n = 3)
|
Ratio of Gene/GAPDH |
TM (GAPDH Cp = 17.3) |
SC (GAPDH Cp = 18.7) |
SC/TM, Fold |
||
|
Mean |
SD |
Mean |
SD |
||
| EP1 | 6.6E-04 | 3.1E-04 | 1.6E-03 | 7.0E-04 | 2.4 |
| EP2 | 1.9E-03 | 3.0E-04 | 8.2E-04 | 3.7E-04 | 0.4 |
| EP3 | 6.3E-06 | 2.6E-06 | 2.3E-03 | 1.4E-03 | 367.7 |
| EP4 | 6.7E-04 | 1.5E-04 | 7.7E-04 | 2.2E-05 | 1.2 |
| FP | 1.9E-03 | 2.8E-04 | 7.3E-03 | 3.6E-03 | 3.8 |
| IP | 2.4E-05 | 7.6E-06 | 3.6E-04 | 7.0E-05 | 14.7 |
| TP | 6.1E-04 | 1.1E-04 | 2.0E-03 | 5.1E-04 | 3.3 |
Figure 1.
PG receptor RNA expression profile in primary cultures of human TM and SC cells by RT-qPCR. Gene expression was normalized to GAPDH. Each condition was tested in triplicate from three cell strains of each cell type (n = 3, mean ± SD).
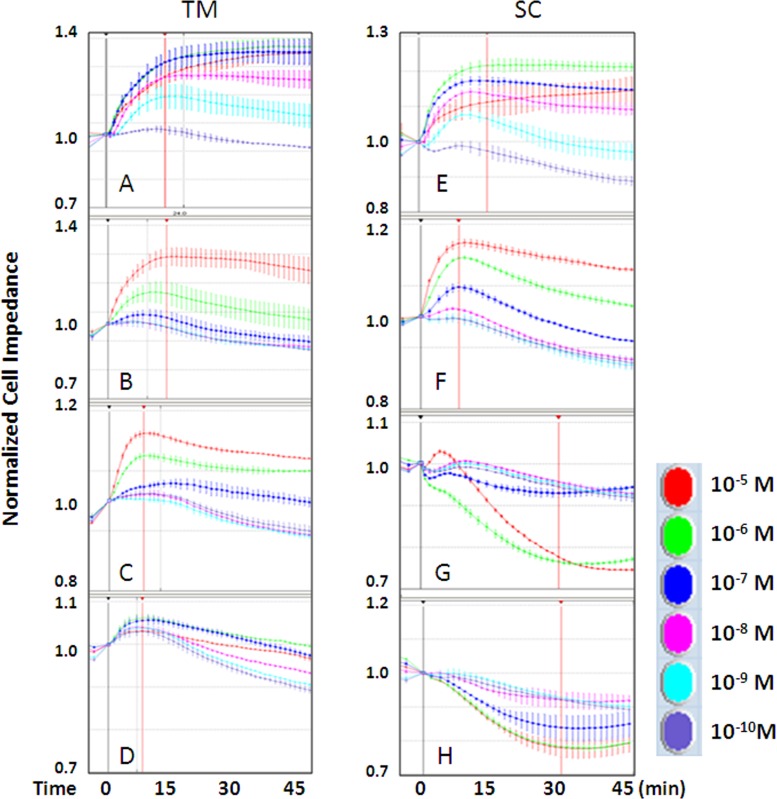
To examine the relationship between RNA expression profile and functional activity of the PG receptors, cell impedance assays were performed in human primary TM and SC cells using selective agonists. Figure 2 depicts the traces of cell impedance in TM and SC cells after FP agonist 17-phenyl PGF2α (Fig. 2A, 2E), EP1/3 agonist Sulprostone (Fig. 2B, 2F), EP2 agonist Butaprost (Fig. 2C, 2G), and EP4 agonist L-902688 (Fig. 2D, 2H) treatment over time. The data showed that, firstly, stimulation of EP1 and/or EP3 resulted in an increase of cell impedance in both TM and SC cells; secondly, the EP2 agonists Butaprost robustly induced cell impedance to increase in TM versus a decrease in SC cells; thirdly, the EP4 agonist L-902688 induced minimal cell impedance increase in TM compared with the obvious decrease in SC cells. Also, as seen previously, 17-phenyl PGF2α potently increases impedance in both TM and SC cells.4 The signature of cell impedance, in terms of the time (x-axis) and the vertical direction of the curve (y-axis) at CImax (indicated by the red vertical line) within 30 minutes of agonist addition was not only receptor dependent, but also cell type dependent.
Figure 2.
Individual traces of human TM and SC cell impedance in response to PG drugs over time. Cell impedances induced by graded dose (as shown in colors) of the FP agonist 17-phenyl PGF2α (A, E), the EP1/3 agonist Sulprostone (B, F), the EP2 agonist Butaprost (C, G), and the EP4 agonist L-902688 (D, H) were normalized to 1 at time 0 of agonist addition (black vertical line). Maximum changes in cell impedance within 30 minutes of agonist addition (red vertical line) were exported for data analysis. Shown are representative traces of responses from a single cell strain for each cell type. Similar results were observed in all three strains for each cell type (n = 3).
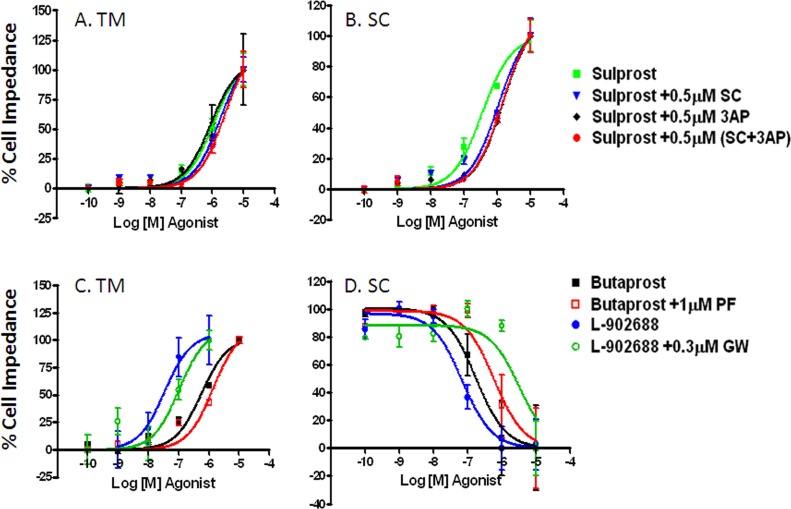
For comparisons, the dose response curves of EP receptor agonists on cell impedance were plotted using 10−6 M 17-phenyl PGF2α as the 100% reference (Fig. 3). Sulprostone elevated cell impedance in both cell types (TM Emax = 101%, SC Emax = 86%) with more than half a log lower potency in TM (EC50 = 1.1 μM) versus SC (EC50 = 0.4 μM). In contrast to the increased impedance in TM cells (EC50 = 0.6 μM, Emax = 77%), Butaprost decreased cell impedance in SC cells (EC50 = 0.2 μM, Emax = 47%). Similarly, L-902688 produced an upward response in TM and downward response in SC cells, with only minimal activity in TM cells (Emax < 15%) and midrange activity in SC (Emax = 23%, EC50 = 69 nM) (Table 2).
Figure 3.
Dose response curves of EP receptor agonists on cell impedance of human TM and SC cells. Maximum change in cell impedance within 30 minutes of agonist addition was exported for data analysis. Cell impedance induced by graded dose of selective agonists Sulprostone (for EP1/3), Butaprost (for EP2), L-902688 (for EP4) was normalized to 10−6M 17-phenyl PGF2α, a reference control. Each condition was tested in duplicate using three cell strains of each cell type (n = 3, mean ± SEM).
Table 2.
Agonist and Antagonist Activity of EP Receptors in Human TM and SC Cells
|
PG Receptor Agonist |
Versus |
TM |
SC |
||||
|
Emax, % |
EC50/IC50, M |
Kb, nM |
Emax, % |
EC50/IC50, M |
Kb, nM |
||
| 17-phenyl PGF2α | Vehicle | 88 | 7.90E-10 | - | 81 | 1.30E-09 | - |
| EP1/3 agonist Sulprostone | Vehicle | 101 | 1.1E-06 | - | 86 | 3.6E-07 | - |
| 0.5 μM SC-51322 | 137 | 1.8E-06 | 770 | 139 | 1.1E-06 | 244 | |
| vs. | 0.5 μM compound 3ap | 86 | 8.8E-07 | NA | 135 | 1.6E-06 | 148 |
| 0.5 μM (SC51322+3ap) | 105 | 2.6E-06 | 377 | 104 | 1.5E-06 | 154 | |
| EP2 agonist Butaprost | Vehicle | 77 | 5.6E-07 | - | 47 | 1.7E-07 | - |
| PF-04418948 | 106 | 1.4E-06 | 674 | 44 | 5.9E-07 | 420 | |
| EP4 agonist L-902688 | Vehicle | 14 | 3.4E-08 | - | 23 | 6.9E-08 | - |
| 0.3 μM GW-627368 | 17 | 1.0E-07 | 148 | 20 | 3.2E-06 | 7 | |
NA, no antagonism. E max is relative to 10−6 M 17-phenyl PGF2α.. EC50, IC50, and Kb are described in the Cell Impedance Assay of the Methods section. Data shown as mean of n = 3 cell strains for each cell type.
Next, to complete pharmacologic characterization of EP receptor subtypes in cell impedance assay, antagonist studies were performed using 0.5 μM of EP1 antagonist SC-51322, 0.5 μM of EP3 antagonist compound 3ap, 1 μM of EP2 antagonist PF-04418948, and 0.3 μM of EP4 antagonist GW-627368. Each antagonist was used at a concentration that did not impact baseline impedance of TM and SC cells (data not shown). As shown in Figure 4 and Table 2, Sulprostone-mediated TM cell responses were not blocked by compound 3ap and were only weakly antagonized by 0.5 μM SC-51322 alone (Kb = 770 nM). A fraction more inhibition was obtained when 0.5 μM SC-51322 and 0.5 μM compound 3ap were combined (Kb = 377 nM). In SC cells, the blockade of Sulprostone by SC-51322 (Kb = 244 nM), compound 3ap (Kb = 148 nM), or a combination of SC-51322 and compound 3ap (Kb = 154) was more substantial. Antagonism of Butaprost by 1 μM PF-04418948 was weak in both TM and SC cells (TM Kb = 674 nM, SC Kb = 419 nM). With an EC50 value in the two digit nanomolar range (34 nM for TM, 69 nM for SC), the response of L-902688 in SC cells rightward shifted more than 2 log10 in the presence of 0.3 μM GW-627368 (Kb = 7 nM), which displayed by far the strongest EP receptor antagonism in the present study. Whereas the minimal impedance change induced by L-902688 in TM (Emax <15%) was less profound but still significantly inhibited by 0.3 μM GW-627368 (Kb = 144 nM).
Figure 4.
Effects of EP receptor-selective antagonists on TM and SC cell impedance induced by EP receptor agonists. (A, B) Graded dose of Sulprostone with vehicle (green) versus 0.5 μM SC-51322 (blue), 0.5 μM compound 3ap (black), or 0.5 μM SC-51322, and 0.5 μM compound 3ap (red). (C, D) Graded dose of Butaprost with vehicle (black) versus 0.5 μM PF-04418948 (red); and graded dose of L-902688 with vehicle (blue) versus 0.3μM GW-627368 (green). Maximum change in cell impedance within 30 minutes of agonist addition was exported for data analysis. Data are normalized such that the smallest value in each data set = 0%, and the largest value in each data set = 100%. Each condition was tested in duplicate using three cell strains of each cell type (n = 3, mean ± SEM).
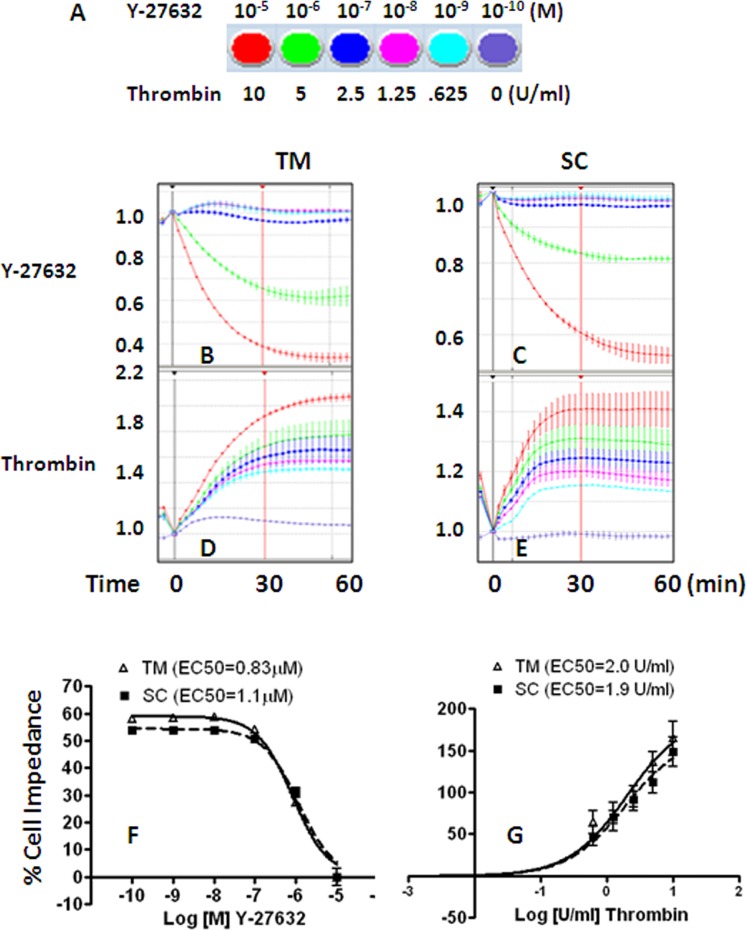
The relationship between cell impedance and cell contractility (stiffness) on TM and SC cells was investigated using two well characterized Rho/Rho kinase regulators, Y-27632 and thrombin, at graded doses (Fig. 5A). Y-27632 at 10 μM produced about a 60% drop in cell impedance (Figs. 5B, 5C) in both TM and SC cells (TM EC50 = 0.83 μM, SC EC50 = 1.1 μM, Fig. 5F), while 10 U/mL thrombin caused a 190% and 168% increase in cell impedance (Figs. 5D, 5E) in TM and SC cells respectively (TM EC50 = 2.0 U/mL, SC EC50 = 1.9 U/mL, Fig. 5G). Thus, responses of TM and SC cells to two agents that change cell contractility in opposite directions were indistinguishable.
Figure 5.
Effects of Y-27632 and thrombin on TM and SC cell impedance. Representative traces of cell impedance induced by graded doses (shown as color coded in (A) of Y-27632 (B, C) and thrombin (D, E) were normalized to 1 at time 0 of drug addition (black vertical line). Maximum change in cell impedance within 30 minutes of agonist addition (red vertical line) was exported for data analysis. Dose response curves for Y-27632 (F) and thrombin (G) were normalized to 10−6 M 17-phenyl PGF2α. Each condition was tested in duplicate using three cell strains for both cell types (n = 3, mean ± SEM).
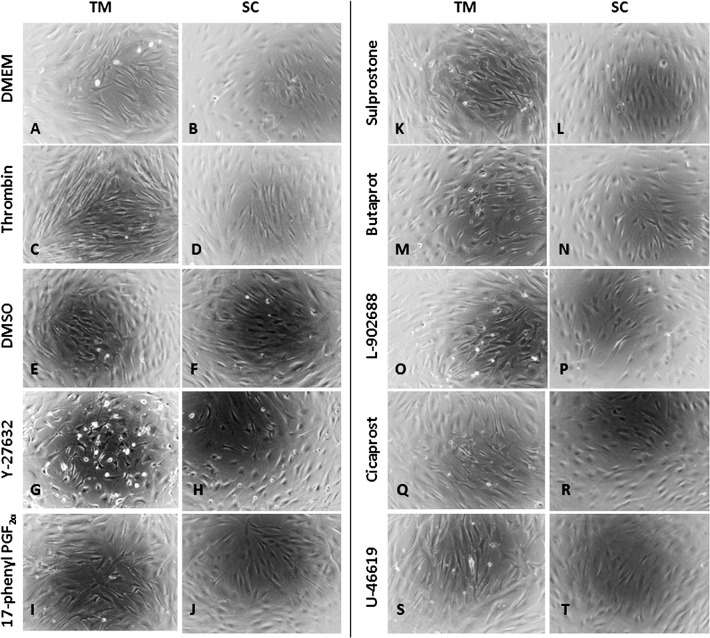
Consistent with previous observations,12 treatment with the Rho kinase inhibitor Y-27632 at 1, 5, or 10 μM caused dose- and time-dependent cell–cell detachment/retraction on TM and SC cells (Figs. 6G, 6H, shown at 10 μM overnight treatment only) compared with DMSO vehicle treatment (Figs. 6E, 6F), which was more profound in TM cells. Although a robust impedance reduction in TM and SC cells was induced by 1 μM Y-27632 within 30 minutes of drug addition (green curve, Figs. 5B, 5C), cell morphology change was not obvious 2 and 4 hours after treatment (data not shown). Comparing with DMEM vehicle control (Figs. 6A, 6B), the Rho GTPase activator thrombin caused cellular stretch and tighter cell–cell attachment on both TM and SC cells at 10 U/mL (Figs. 6C, 6D, showing overnight treatment only), and little morphology changes at 2.5 or 5 U/mL (data not shown). When cells were dosed with 1, 5, or 10 μM of 17-phenyl-PGF2α, Sulprostone, Butaprost, L-902688, Cicaprost, or U46619, no obvious changes in cell shape were observed (Figs. 6I–6T, shown at 10 μM overnight treatment only). No cytotoxicity was found according to Alamar Blue viability assay in all conditions after overnight dosing (data not shown).
Figure 6.
Microscopy of TM and SC cell morphology after overnight drug treatments. Serum free DMEM medium was the vehicle control for 10 U/mL thrombin. The vehicle control for 10 μM Y-27632 or 10 μM PG receptor agonists was 0.1% DMSO. Magnification: ×40.
Discussion
The present study shows the most complete characterization to date of endogenous PG receptors in the two cell types, TM and SC that populate the conventional outflow tract and are responsible for outflow resistance/IOP generation. PG receptor expression profiles using RT-qPCR and functional activities of PGE2-sensitive receptors using the cell impedance assay were examined in these cells. Results show that TM and SC differ with respect to abundance and expression distribution of PG receptors. The highest relative gene expression of FP receptors in both cell types correlated with the strong and potent functional activity displayed in the cell impedance study. In contrast, the low abundance of IP receptor gene in TM compared with SC cells correlated with the very modest Cicaprost activity in TM versus obvious activity in SC cells.4 The EP1–4 receptor expression and responses were variable. TM and SC cells differ in their response to PG subtype-selective agonists. In particular, treatment of TM cells with the EP2 or EP4 receptor-selective agonists, Butaprost or L-902688, respectively resulted in impedance changes consistent with increased cell contractility/stiffness. In contrast, Butaprost or L-902688 treatment of SC cells resulted in decreased cell contractility, in agreement with conventional outflow effects of EP4 and possibly EP2 receptor agonists at the level of the inner wall of SC.
Recently, optical magnetic twisting cytometry and traction force microscopy was used to examine cell stiffness of primary cultures of human SC cells. Thrombin was reported to increase cell stiffness by up to 200%, while exposure to Y-27632 reduced cell stiffness by 40% to approximately 80%.13 These data are nearly identical with our observation that 10 U/mL thrombin increased cell impedance by approximately 160%, promoting cellular stretch and cell–cell attachment, whereas 10 μM Y-27632 decreased the impedance by 50% to approximately 60% and caused cell–cell separation or retraction in human primary SC and TM cells. Importantly, these data suggest that cell impedance positively correlates with cellular stiffness, and therefore may relate to resistance to aqueous outflow facility. Significantly, the cell impedance technology can quantitatively detect subtle changes in cytoskeletal stiffness prior to, or in the absence of, any cell morphology changes, thus, has potential value as a high throughput screening method for glaucoma therapy targeting the conventional outflow pathway.
All EP antagonists used in the current study have been tested by others24–26,28 and confirmed at Allergan (data not shown) to be potent with Kb or inhibition constant Ki in less than or equal to two digit nanomolar range and at least 35-fold higher selectivity for their corresponding human EP receptors over other PG receptors in recombinant systems. In contrast, some PG receptor agonists cross react with other PG receptors,24 particularly at high concentrations. For example, Sulprostone binds to recombinant FP receptor at a similar affinity as the EP1 receptor,24 possibly explaining the partial blockade of Sulprostone signaling by the EP1 receptor antagonist SC-51322 alone or combination with the EP3 receptor antagonist compound 3ap in TM and SC cells, as well as the partial blockade of Sulprostone by 3ap alone in SC cells. Nonetheless, the partial antagonism confirmed that there are functional EP1 receptors in TM, and EP1/3 receptors in SC cells. The lack of functional EP3 receptors in TM cells was evidenced by the low gene expression and absence of compound 3ap antagonism to Sulprostone responses.
Partial attenuation of Butaprost signaling by the potent and highly selective EP2 receptor antagonist PF-0441894825 was also observed, despite the high selectivity of Butaprost for EP2 receptors over other PG receptors.24 One possibility may be that the endogenous EP2 receptors in these cells may form heterodimers of a wild type EP2 subunit with an alternatively spliced EP2 subunit (alt-EP2). The resultant heterodimers may be sensitive to Butaprost stimulation, but insensitive to PF-04418948 antagonism, which is different from homodimer behavior in the recombinant systems. The precedents of altered receptor pharmacology because of altered ligand selectivity and/or signal transduction pathway by heterodimerization of FP/alt-FP, IP/TP, and other G-protein coupled receptors have been previously discussed.30–32
The low potency of Sulprostone and Butaprost in TM (EC50 0.6 ∼ 1.1 μM) and SC (EC50 0.2 ∼ 0.4 μM) cells may be another contributing factor for the overall weak blockade by using only 0.5 to approximately 1 μM of the selective antagonists. Usually, a greater than 10-fold ratio of antagonist concentration to agonist EC50 is necessary for a 1log10 rightward shift, requiring an antagonist concentration of 2 to 10 μM in this case. Unfortunately, antagonist concentrations in the μM range resulted in significant effects on basal impedance activity, which prevented use at adequate concentrations.
In cases where significant antagonism was achieved (e.g., 0.3 μM GW-627368), higher potency of the corresponding agonist L-902688 on native EP4 receptors in TM and SC cells was observed (EC50 30 ∼ 70 nM). Interestingly, L-902688 only increased basal cell impedance in TM cells while decreasing cell impedance in SC cells. This indicates the presence of more functional EP4 receptors in SC than in TM cells; although gene expression was similar. These data are consistent with the previously published results showing differential effects of EP4 receptor activation in SC versus TM cells on Gαs-coupled cAMP accummulation.3
We have reported previously that the TP receptor agonist U46619 lowers impedance, while the FP agonist 17-phenyl PGF2α and the IP agonist Cicaprost increase cell impedance in human primary cultures of TM and SC cells.4 If increased cell impedance correlates with increased cell stiffness/contractility and resistance to conventional outflow, the activity of FP agonists on this pathway may be the spillover effect on other PG receptors that mediate cell impedance decrease, such as EP2/4 receptors (PGF2α binding affinity is 964 nM on EP2 and 288 nM on EP4).24 This may also be the reason why the total cell Impedance usually dropped by 10% to 20% at 10 μM compared with 1 μM of 17-phenyl-PGF2α in both TM and SC cells (Fig. 3). Moreover, although IP and TP agonists have shown potential for lowering IOP,33,34 only TP agonist is likely to act through the trabecular meshwork pathway according to its ability to reduce cell impedance in both TM and SC cells.
Cell impedance technology successfully detected functional differences between primary cultures of human TM and SC cells. This was made manifest by comparing the responses of native PGE2-sensitive receptors. We observed increased cell stiffness (contractility) upon agonist-activation of EP1/EP2 receptors in TM cells, with EP4 receptors exhibiting minimal activity and EP3 receptors were functionally inoperative. Of all four EP receptor subtypes functionally active in SC cells, only EP1 and EP3 increased cell stiffness, which may be the reason why EP1/EP3 receptors showed no IOP-lowering effects in monkeys.35 On the other hand, the decrease in cell stiffness mediated by EP2 and EP4 is a unique and important property of the SC cells, not found in TM cells, which may have major impact on the conventional outflow dynamics. It is well known that EP2 and EP4 analogs lower IOP better than FP analogs, and that the EP4 agonist 3,7-dithia PGE1-isopropyl ester lowers IOP by increasing conventional outflow.9,11 Although Butaprost was found to lower IOP via the uveoscleral outflow pathway in nonhuman primates,11 future perfusion studies using human anterior chamber segments may be able to determine whether Butaprost also functions through the conventional pathway. Our data suggest that the IOP lowering effects of EP2 and EP4 analogs are mediated predominantly by the SC cells in the pressure dependent, conventional outflow pathway.
In conclusion, SC cell contractility appears to play a vital role in conventional outflow resistance. Results support the idea that decreased conventional outflow facility is characterized by cytoskeletal stiffening of SC,13,18 and the hypothesis that the inner wall of Schlemm's canal is responsible for most of the resistance in the aqueous outflow tract.36 These findings have profound implications concerning future strategies for therapeutic targeting of the conventional outflow pathway and IOP lowering for glaucoma patients.
Acknowledgments
The authors thank Kristin Perkumas for providing, and Elaine Tang for culturing, primary cultures of human trabecular meshwork and Schlemm's canal cells for the experiments. They also thank Neil Poloso for providing the primers and advice on qPCR.
Supported by Allergan and partially by NIH Grant EY019696, Duke.
Disclosure: J.W. Wang, Allergan (F, E); D.F. Woodward, Allergan (F, E); W.D. Stamer, Allergan (F)
References
- 1. Kaufman PL, Alm A, Adler FH. Adler's Physiology of the Eye: Clinical Application. 10th ed. St. Louis: Mosby, Inc.; 2003. [Google Scholar]
- 2. Johnson M. ‘What controls aqueous humour outflow resistance?' Exp Eye Res. 2006; 82: 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Millard LH, Woodward DF, Stamer WD. The role of the prostaglandin EP4 receptor in the regulation of human outflow facility. Invest Ophthalmol Visual Sci. 2011; 52: 3506–3513 [DOI] [PubMed] [Google Scholar]
- 4. Stamer WD, Piwnica D, Jolas T, et al. Cellular basis for bimatoprost effects on human conventional outflow. Invest Ophthalmol Vis Sci. 2010; 51: 5176–5181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christiansen GA, Nau CB, McLaren JW, Johnson DH. Mechanism of ocular hypotensive action of bimatoprost (Lumigan) in patients with ocular hypertension or glaucoma. Ophthalmology. 2004; 111: 1658–1662 [DOI] [PubMed] [Google Scholar]
- 6. Toris CB, Zhan G, Fan S, et al. Effects of travoprost on aqueous humor dynamics in patients with elevated intraocular pressure. J Glaucoma. 2007; 16: 189–195 [DOI] [PubMed] [Google Scholar]
- 7. Bahler CK, Howell KG, Hann CR, Fautsch MP, Johnson DH. Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am J Ophthalmol. 2008; 145: 114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wan Z, Woodward DF, Cornell CL, et al. Bimatoprost, prostamide activity, and conventional drainage. Invest Ophthalmol Vis Sci. 2007; 48: 4107–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodward DF, Nilsson SF, Toris CB, Kharlamb AB, Nieves AL, Krauss AH. Prostanoid EP4 receptor stimulation produces ocular hypotension by a mechanism that does not appear to involve uveoscleral outflow. Invest Ophthalmol Vis Sci. 2009; 50: 3320–3328 [DOI] [PubMed] [Google Scholar]
- 10. Boussommier-Calleja A, Bertrand J, Woodward DF, Ethier CR, Stamer WD, Overby DR. Pharmacologic manipulation of conventional outflow facility in ex vivo mouse eyes. Invest Ophthalmol Vis Sci. 2012; 53: 5838–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nilsson SF, Drecoll E, Lutjen-Drecoll E, et al. The prostanoid EP2 receptor agonist butaprost increases uveoscleral outflow in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 2006; 47: 4042–4049 [DOI] [PubMed] [Google Scholar]
- 12. Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001; 42: 1029–1037 [PubMed] [Google Scholar]
- 13. Zhou EH, Krishnan R, Stamer WD, et al. Mechanical responsiveness of the endothelial cell of Schlemm's canal: scope, variability and its potential role in controlling aqueous humour outflow. J R Soc Interface. 2012; 9: 1144–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu Z, Overby DR, Scott PA, Freddo TF, Gong H. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008; 86: 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian B, Kaufman PL. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp Eye Res. 2005; 80: 215–225 [DOI] [PubMed] [Google Scholar]
- 16. Honjo M, Tanihara H, Inatani M, et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001; 42: 137–144 [PubMed] [Google Scholar]
- 17. Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem. 2007; 282: 7833–7843 [DOI] [PubMed] [Google Scholar]
- 18. Kumar J, Epstein DL. Rho GTPase-mediated cytoskeletal organization in Schlemm's canal cells play a critical role in the regulation of aqueous humor outflow facility. J Cell Biochem. 2011; 112: 600–606 [DOI] [PubMed] [Google Scholar]
- 19. Mettu PS, Deng PF, Misra UK, Gawdi G, Epstein DL, Rao PV. Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2004; 45: 2263–2271 [DOI] [PubMed] [Google Scholar]
- 20. Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res. 2005; 80: 197–206 [DOI] [PubMed] [Google Scholar]
- 21. Chudgar SM, Deng P, Maddala R, Epstein DL, Rao PV. Regulation of connective tissue growth factor expression in the aqueous humor outflow pathway. Mol Vis. 2006; 12: 1117–1126 [PubMed] [Google Scholar]
- 22. Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture, and characterization of endothelial cells from Schlemm's canal. Invest Ophthalmol Vis Sci. 1998; 39: 1804–1812 [PubMed] [Google Scholar]
- 23. Stamer WD, Seftor RE, Snyder RW, Regan JW. Cultured human trabecular meshwork cells express aquaporin-1 water channels. Curr Eye Res. 1995; 14: 1095–1100 [DOI] [PubMed] [Google Scholar]
- 24. Abramovitz M, Adam M, Boie Y, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000; 1483: 285–293 [DOI] [PubMed] [Google Scholar]
- 25. af Forselles KJ, Root J, Clarke T, et al. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP(2) receptor antagonist. Br J Pharmacol. 2011; 164: 1847–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Connell M, Zeller W, Burgeson J, et al. Peri-substituted hexahydro-indolones as novel, potent and selective human EP3 receptor antagonists. Bioorg Med Chem Lett. 2009; 19: 778–782 [DOI] [PubMed] [Google Scholar]
- 27. Billot X, Chateauneuf A, Chauret N, et al. Discovery of a potent and selective agonist of the prostaglandin EP4 receptor. Bioorg Med Chem Lett. 2003; 13: 1129–1132 [DOI] [PubMed] [Google Scholar]
- 28. Wilson RJ, Giblin GM, Roomans S, et al. GW627368X ((N-{2-[4-(4, 9-diethoxy-1-oxo-1, 3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl] acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol. 2006; 148: 326–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poloso NJ, Urquhart P, Nicolaou A, Wang J, Woodward DF. PGE(2) differentially regulates monocyte-derived dendritic cell cytokine responses depending on receptor usage (EP(2)/EP(4)). Mol Immunol. 2013; 54: 284–295 [DOI] [PubMed] [Google Scholar]
- 30. Wilson SJ, Roche AM, Kostetskaia E, Smyth EM. Dimerization of the human receptors for prostacyclin and thromboxane facilitates thromboxane receptor-mediated cAMP generation. J Biol Chem. 2004; 279: 53036–53047 [DOI] [PubMed] [Google Scholar]
- 31. Liang Y, Woodward DF, Guzman VM, et al. Identification and pharmacological characterization of the prostaglandin FP receptor and FP receptor variant complexes. Br J Pharmacol. 2008; 154: 1079–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prinster SC, Hague C, Hall RA. Heterodimerization of g protein-coupled receptors: specificity and functional significance. Pharmacol Rev. 2005; 57: 289–298 [DOI] [PubMed] [Google Scholar]
- 33. Hoyng PF, de Jong N. Iloprost, a stable prostacyclin analog, reduces intraocular pressure. Invest Ophthalmol Vis Sci. 1987; 28: 470–476 [PubMed] [Google Scholar]
- 34. Krauss AH, Woodward DF, Chen J, et al. AGN 191976: a novel thromboxane A2-mimetic with ocular hypotensive properties. J Ocul Pharmacol Ther. 1995; 11: 203–212 [DOI] [PubMed] [Google Scholar]
- 35. Gabelt BT, Hennes EA, Bendel MA, Constant CE, Okka M, Kaufman PL. Prostaglandin subtype-selective and non-selective IOP-lowering comparison in monkeys. J Ocul Pharmacol Ther. 2009; 25: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson MC, Kamm RD. The role of Schlemm's canal in aqueous outflow from the human eye. Invest Ophthalmol Vis Sci. 1983; 24: 320–325 [PubMed] [Google Scholar]