Abstract
Background
Pulsatile tinnitus, unlike idiopathic tinnitus, usually has a specific, identifiable cause. Nonetheless, uncertainty often arises in clinical practice about the findings to be sought and the strategy for work-up.
Methods
Selective literature review and evaluation of our own series of patients.
Results
Pulsatile tinnitus can have many causes. No prospective studies on this subject are available to date. Pulsatile tinnitus requires both a functional organ of hearing and a genuine, physical source of sound, which can, under certain conditions, even be objectified by an examiner. Pulsatile tinnitus can be classified by its site of generation as arterial, arteriovenous, or venous. Typical arterial causes are arteriosclerosis, dissection, and fibromuscular dysplasia. Common causes at the arteriovenous junction include arteriovenous fistulae and highly vascularized skull base tumors. Common venous causes are intracranial hypertension and, as predisposing factors, anomalies and normal variants of the basal veins and sinuses. In our own series of patients, pulsatile tinnitus was most often due to highly vascularized tumors of the temporal bone (16%), followed by venous normal variants and anomalies (14%) and vascular stenoses (9%). Dural arteriovenous fistulae, inflammatory hyperemia, and intracranial hypertension were tied for fourth place (8% each).
Conclusion
The clinical findings and imaging studies must always be evaluated together. Thorough history-taking and clinical examination are the basis for the efficient use of imaging studies to reveal the cause of pulsatile tinnitus.
Tinnitus is the conscious, usually unwanted perception of sound that arises or seems to arise involuntarily in the ear of the affected individual. In most cases there is no genuine physical source of sound. This nonpulsatile tinnitus is caused by a hearing malfunction (1). Less than 10% of tinnitus patients suffer from pulsatile tinnitus (2). If tinnitus can also be detected by a clinician, it is described as objective. Pulsatile tinnitus requires hearing, as there is usually a genuine physical source of sound (3). Pulsatile tinnitus is therefore included under the umbrella terms “physical tinnitus” and “somatosounds” (4). There are two plausible causes of pulsatile tinnitus:
Bloodflow accelerates, or changes in bloodflow disrupt laminar flow, and the resulting local turbulence is audible.
Normal flow sounds within the body are perceived more intensely, either as a result of alterations in the inner ear with increased bone conduction or as a result of disturbance of sound conduction leading to loss of the masking effect of external sounds.
Pulsatile tinnitus is usually unilateral, unless the underlying vascular pathology is bilateral. Recently, a disorder known as “somatosensory pulsatile tinnitus” has been discussed. This is bilateral tinnitus with no vascular cause (5).
It is often possible to identify the cause of pulsatile tinnitus. In addition to the patient’s medical history and targeted clinical examination, imaging procedures also play an important role in diagnosis. However, despite careful examination, no cause is found in up to 30% of patients (6).
This review article is based on a selective search of the literature and analysis of our patient records. The search of the literature was performed using PubMed and included review articles, case series, and case studies, with no restrictions on date of publication. We performed a retrospective search of our own patients’ radiology reports for 2003 to 2012 using the keywords “pulssynchron” or “pulsierend” (“pulsatile”) and “Ohrgeräusch” or “Tinnitus” (“tinnitus”).
Table 1 shows the results for the 77 identified patients (male/female 26/51, mean age 56 years). Tinnitus was right-sided in 38 cases, and left-sided in 27. It was bilateral in 12 cases. A cause was found significantly less frequently in these cases of bilateral tinnitus than in unilateral tinnitus (42% versus 88%, Fisher’s exact test, p = 0.001). Frequencies reported in the largest case series published to date vary enormously, as a result of both differing patient selection and different diagnostic pathways. There are no prospective studies.
Table 1. Frequency of causes of pulsatile tinnitus.
| Location | Cause | Absolute frequency | Relative frequency | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dietz 1994 (15) | Herraiz 2007 (4) | Mattox 2008 (6) | Sismanis 1998 (24) | Sonmez 2007 (26) | Waldvogel 1998 (18) | Pooled data from columns 1 to 6 | Authors’ own patient data | ||
| Arterial | Stenoses | 2 | 15 | 13 | 24 | 16 | 17 | 18% | 9% |
| Aneurysms | 0 | 1 | 0 | 2 | 3 | 1 | 1% | 4% | |
| Anatomical variants/abnormalities | 2 | 6 | 1 | 7 | 1 | 1 | 4% | 1% | |
| Arteriovenous transition | Dural arteriovenous fistulas | 10 | 3 | 0 | 3 | 2 | 17 | 7% | 8% |
| Direct arteriovenous fistulas | 3 | 0 | 0 | 0 | 0 | 6 | 2% | 3% | |
| Arteriovenous malformations | 1 | 0 | 0 | 1 | 0 | 0 | 0% | 1% | |
| Vessel-rich tumors | 5 | 2 | 0 | 17 | 2 | 5 | 6% | 16% | |
| Capillary hyperemia | 0 | 11 | 0 | 4 | 0 | 0 | 3% | 8% | |
| Venous | Intracranial hypertension | 0 | 8 | 1 | 61 | 0 | 6 | 16% | 8% |
| Anatomical variants/abnormalities | 5 | 3 | 23 | 0 | 25 | 1 | 12% | 14% | |
| Other | Semicircular canal dehiscence | 0 | 0 | 1 | 0 | 0 | 0 | 0% | 5% |
| Other | 0 | 21 | 0 | 13 | 1 | 3 | 8% | 4% | |
| Unknown | 21 | 10 | 15 | 13 | 24 | 27 | 23% | 20% | |
| Total | 49 | 80 | 54 | 145 | 74 | 84 | |||
Types of pulsatile tinnitus
The most common classification of tinnitus cases in the literature is subjective (heard by the patient only) versus objective (perceptible to the examiner also). This distinction depends on how hard the clinician searches for the sound and does not reflect etiology. We therefore use a different classification, one oriented more towards where the sound emanates from and its pathophysiology: Pulsatile tinnitus can be arterial or venous in origin, or it may originate between arteries and veins, i.e. in capillaries or the arteriovenous transition.
Tinnitus arising in the arteries
Vascular stenoses: Arteriosclerotic plaques and stenoses in the vessels of the head and neck are the most common cause of pulsatile tinnitus in the elderly (1). It is perfectly possible for the cause of tinnitus to lead to contralateral symptoms: Closure of a vessel on one side of the body may lead to a compensatory acceleration in flow in the open vessel, which then becomes symptomatic as tinnitus.
Fibromuscular dysplasia, a segmental, nonatheromatous vascular disease that often leads to stenosis, can cause pulsatile tinnitus, particularly in younger persons. Stenoocclusive vascular diseases found mainly in younger patient groups also include vascular dissection: The vascular lumen is narrowed by a hematoma on the vessel wall. Patients usually complain of acute-onset pain in the back of the neck. Damage to the cervical sympathetic trunk running alongside the vessels leads to ipsilateral Horner syndrome. There is a risk of cerebral infarction resulting from cerebral thromboembolism or hemodynamic instability of cerebral circulation. Angiography reveals tears of the intima, with membranes and intimal cusps, or segmental narrowings of the lumen over longer distances, caused by the intramural hematoma. Magnetic resonance imaging (MRI) often reveals this intramural bleeding directly (Figure 1). In late stages, pseudoaneurysms may form at the location of the intimal tear (7).
Figure 1.
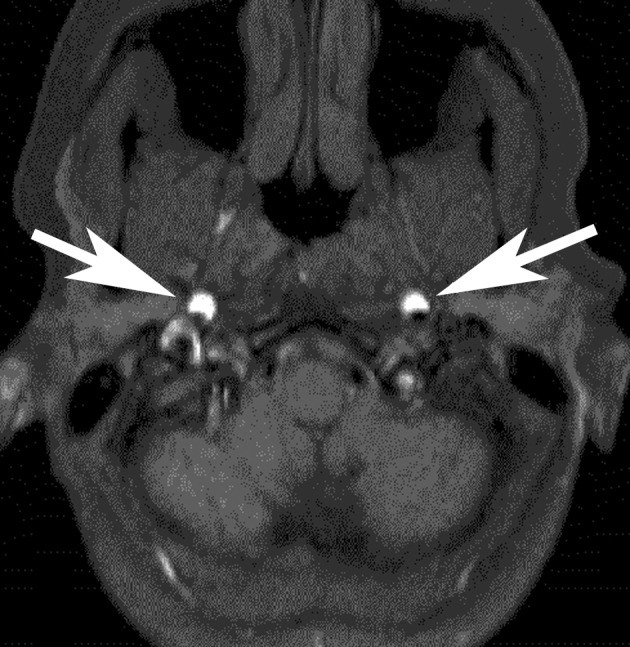
Bilateral carotid dissection in a 41-year-old woman.
Clinical symptoms: bilateral pulsatile tinnitus, pain in the back of the neck, and weakness in both arms. Axial T1-weighted MRI showed direct evidence of intramural hematoma (arrow)
Elongations and loops in the arteries that supply the brain are occasionally described as a cause of pulsatile tinnitus (3). However, because such findings are also common in asymptomatic patients, particularly the elderly, they must be evaluated cautiously and should not be grounds for not searching carefully for another cause.
Aneurysms: Aneurysms of the internal carotid artery or the vertebral artery often lead to turbulent bloodflow, but it is surprisingly rare for them to become clinically manifest as pulsatile tinnitus. Dissecting aneurysms are exceptions to this (3).
Anatomical variants and abnormalities of the arteries: The rare ectopic internal carotid artery, carotid-cochlear dehiscence, and persistent stapedial artery can be diagnosed using computed tomography (CT) (8– 10). The frequency of vascular loops in the inner ear is higher in individuals with pulsatile tinnitus than chance alone would predict (11). The transfer of flow sounds to the inner ear by bone conduction may be a cause of pulsatile tinnitus (12). Microscopic vascular abnormalities in the inner ear should be mentioned for the sake of completeness (13).
Tinnitus arising in the arteriovenous transition
Arteriovenous fistulas can cause unbearably loud pulsatile roaring sounds that can often be heard by the clinician too. Many patients are diagnosed only after a long, involved process. However, the real risk of damage posed by fistulas lies not in short-circuit but in the anatomy of venous drainage. This determines whether neurological complications (focal symptoms, elevated intracranial pressure, intracranial hemorrhage) may arise in addition to tinnitus (14).
With the exception of headaches, pulsatile tinnitus is the most common clinical symptom in dural arteriovenous fistulas and acquired arteriovenous short-circuits to the cerebral veins or sinuses (3). Arterial inflows arise mainly at dural branches of the carotid artery. The occipital artery is most frequently involved. Compression of the occipital artery against the mastoid process therefore often reduces tinnitus.
As short-circuits lie within the dura mater, CT and MRI often yield only indirect evidence of them (15, 16). Even on MRA (magnetic resonance angiography), alterations are usually subtle (see Case Study). As a result, the diagnostic gold standard is digital subtraction angiography (DSA). Dural arteriovenous fistulas are the classic cause of objective pulsatile tinnitus, but not all arteriovenous fistulas cause tinnitus that can be heard by the clinician (17, 18). Treatment consists of endovascular embolization and/or neurosurgical extirpation.
Case Study.
The 81-year-old female patient had been complaining of unbearable right-sided pulsatile tinnitus for five months, leading to considerable sleep disorders. She had consulted several specialists but not obtained any specific conclusion. The patient then attempted suicide. MRA provided initial evidence of the cause of the tinnitus (Figure). Physical examination revealed a pulsatile sound behind the right ear that was detectable on auscultation. Arterial compression in the right side of the neck and the right mastoid process led the noise to cease. Catheter angiography was indicated due to a suspected dural arteriovenous fistula.
Digital subtraction angiography confirmed a dural arteriovenous fistula fed by the right occipital artery as the main source. Venous drainage was occurring only orthogradely through the right transverse/sigmoid sinus into the right jugular vein. Because there was no risk of
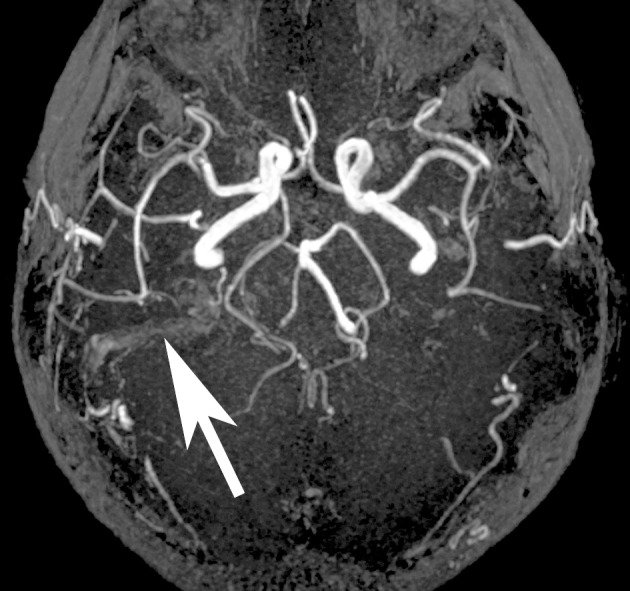
Dural arteriovenous fistula, MRA showed only subtle alterations as a result of atypical flows in the right transverse sinus (arrow).
neurological complications (increased intracranial pressure, cerebral hemorrhage, focal neurological symptoms), complete obliteration of the fistula, which would have been resource-intensive and potentially very risky, was not indicated; instead, only symptomatic endovascular treatment was indicated, as the least invasive possible surgery: The fistula vessels leading from the occipital artery were embolized using polyvinyl alcohol particles, under local anesthesia. This resolved the unbearable tinnitus. Six months after treatment, the patient spontaneously sent a thank-you card and reported that the tinnitus had disappeared long-term.
Dural arteriovenous fistula. MRA showed only subtle alterations as a result of atypical flows in the right transverse sinus (arrow).
Direct arteriovenous fistulas result either from damage to major arteries that supply the brain or from the rupture of an extradural aneurysm into the surrounding venous plexus. The classic cause is a carotid-cavernous fistula in cases of fracture of the base of the skull. However, vertebrovertebral fistulas (between the vertebral artery and the vertebral venous plexus) must also be considered (Figure 2). As with dural arteriovenous fistulas, venous outflow is critical to clinical symptoms. A vascular steal phenomenon involving the vessels that supply the brain poses an additional risk of damage. As with dural arteriovenous fistulas, endovascular surgery, the usual form of treatment, cannot be done without prior diagnostic DSA.
Figure 2.
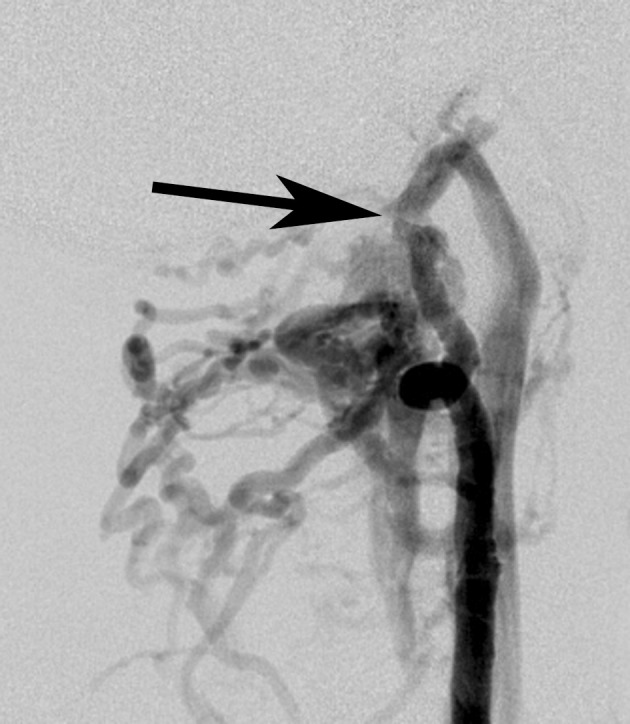
Digital subtraction angiography (DSA) of a direct arteriovenous short-circuit (arrow) between the vertebral artery and the vertebral venous plexus. Subsequent closure with a detachable balloon led to immediate, long-term cessation of the tinnitus, which had been unbearable
Pial arteriovenous vascular malformations are congenital and are found within the brain. They can cause neurological symptoms but rarely cause pulsatile tinnitus (19).
Typical tumors that are rich in blood vessels are paragangliomas (glomus tumors), benign tumors of the base of the skull. Pulsatile tinnitus is one of the symptoms of tympanic and jugular paragangliomas. Paraganglioma occurs bilaterally in 10% of cases; it can cause bilateral symptoms in these patients (20). Tympanic paragangliomas are otoscopically visible as a reddish pulsating space-occupying lesion behind the tympanic membrane. Larger jugular paragangliomas grow primarily in the jugular foramen. They are not otoscopically visible unless they penetrate the tympanic cavity. In contrast, otoscopically visible paragangliomas may be only the tip of the iceberg if the main tumor mass is extratympanic. Suspected paraganglioma is therefore always grounds for performing subtle cross-sectional imaging. DSA is required only as part of preoperative tumor embolization.
Pulsatile tinnitus can also be caused by other vessel-rich tumors of the base of the skull, particularly tumors of the temporal bone (metastases, basal meningiomas, hemangiomas, Heffner tumors) or Paget’s disease (21, 22).
Capillary hyperemia: Throbbing sounds in the ear that are synchronous with the pulse in acute otitis is easy to clarify using the patient’s medical history and results of clinical examinations. In otosclerosis, arteriovenous microfistulas over the oval window lead to pulsatile tinnitus (1).
Tinnitus arising in the veins
The bloodflow in the human body causes constant flow sounds. These are not usually consciously perceived (10). They are heard only when they are so loud that they can no longer be suppressed by the hearing organs and auditory pathway, usually as venous tinnitus. Venous hum, often overlooked, can be heard through a stethoscope and is thought to be caused by altered bloodflow behaviors, usually in anemia. The resulting turbulences are perceived as humming sounds.
If there are no other venous abnormalities, venous tinnitus is perceived as right-sided more frequently than left-sided, because the right jugular vein is dominant in 70% to 80% of cases (23). In very general terms, it seems that venous tinnitus is often favored by anatomical predisposition and triggered by physiological conditions. This also explains why it can disappear as spontaneously as it begins. Ligature of the jugular vein, the first-line therapy, should therefore be reserved for persistent cases that cause a high degree of suffering.
Intracranial hypertension: Pulsatile tinnitus can be caused by an increase in intracranial pressure (24). One of the causes, particularly in young, overweight women, is pseudotumor cerebri, more accurately described as idiopathic intracranial hypertension. Symptoms are headaches and visual disturbance. Pulsatile tinnitus occurs in 65% of patients (25). MRI often reveals empty sella syndrome, a prolapse of CSF-filled arachnoid membranes from the suprasellar cisterns through the sellar diaphragm and into the sella turcica. Particular care should be taken to detect any stenosis of the venous sinuses. Blood vessels can become narrowed from outside as a result of intracranial hypertension, but the reverse is also true: A primary sinus stenosis can also be the cause of intracranial hypertension. In addition to clinical symptoms, lumbar puncture with measurement of CSF pressure, which imaging cannot reveal, can guide diagnosis. Treatment consists of lumbar punctures to relieve CSF pressure or surgical CSF drainage (ventriculoperitoneal or lumboperitoneal shunt, optic nerve sheath fenestration).
It should never be forgotten that intracranial hypertension can also be caused by cerebral sinus thrombosis. If there is a unilateral transverse sinus thrombosis, venous blood has to flow out through the open opposing side, where the increase in blood flow can lead to tinnitus.
Other factors that should be mentioned as causes of increased intracranial pressure are cerebral neoplasias and other intracranial space-occupying lesions, craniocervical transition disorders, cranial stenoses, and hydrocephalic CSF flow disorders.
Anatomical variants and abnormalities of the veins and sinuses: Atypical formations of the jugular bulb favor the development of venous tinnitus. These include a high-riding jugular bulb, a jugular bulb in an unusually lateral location, enlarged jugular bulb, and jugular bulb diverticulum. However, there are huge variations between individuals, and these variants are common, asymptomatic incidental findings (26– 28). This is also true of emissary veins (condylar or mastoid), which might be associated with tinnitus but are also found frequently.
A high, dehiscent bulb is otoscopically visible as a livid structure behind the eardrum. Patients with a dehiscent bulb may present with conductive hearing loss if the bulb is in contact with the ossicles and restricts their mobility. If the bulb invades the bony labyrinth, a third window appears, through which sound waves escape. This also reduces sound conduction, in a similar way to semicircular canal dehiscence or cholesteatoma (29).
Diverticula of the sigmoid or transverse sinus are venous excrescences that bulge out through the internal bony cortex of the cranium into the diploë (Figure 3). Local flow turbulences are perceived as venous tinnitus (30). Stenoses, strictures, and segmentation of the sinus (particularly the transverse sinus) are also associated with pulsatile tinnitus (31).
Figure 3.
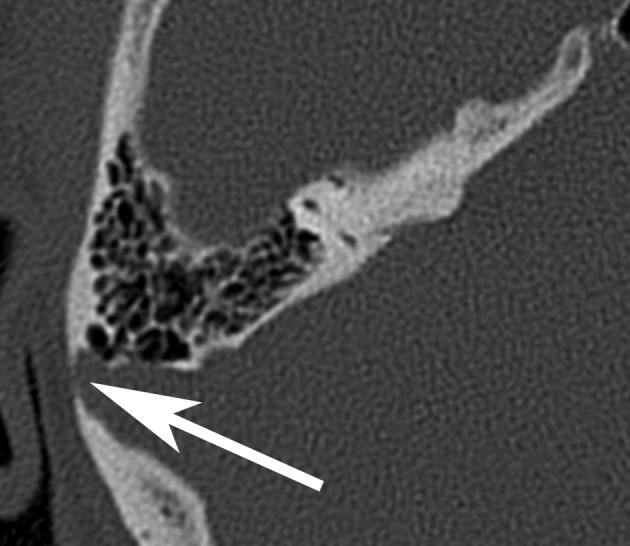
Diverticulum of the right transverse sinus (arrow)
Signs of semicircular canal dehiscence are an absence of bone covering a semicircular canal, usually the anterior. Diagnosis is made by CT (Figure 4). However, only a fraction of semicircular canal dehiscences actually cause audiovestibular symptoms, some of which are quite odd:
Figure 4.
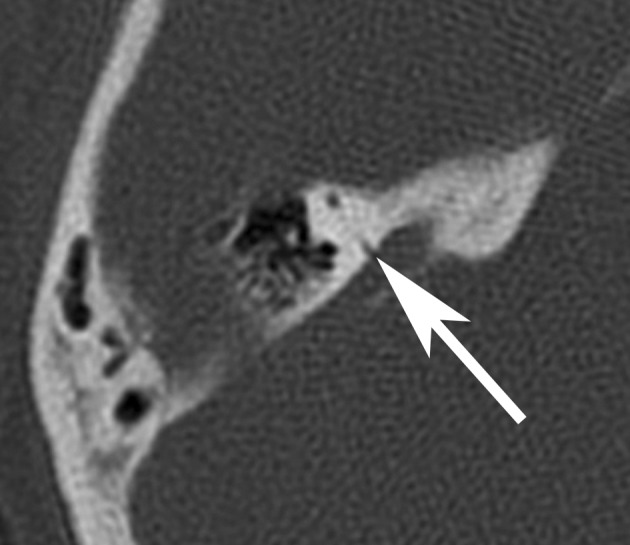
Dehiscence of the posterior portion of the anterior semicircular canal to the superior petrosal sinus (arrow). Clinical symptoms: right-sided pulsatile tinnitus, unpleasant perception of the patient’s own footsteps
Tullio’s phenomenon (vestibular symptoms that can be caused by loud sounds, such as dizziness, nystagmus, oscillopsia)
Conductive hearing loss caused by sound waves escaping at an area of dehiscence (third window)
Increased bone conduction with increased perception of sounds produced by the body itself (somatosounds) such as bloodflow sounds, autophony, eye movement sounds, and acoustic perception of the patient’s own footsteps (32, 33).
Treatment for semicircular canal dehiscence is surgery to cover the affected semicircular canal or obliteration of it.
Other tinnitus
Rare causes of pulsatile tinnitus include meningocele of the temporal bone (34), cholesterol granulomas (35), and perilymph fistulas (21).
Examination procedures
Clinical
In addition to questions concerning the duration and cause of tinnitus and any previous cerebrocranial trauma, the patient’s drug history is important, as some substances (ACE inhibitors, calcium antagonists) favor pulsatile tinnitus (24). Naturally, it must be determined whether the tinnitus is actually synchronous with the pulse. Careful auscultation of the head and neck region and the heart should be performed in a completely quiet environment with no disrupting external sounds. Provocation and rotation maneuvers (Table 2) can be used to distinguish whether the tinnitus sounds are arterial or venous in origin. Essential examinations include taking blood pressure, determining body mass index, testing for anemia, and ruling out hyperthyroidism.
Table 2. Clinical procedures for distinguishing between arterial and venous tinnitus.
| Venous | Arterial | |
|---|---|---|
| Strong arterial compression of the carotid artery | No effect | Resolution or decrease |
| Slight venous compression (ipsilateral) | Resolution or decrease | No effect |
| Slight venous compression (contralateral) | Increase | Usually no effect, rarely decrease |
| Valsalva maneuver | Decrease | No effect |
| Müller maneuver | Increase | No effect |
| Rotation of head towards side of tinnitus | Decrease | No effect |
| Rotation of head towards opposing side | Increase | No effect |
Hearing must be tested. Otoscopy must check for otitis and search for any vascular structure behind the eardrum. A search for neurological symptoms of increased intracranial pressure (headache, visual disturbance with papilledema, double vision, and in the case of serious intracranial hypertension nausea and vomiting) should be performed, as should lumbar puncture measuring CSF pressure if required. Full Doppler ultrasound of the head and neck vessels is also essential.
Clinical warning signs are focal neurological symptoms, signs of intracranial pressure, and objective pulsatile tinnitus. Stenosing diseases of vessels that supply the brain must be searched for. If venous hum caused by anemia has been ruled out, an arteriovenous fistula must also always be considered.
Imaging
Imaging must include at least CT and MRI, which complement each other. Magnetic resonance angiography (MRA) is useful in imaging arteries that supply the brain, while veins and sinuses are better represented by CT angiography (CTA) (36). Imaging must never be considered in isolation: It must always be interpreted in the context of clinical findings. If no other causes can be identified for confirmed pulsatile tinnitus that is synchronous with the pulse, DSA is indicated. The risk entailed in catheter angiography, which with an experienced clinician is low, must be weighed against the possibly risky spontaneous course of an undetected arteriovenous fistula. The Box provides an overview of the anatomical compartments and the imaging findings to be expected in each one.
Box. Checklists of imaging findings in various anatomical compartments*.
-
Epicranium
Dilatation of branches of the external carotid artery (in dural arteriovenous fistulas)
-
Neck
Vascular stenosis (arteriosclerosis, fibromuscular dysplasia)
Vascular dissection
Aneurysm
Carotid or vagal paraganglioma
Jugular vein abnormality
-
Temporal bone
Aberrant or dehiscent internal carotid artery
Persistent stapedial artery
Anatomical variants/abnormalities of the jugular bulb
Tympanic/jugular paraganglioma
Other strongly vascularized tumor of the temporal bone
Otosclerosis
Otitis
Semicircular canal dehiscence
Labyrinth fistula
Meningocele, meningoencephalocele
Cholesterol granuloma
-
Other skull
Strongly vascularized tumor, vessel-rich metastasis
Paget’s disease
Empty sella
Large emissary vein
Dilated transossial vascular canals (in dural arteriovenous fistulas)
-
Dura mater
Dural arteriovenous fistula
Sinuvenous thrombosis
Stenosis or diverticulum of dural sinus
-
Endocranium
Space-occupying lesion
Disturbance of CSF circulation
Craniocervical transition disorder
Vascular loops in the internal auditory meatus
Pial arteriovenous vascular malformation
Venous congestion (in dural arteriovenous fistulas)
*Modified according to (37)
As a symptom, pulsatile tinnitus has many, highly varied causes and involves several clinical disciplines. This gives rise to the interface problem: Diagnosis is often only possible if all clinical findings are collated and critically assessed in conjunction with imaging results. Ideally, this should be performed by a multidisciplinary team with structured diagnostic pathways.
Key Messages.
Pulsatile tinnitus is a syndrome with multiple etiologies. A specific cause can be found in three-quarters of cases.
Tinnitus can be classified as arterial, arteriovenous, or venous, depending on the source of the sound. Targeted clinical examination must attempt to locate the sound according to this classification.
Clinical warning signs that are grounds for suspecting a potentially serious underlying disease are focal neurological symptoms, signs of increased intracranial pressure, and objective tinnitus. Pulsatile tinnitus can also be the first indication of stenosis of arteries serving the brain, so examination for such stenosis must be performed.
CT angiography and MRI/MR angiography are the essential imaging procedures to be used. Digital subtraction angiography is indicated if an arteriovenous fistula is suspected.
Imaging results must always be interpreted together with the results of clinical examination. This requires multidisciplinary teamwork.
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
We would like to thank Dipl.-Ing. O. Berboth of the IT Department at Fulda Hospital for his search of medical reports.
We are also grateful to Dr. A. Straube of the Magnetic Resonance Imaging Practice at St. Joseph’s Hospital, Berlin for her permission to use the image in the Case Study.
Footnotes
Conflict of interest statement
All authors declare that no conflict of interest exists.
References
- 1.de Ridder D, Menovsky D, van de Heyning P. An otoneurosurgical approach to non-pulsatile and pulsatile tinnitus. B-ENT. 2007;3(Suppl 7):79–86. [PubMed] [Google Scholar]
- 2.Kircher ML, Standring RT, Leonetti JP. Neuroradiologic assessment of pulsatile tinnitus. Otolaryngol Head Neck Surg. 2008;139(Suppl) [Google Scholar]
- 3.Sismanis A. Pulsatile tinnitus. Otolaryngol Clin N Am. 2003;36:389–402. doi: 10.1016/s0030-6665(02)00169-x. [DOI] [PubMed] [Google Scholar]
- 4.Herraiz C, Aparicio JM. Diagnostic clues in pulsatile tinnitus. Acta Otorrinolaringol Esp. 2007;58:426–433. [PubMed] [Google Scholar]
- 5.Levine RA, Nam EC, Melcher J. Somatosensory pulsatile tinnitus syndrome: Somatic testing identifies a pulsatile tinnitus subtype that implicates the somatosensory system. Trends Amplif. 2008;12:242–253. doi: 10.1177/1084713808321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattox DE, Hudgins P. Algorithm for evaluation of pulsatile tinnitus. Acta Oto-Laryngol. 2008;128:427–431. doi: 10.1080/00016480701840106. [DOI] [PubMed] [Google Scholar]
- 7.Nüssel F, Bradac GB, Schuierer G, Huk WJ. Klinische und neuroradiologische Aspekte bei Karotis- und Vertebralisdissektionen. Clin Neuroradiol. 1991;1:26–33. [Google Scholar]
- 8.Schröck A, Strach K, Kühnemund M, Bootz F, Eichhorn KW. Seltene Ursache eines pulssynchronen Tinnitus. HNO. 2008;56:714–716. doi: 10.1007/s00106-008-1713-2. [DOI] [PubMed] [Google Scholar]
- 9.Lund AD, Palacios SD. Carotid artery-cochlear dehiscence: A review. Laryngosope. 2011;121:2658–2660. doi: 10.1002/lary.22391. [DOI] [PubMed] [Google Scholar]
- 10.Lo WWM, Maya MM. Som PM, Curtin HD, editors. Temporal bone: Vascular tinnitus. Head and neck imaging. St. Louis: Mosby. 2003:1361–1374. [Google Scholar]
- 11.Chadha NK, Weiner GM. Vascular loops causing otological symptoms: a systematic review and meta-analysis. Clin Otolaryngol. 2008;33:5–11. doi: 10.1111/j.1749-4486.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 12.de Ridder D, de Ridder L, Nowe V, Thierens H, van de Heyning P, Moller A. Pulsatile tinnitus and the intrameatal vascular loop: why do we not hear our carotids? Neurosurgery. 2005;57:1213–7. doi: 10.1227/01.neu.0000186035.73828.34. [DOI] [PubMed] [Google Scholar]
- 13.Gulya AJ, Schuknecht HF. Letter to the editor. Am J Otol. 1984;5 [Google Scholar]
- 14.Zipfel GJ, Shah MN, Refai D, Dacey RG, Derdeyn CP. Cranial dural arteriovenous fistulas: modification of angiographic classification scales based on new natural history data. Neurosurg Focus. 2009;26 doi: 10.3171/2009.2.FOCUS0928. [DOI] [PubMed] [Google Scholar]
- 15.Dietz RR, Davis WL, Harnsberger HR, Jacobs JM, Blatter DD. MR imaging and MR angiography in the evaluation of pulsatile tinnitus. AJNR. 1994;15:879–889. [PMC free article] [PubMed] [Google Scholar]
- 16.Alatakis S, Koulouris G, Stuckey S. CT-demonstrated transcalvarial channels diagnostic of dural arteriovenous fistula. AJNR. 2005;26:2393–2396. [PMC free article] [PubMed] [Google Scholar]
- 17.Park IH, Kang HJ, Suh SI, Chae SW. Dural arteriovenous fistula presenting as subjective pulsatile tinnitus. Arch Otolaryngol Head Neck Surg. 2006;132:1148–1150. doi: 10.1001/archotol.132.10.1148. [DOI] [PubMed] [Google Scholar]
- 18.Waldvogel D, Mattle HP, Sturzenegger M, Schroth G. Pulsatile tinnitus - a review of 84 patients. J Neurol. 1998;245:137–142. doi: 10.1007/s004150050193. [DOI] [PubMed] [Google Scholar]
- 19.Grzyska U, Fiehler J. Pathophysiology and treatment of brain AVMs. Clinical Neuroradiology. 2009;19:82–90. doi: 10.1007/s00062-009-8035-y. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann E, Arps H, Schwager K. Paragangliome der Kopf-Hals-Region („Glomustumoren“) Radiologie Up2Date. 2009;4:339–353. [Google Scholar]
- 21.Gehrking E, Gliemroth J, Missler U, Remmert S. Hauptsymptom pulssynchrones Ohrgeräusch. Laryngo-Rhino-Otol. 2000;79:510–516. doi: 10.1055/s-2000-6944. [DOI] [PubMed] [Google Scholar]
- 22.Swartz JD. An approach to the evaluation of the patient with pulsatile tinnitus with emphasis on the anatomy and pathology of the jugular foramen. Semin Ultrasound CT MRI. 2004;25:319–331. doi: 10.1053/j.sult.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Friedmann DR, Eubig J, McGill M, Babb SB, Pramanik BK, Lalwani AK. Development of the jugular bulb: A radiologic study. Otol Neurotol. 2011;32:1389–1395. doi: 10.1097/MAO.0b013e31822e5b8d. [DOI] [PubMed] [Google Scholar]
- 24.Sismanis A. Pulsatile tinnitus. Am J Otol. 1998;19:472–477. [PubMed] [Google Scholar]
- 25.Skau M, Sander B, Milea D, Jensen R. Disease activity in idiopathic intracranial hypertension: a 3-month follow-up study. J Neurol. 2011;258:277–283. doi: 10.1007/s00415-010-5750-x. [DOI] [PubMed] [Google Scholar]
- 26.Sonmez G, Basekim CC, Öztürk E, Güngör A, Kizilkaya E. Imaging of pulsatile tinnitus: a review of 74 patients. Clinical imaging. 2007;31:102–108. doi: 10.1016/j.clinimag.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Fiedler KL. Anatomische Landmarken des Felsenbeins im hochauflösenden CT. Dissertation Würzburg. 2005 [Google Scholar]
- 28.Kang M, Escott E. Imaging of tinnitus. Otolaryngol Clin N Am. 2008;41:179–193. doi: 10.1016/j.otc.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Friedmann DR, Le BT, Pramanik BK, Lalwani AK. Clinical spectrum of patients with erosions of the inner ear by jugular bulb abnormalities. Laryngoscope. 2010;120:365–372. doi: 10.1002/lary.20699. [DOI] [PubMed] [Google Scholar]
- 30.Otto KJ, Hudgins PA, Abdelkafy W, Mattox DE. Sigmoid sinus diverticulum: a new surgical approach to the correction of pulsatile tinnitus. Otol Neurotol. 2006;28:48–53. doi: 10.1097/01.mao.0000247814.85829.f6. [DOI] [PubMed] [Google Scholar]
- 31.Russell EJ, De Michaelis BJ, Wiet R, Meyer J. Objective pulse-synchronous essential tinnitus due to narrowing of the transverse dural venous sinus. Int Tinnitus J. 1995;1:127–137. [PubMed] [Google Scholar]
- 32.Adams ME, Levine SC. The first new otologic disorder in a century: superior canal dehiscence syndrome. Minn Med. 2011;94:29–32. [PubMed] [Google Scholar]
- 33.Koo JW, Hong SK, Kim DK, Kim JS. Superior semicircular dehiscence syndrome by the superior petrosal sinus. J Neurol Neurosurg Psychiat. 2010;81:465–467. doi: 10.1136/jnnp.2008.155564. [DOI] [PubMed] [Google Scholar]
- 34.Doshi J, Anari S, Carrie S. Objective pulsatile tinnitus: a video clip demonstration of the condition. Laryngoscope. 2006;116 doi: 10.1097/01.mlg.0000240968.55613.5d. [DOI] [PubMed] [Google Scholar]
- 35.Remley KB, Coit WE, Harnsberger HR, Smoker WRK, Jacobs JM, McIff EB. Pulsatile tinnitus and the vascular tympanic membrane. Radiology. 1990;174:383–389. doi: 10.1148/radiology.174.2.2296650. [DOI] [PubMed] [Google Scholar]
- 36.Osborn A. Venous anatomy and occlusions. In: Osborn A, editor. Osborn's brain: Imaging, pathology, and anatomy. Philadelphia: Lippincott; 2013. pp. 215–241. [Google Scholar]
- 37.Vattoth S, Shah R, Curé JK. A compartment-based approach for the imaging evaluation of tinnitus. Am J Neuroradiol. 2010;31:211–218. doi: 10.3174/ajnr.A1704. [DOI] [PMC free article] [PubMed] [Google Scholar]


