Abstract
It is already known that the Maitake (D-Fraction) mushroom is involved in stimulating the immune system and activating certain cells that attack cancer, including macrophages, T-cells, and natural killer cells. According to the U.S. National Cancer Institute, polysaccharide complexes present in Maitake mushrooms appear to have significant anticancer activity. However, the exact molecular mechanism of the Maitake antitumoral effect is still unclear. Previously, we have reported that Maitake (D-Fraction) induces apoptosis in breast cancer cells by activation of BCL2-antagonist/killer 1 (BAK1) gene expression. At the present work, we are identifying which genes are responsible for the suppression of the tumoral phenotype mechanism induced by Maitake (D-Fraction) in breast cancer cells. Human breast cancer MCF-7 cells were treated with and without increased concentrations of Maitake D-Fraction (36, 91, 183, 367 μg/mL) for 24 h. Total RNA were isolated and cDNA microarrays were hybridized containing 25,000 human genes. Employing the cDNA microarray analysis, we found that Maitake D-Fraction modified the expression of 4068 genes (2420 were upmodulated and 1648 were downmodulated) in MCF-7 breast cancer cells in a dose-dependent manner during 24 h of treatment. The present data shows that Maitake D-Fraction suppresses the breast tumoral phenotype through a putative molecular mechanism modifying the expression of certain genes (such as IGFBP-7, ITGA2, ICAM3, SOD2, CAV-1, Cul-3, NRF2, Cycline E, ST7, and SPARC) that are involved in apoptosis stimulation, inhibition of cell growth and proliferation, cell cycle arrest, blocking migration and metastasis of tumoral cells, and inducing multidrug sensitivity. Altogether, these results suggest that Maitake D-Fraction could be a potential new target for breast cancer chemoprevention and treatment.
Key Words: breast cancer cells, gene expression, Maitake D-Fraction, malignant phenotype, microarrays
Introduction
Numerous studies have confirmed that Maitake has prominent beneficial effects on immune function.1–9 It promotes the action of not only macrophages, but also a variety of other immune-related cells, such as natural killer (NK) cells and cytotoxic T-cells that can attack tumor cells. Maitake also increases the immune-related efficiency of these cells by increasing interleukin-1, interleukin-2, and lymphokines.1–9
The specific proteoglucan in the Maitake mushroom extract for fighting cancer tumors is called D-Fraction. Maitake D-Fraction has been reported to exert its antitumor effect in tumor-bearing mice by enhancing the immune system through activation of macrophages, T cells, and NK cells. The proteoglucan shows anticarcinogenic activity, prevents oncogenesis, and prevents metastasis. However, the exact molecular mechanisms and gene expression profiles generated by Maitake D-Fraction in the anticarcinogenesis process are still unclear. In our previous work, we have demonstrated that D-Fraction of Maitake mushroom was able to effectively induce apoptosis in MCF-7 breast cancer cells.10 These findings were corroborated by a reduction in cell viability upon treatment with different concentrations of this fraction. Several genes promoting apoptosis were upregulated as assessed by cDNA microarray analysis. We have reported that D-Fraction Standard (Mushrooom Wisdom, Inc.) induces apoptosis in breast cancer cells by activation of BAK1 gene expression; we also found that cytochrome C1 was released to the cytoplasm in a dose-dependent manner.10 In the present work, we are trying to clarify the genomic expression induced by Maitake (D-Fraction) in MCF-7 breast cancer cells. We also want to demonstrate which genes are involved in the mechanism of suppression of the tumoral breast phenotype. We performed a new set of human cDNA microarray assays, including 25,000 genes employing MCF-7 cells treated with and without (control) increased doses of Maitake (D-Fraction) (36, 91, 183, and 367 μg/mL), during 24 h. The gene expressions are corroborating by real-time reverse transcription (RT)–polymerase chain reaction (PCR) assay employing commercial reagents and custom primers designed by Applied Biosystems, Inc.
Materials and Methods
Bioactive Maitake D-Fraction
The bioactive D-Fraction was obtained as a commercially available bottled liquid, product developed by Mushroom Wisdom, Inc. Basically, Maitake D-Fraction was ethanol extracted from Grifola frondosa mushroom, corresponding to the protein-bound polysaccharide compound, and was prepared by a standardized procedure developed by Maitake Products, Inc.
Cell culture
The human breast cancer MCF-7 cell line was obtained from the American Type Culture Collection (ATCC). MCF-7 cells were routinely cultured in the DMEM containing 10% inactivated FBS and 1% penicillin/streptomycin. Cell culture media, fetal bovine serum, and penicillin/streptomycin were purchased from Invitrogen Life Technologies. Cells were grown at 37°C in a humidified 5% CO2 atmosphere.
MCF-7 cells Maitake D-Fraction treatment
MCF-7 cells were treated with and without (control) increased concentrations of Maitake D-Fraction for 24 h, such as 36, 91, 187, or 367 μg/mL.
Total RNA isolation
The RNA was isolated by duplicate using Trizol (Invitrogen) following the classic phenol purification method.11 The concentration and the quality of total isolated RNA were measured in the Nanodrop (Nanodrop Technologies) and in the Bioanalyzer (Agilent Technologies).
Labeling and cDNA human microarray hybridization
We employed direct labeling of probes with amine-modified random primers using 5 μg of RNA followed the protocol indicated previously.10 Probes were purified, before hybridization, Cy3- and Cy5-labeled products were combined and 30 μL of water was added. The purified probes were pipetted onto microarrays, coverslips were applied, and the slides were placed in a hybridization chamber (Corning). Arrays were incubated at 42°C water bath for 16 h, and subsequently washed with 0.5× saline–sodium citrate buffer (SSC), 0.01% (w/v) SDS, followed by 0.06× SSC, at room temperature for 10 min each. Slides were spun for 5 min at 800 rpm (130 g) at room temperature.
Array scanning and data analysis
Arrays were read with a Affymetrix 428 fluorescent scanner (MWG Technologies) at a 10-μm resolution and variable photomultiplier tube voltage settings to obtain the maximal signal intensities with <1% probe saturation. The resulting images were analyzed and normalized by the Lowess Method employing the Nexus Expression Software (Biodiscovery). Hierarchical clustering was done by using the neighbor joining method applied to the Pearson correlation distance matrix. In addition, other hierarchical clustering methods and other distance-generating functions (such as Euclidean) were used. Only marginal deviations between the methods and the distance matrices used were observed. We found the most stable results for the neighbor-joining method applied to the Pearson correlation distance matrix.
Gene validation by RT-PCR amplification assay
To corroborated the gene expression induced by Maitake D-Fraction in MCF-7 cells, we validated by real-time RT-PCR assay, three upregulated genes (ITGA2, SOD2, and Cul3) and one downmodulated gene (intercellular adhesion molecule 3 [ICAM3]) using nucleotide sequences that we found by the gene accession number obtained from cDNA glass microarray and searching the National Cancer Institute website. TaqMan primer and probe sequences are listed below. The sense and antisense sequence primers were designed using Primer3 software and are the following. ITAG2 (sense primer: TCT CAT CTG GAT TTT TGG TCA TC, antisense primer: AAC CTG ATG AGA AAG CCG AAG), SOD2 (sense primer: TGC CTC CAG TCA ACA AGA TG, antisense primer: CGT TAG TGG TTT GCA CAA GG), Cul3 (sense primer: GAC CCT AAA ACT GAG CAT CAA A, antisense primer: AGA CGT TAA GAA TGG CAG ATA AA), ICAM3 (sense primer: GTA ACT GCC GCT CCG TTG, antisense primer: ACT TTG TCC CCG TCT TCG T). A β-actin primer was included as a control for gene expression. Primers were labeled with SyBro Green dye (Applied Biosystems). All RT-PCR reactions were performed on the ABI Prism 7000 Sequence Detection System.
Statistical analysis
Normalization and statistical analysis of the expression data were carried out using Linear Models for Microarray Data.12–14 For detecting the differential expression of genes that might not necessarily be highly expressed, background correction using the normexp method in Linear Models for Microarray Data was done for adjusting the local median background estimates, a correction strategy that avoids problems with background estimates that are greater than foreground values and ensures that there were no missing or negative corrected intensities. An offset of 100 was used for both channels to further dampen down the variability of log ratios for low-intensity spots. The resulting log ratios were normalized by using the print-tip group Lowess method with a span of 0.4, as recommended by Smyth.14 Moderated t statistic was used as the basic statistic for significance analysis; it was computed for each probe and for each contrast.14 The false discovery rate was controlled using the BH adjustment of Benjamini and Hochberg.15,16 All genes with P value below a threshold of .05 were selected as differentially expressed, maintaining the proportion of false discoveries in the selected group below the threshold value, in this case 5%.17
Results
cDNA microarray analysis
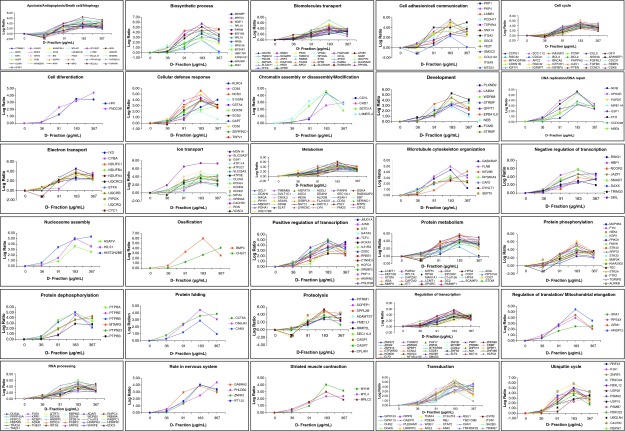
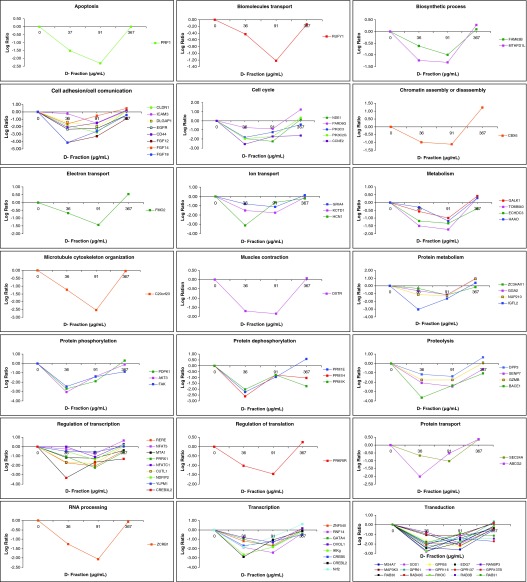
Employing the cDNA microarray analysis, we demonstrated that Maitake D-Fraction modified the expression of 4068 genes (2420 were upmodulated and 1648 downmodulated) in MCF-7 breast cancer cells in a dose-dependent manner compared to control (untreated cells) during 24 h of treatment. Under more stringent conditions, we found that 505 genes modified their expression, 430 genes of them were found upregulated, and 75 genes were downregulated at 367 μg/mL of Maitake D-Fraction after 24 h of treatment with a log2 ratio ≥2.0 for the upmodulated and a log2 ratio <−2.0 for the downmodulated genes, respectively. Those genes are related to several biological functions as the following (Fig. 1 and Supplementary Table S1; Supplementary Data are available at www.liebertpub.com/jmf): 31 genes related to apoptosis/antiapoptosis/cell death/autophagy, 14 related to biosynthetic process, 59 related to transport, 13 related to cell adhesion/cell communication, 30 genes related to cell cycle, 2 related to cell differentiation, 11 related to cellular defense response, 4 genes related to chromatin assembly/disassembly or modification, 9 genes related to development, 8 related to DNA replication/DNA repair, 65 related to metabolism, 7 related to microtubule cytoskeleton organization, and 56 to regulation of transcription. Three genes are related to nucleosome assembly, 2 related to ossification, 22 to protein phosphorylation/dephosphorylation, 3 genes related to protein folding, and 10 genes related to proteolysis. Four genes are related to regulation of translation/mitochondrial translational elongation, 32 related to RNA processing, 4 genes with roles in nervous system, 3 to striated muscle contraction, 24 to transduction, and 14 genes related to ubiquitin cycle (Fig. 1 and Supplementary Table S1). Also, we detected that 75 genes were downmodulated in a dose-dependent manner in MCF-7 cells. After Maitake D- Fraction treatment, we found that 91 μg/mL of Maitake (D-Fraction) during 24 h induces the maximum downmodulation in those genes (Fig. 2 and Supplementary Table S2) that were related to several biological functions as the following: 1 genes related to apoptosis, 2 related to biosynthetic process, 5 genes related to cell adhesion/cell communication, 5 related to cell cycle, 1 related to chromatin assembly or disassembly, and 7 genes related to transport. Eight genes related to metabolism, 1 related to microtubule cytoskeleton organization, 1 to muscle contraction, and 6 related to protein phosphorylation/dephosphorylation. Four genes related to proteolysis, 9 related to regulation of transcription, 1 to regulation of translation, 1 to RNA processing, 8 to transcription, and 15 genes related to signal transduction (Fig. 2 and Supplementary Table S2).
FIG. 1.
Gene expression profile of upmodulated genes induced by Maitake D-Fraction in MCF-7 cells. Human breast cancer MCF-7 cells were treated with and without (control) increased concentrations of Maitake D-Fraction for 24 h, namely, 36, 91, 187, or 367 μg/mL. Genomic analysis using complementary DNA (cDNA) microarrays was used to monitor expression levels of 25,000 human known genes. The resulting array images were analyzed and normalized employing the Nexus Expression Software (Biodiscovery). Color images available online at www.liebertpub.com/jmf
FIG. 2.
Gene expression profile of downmodulated genes induced by Maitake D-Fraction in MCF-7 cells. Human breast cancer MCF-7 cells were treated with and without (control) increased concentrations of Maitake D-Fraction for 24 h, namely, 36, 91, 187, or 367 μg/mL. Genomic analysis using complementary DNA (cDNA) microarrays was used to monitor expression levels of 25,000 human known genes. The resulting array images were analyzed and normalized employing the Nexus Expression Software (Biodiscovery). Color images available online at www.liebertpub.com/jmf
Validation of differential expression
To confirm the differential expression results, a subset of four genes, three of them upregulated (ITGA2, SOD2, and Cullin 3 [CUL3]) and one downregulated (ICAM3), were selected and their mRNA expression were corroborated by real-time quantitative PCR assay employing reagents acquired from Applied Biosystems.
Discussion
Out of 505 genes that showed statistically significant differential expression in MCF-7 cells after treatment with Maitake (D-Fraction) in a dose-dependent manner compared to control, we investigate in the public literature the relationship between those genes and neoplasic disease, specifically in breast cancer. In this manner, we identify genes that are responsible for the suppression of the tumoral phenotype mechanism induced by Maitake (D-Fraction) in breast cancer cells. Thus, we can elucidate the molecular mechanism induced by Maitake in the anti-tumoral action. In the following section, we will describe certain genes that were considered more important in the suppression of the tumoral phenotype mechanism.
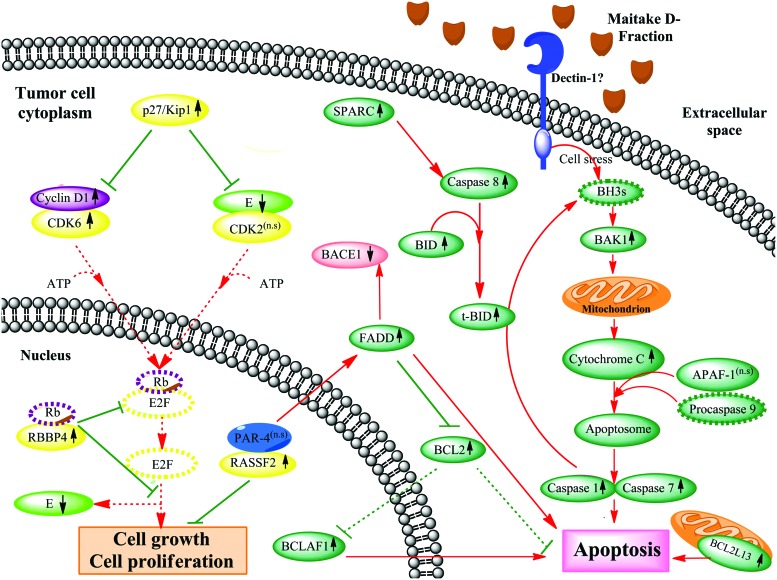
Maitake D-Fraction induces apoptosis in MCF-7 cells through activation of BAK1, BCLAF1, RASSF2, FADD, SPARC, and BCL2L13 genes and by downregulated PI3K-AKT signaling
Evidence demonstrates that BAK1 promotes cell death and counteracts the protection from apoptosis provided by BCL2.18,19 Chittenden et al.18 found that enforced expression of BAK1 induced rapid and extensive apoptosis of serum-deprived fibroblasts, suggesting that BAK1 may be directly involved in activating cell death machinery. Accordingly, Kiefer et al.19 reported that the BAK1 gene product primarily enhances apoptotic cell death after an appropriate stimulus. Previously, we have published that Maitake (D-Fraction) induces apoptosis in breast cancer cells by activation of BAK1 gene expression.10 Activated BAK1 proteins oligomerize at the mitochondrial membrane and cause the release of several mitochondrial factors, such as cytochrome C, which in combination with APAF-1 and procaspase 9 form an apoptosome. Activated caspase 9 probably then activates caspase 7 and caspase 1, allowing apoptosis to proceed.10 In concordance with that, in the present work, we found that Maitake D-Fraction induces the overexpression of the proapoptotic BAK1 gene (2.18-fold) at 183 μg/mL in MCF-7 cells (Fig. 3). However, more research must be done to determine the exact apoptotic mechanism and its regulation. Also, here we have shown that Maitake D-Fraction induces the expression of BCL2-associated transcription factor 1 (BCLAF1; 5.43-fold), another proapoptotic gene. BCLAF1 is a nuclear protein that was originally identified in a screening of proteins that interact with BCL2. It has been shown that BCLAF1-deficient cells did not experience cell death after exposure to various apoptotic stimuli.20 Over expression of BCLAF1 was shown to induce apoptosis as well as transcriptional repression, properties that were reversible in the presence of BCL2 or BCL-XL.21 Taken together, these studies potentially support a role for BCLAF1 in apoptosis through events that control transcription.22 In addition to the overexpression of BCLAF1, we also found upmodulation of the antiapoptotic BCL2 gene. This could be due probably in response to controlled or regulated cell death mechanisms already triggered by Maitake D-Fraction through activation of several apoptotic genes in MCF-7 tumoral cells. We reiterate that after 24 h of treatment with this compound, we observed >85% of the MCF-7 cells died because of apoptosis;10 the surviving ∼15% could be explained by the antiapoptotic signal induced by BCL2 gene expression. In conclusion, it is well known that BCLAF1 activation is related to apoptosis; however, its exact mechanism in these events remains unclear (Fig. 3).
FIG. 3.
Maitake D-Fraction induces apoptosis mediated by BAK1 stimulation and blocks tumor cells growth and proliferation in MCF-7 cells through activation of p27/Kip1 and RASSF2 genes. The putative molecular mechanism of apoptosis and anti-tumoral cell proliferation induced by Maitake D-Fraction in breast cancer MCF7 cells is shown, with red lines indicating stimulated pathways, green lines indicating inhibitory pathways, and discontinuous gray lines representing the genes whose expression has not been changed after Maitake treatment. Color images available online at www.liebertpub.com/jmf
Likewise, it is well known that RASSF2 (Ras association [RalGDS/AF-6] domain family member 2) is a novel proapoptotic effector of K-Ras. RASSF2 is implicated as a tumor suppressor, whose inactivation facilitates transformation by disconnecting apoptotic responses from Ras. It has been demonstrated that RASSF2 forms a direct and endogenous complex with the tumor suppressor prostate apoptosis response protein 4 (PAR-4).23 This interaction is regulated by K-Ras and is essential for the full apoptotic effects of PAR-4, a proapoptotic protein capable of selectively inducing apoptosis in cancer cells. It has been shown that PAR-4 sensitized the cells to diverse apoptotic stimuli and causing regression of tumors in animal models.23 PAR-4 induces apoptosis in certain cancer cells by activating Fas TNFRSF6-associated via death domain (FADD) prodeath pathway and coparallel inhibition of NF-κB transcriptional activity.23 In the present work, we found that Maitake D-Fraction treatment stimulates the apoptosis mechanisms by increasing the expression (4.62-fold) of RASSF2 gene and upmodulation of the FADD gene (3.87-fold) through interaction with PAR-4. It is well known that the FADD protein inhibits the expression of the antiapoptotic protein BCL2 and maintains the stimulated apoptotic pathway in these tumor cells. However, we found BCL2 gene expression upmodulated (3.47-fold) after Maitake treatment; based on previous reports, we can suggest that the apoptosis pathways in MCF-7 cells are positively balanced due to the BCL2 interaction with the FADD protein, which could affect the BCL2 pre-mRNA splicing and processing to play a pivotal role in the regulation of apoptosis. By another hand, it has also been demonstrated that FADD may be directly involved in regulating the amyloid precursor protein (APP) cleavage activity of BACE1,24 which was found downmodulated (−2.37-fold) in our experiments; thus, we can also suggest a potential role of Maitake D-Fraction in Alzheimer's disease, preventing APP accumulation. In conclusion, here we show that Maitake D-Fraction significantly increased the expression of RASSF2, a potential tumor suppressor, which may promote apoptosis in MCF-7 cells (Fig. 3). However, the exact molecular mechanism of RASSF2 in apoptosis is not yet defined.
Also, researchers show that SPARC (secreted proteins, acidic, cysteine-rich) potentiates apoptosis in the presence of chemotherapy in colorectal cancer, by augmenting the signaling cascade in a caspase-8-dependent manner. They found that this occurs independently of death receptor activation and leads to downstream involvement of Bid and subsequent apoptosis.25 In this study, we found that Maitake D-Fraction induces the expression of the SPARC gene (5.45-fold) in MCF-7 cells. Thus, we can suggest that Maitake D-Fraction induces the extrinsic pathway of apoptosis by overexpression of the SPARC gene (Fig. 3).
BCL2L13, also called BCL-rambo, is a widely expressed BCL-2 member that shows an overall structural homology containing sequence conservation clustered in BCL-2 homology (BH) domains. In mammalian cells, BCL-rambo was localized to mitochondria, and its overexpression induces apoptosis in those cells.26 Similar to Bax and Bak, overexpression of BCL-rambo causes the release of cytochrome C to the cytosol, indicating that mitochondrial damage occurs during BCL-rambo–induced cell death. Surprisingly, it has been found that overexpression of antiapoptotic BCL-XL and BCL-2 genes had little effect on BCL-rambo–mediated cell death. Thus, BCL-rambo appears to induce apoptosis, at least in part, independently of known mitochondrial signaling pathways.26 In our experiments, we found that Maitake D-Fraction treatment increases the expression of BCL2L13 (3.40-fold) in MCF-7 cells; thus, we suggest that this compound could be activated by the apoptotic pathway independently by the overexpression of the antiapoptotic BCL-2 gene (Fig. 3).
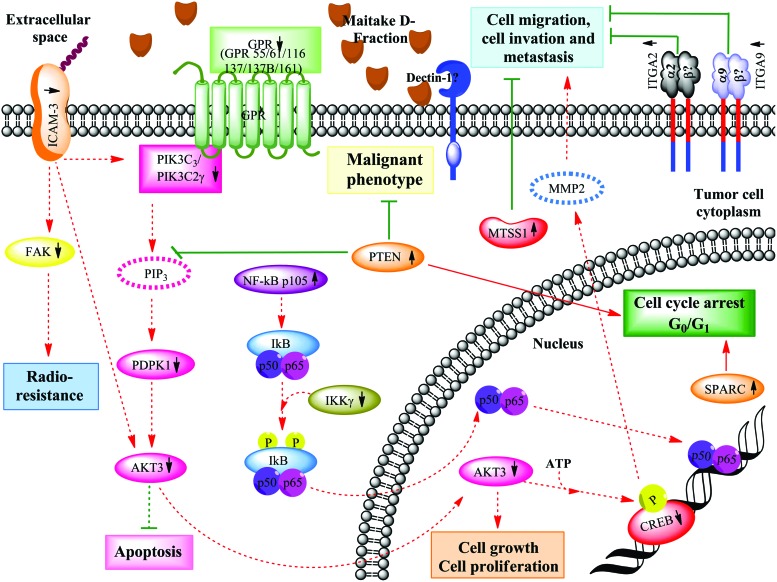
Decades of study have demonstrated an important role of phosphoinositide-3(OH) kinase (PI3K)–Akt signaling in the formation and progression of a wide variety of tumors. There are many diverse mechanisms by which aberrant activation of PI3K-AKT signaling occur in cancer cells, but all lead to similar downstream signaling events: from cell growth and proliferation to survival and motility, which drive tumor progression.27,28 Particularly, the mechanism by which AKT protects cells from death is likely to be multifactorial, because AKT directly phosphorylates several components of the cell death machinery.28 In this work, we found that D-Fraction downregulated PI3K-AKT signaling at 91 μg/mL in MCF-7 cells by downmodulation of PIK3C3 (−1.25-fold), PIK3C2γ, (−1.71-fold), PDPK1 (−1.91-fold), and AKT3 (−1.38-fold) genes. Thus, we can suggest that D-Fraction could decrease activation of the AKT anti-apoptotic pathway, which is frequently overactivated in cancer (Fig. 4). ICAM3 and PTEN genes are also involved in the downregulation of that pathway.
FIG. 4.
Maitake D-Fraction blocks the malignant phenotype, cell invasion, and metastasis in MCF-7 cells through activation of PTEN and MTSS1. The putative molecular mechanism of cell cycle arrest and blocking of malignant phenotype induced by activation of PTEN gene expression after Maitake D-Fraction treatment is depicted. As shown, MTSS1 blocks cell migration, invasion, and metastasis in MCF-7 cells. Stimulated (red) and inhibitory (green) pathways are indicated. Discontinuous gray lines represent the genes whose expression has not been changed after Maitake treatment. Color images available online at www.liebertpub.com/jmf
Maitake D-Fraction blocks tumor cell growth and proliferation
In our experiments, we found that Maitake D-Fraction increases (3.28-fold) the expression of cyclin-dependent kinase (CDK) inhibitor 1B (p27/Kip1) gene in MCF-7 cells. p27 or CDKN1B is a CDK inhibitor directly involved in the cell cycle arrest, and it acts as a typical tumor suppressor that regulates GO to S phase transitions by binding to and regulating the activity of CDKs.29 Recently, it has been reported that an increase in p27 gene expression is associated with cell growth arrest, cell differentiation, and apoptotic pathways, whereas its downmodulation is related to cell proliferation stimulation and breast tumorigenesis.30 Moreover, high levels of p27 are expressed in normal human mammary epithelium, but loss of p27 is frequent in breast cancer and has been demonstrated to have prognostic implications.31 Also, in primary tumors, low levels of p27 have been associated with reduced time to disease relapse and/or reduced overall patient survival.32 It has been demonstrated33 that only patients with tumors expressing high levels of p27 benefited from a combined treatment with tamoxifen and goserelin. Based on these findings and our results, we can postulate that Maitake D-Fraction could be directly involved in the breast tumor cell growth arrest, acting as a tumor suppressor by stimulating the p27/Kip1 gene expression. Also, we can hypothesize that Maitake D-Fraction could benefit patients who receive certain treatment by increasing the p27/Kip1 gene expression (Fig. 3).
It is well known that the retinoblastoma binding protein 4 gene (RBBP4) encodes an integral component of transcriptional silencing corepressor complexes. It was found that RBBP4, among several cellular proteins, binds directly to the retinoblastoma protein to regulate cell proliferation, and also seems to be involved in transcriptional repression of E2F-responsive genes.34 In our experiments, we found that Maitake D-Fraction (183 μg/mL) increases the expression (4.70-fold) of the RBBP4 gene in MCF-7 cells after 24 h of treatment. We can suggest that Maitake D-Fraction inhibits tumor cell growth and proliferation pathways by overexpression of the RBBP4 gene in breast cancer cells by blocking the RB-E2F protein complex formation (Fig. 3).
It has been demonstrated that ICAM-3 expression can contribute to cancer progression by inducing cancer cell proliferation through the PI3K/AKT pathway.35 In this study, we found that Maitake D-Fraction induces downmodulation of the ICAM-3 gene (−1.53-fold) and downregulated PI3K-AKT signaling (as described earlier) in MCF-7 cells. Thus, we can suggest that Maitake D-Fraction could decrease activation of the AKT proliferative pathway by repression of ICAM-3 gene expression (Fig. 4). However, the exact ICAM-3 molecular mechanisms remain unclear.
Many studies have demonstrated that RASSF2 may function as a tumor suppressor gene in in vitro cell migration, cell cycle progression, and colony formation assays. Recently, it has been reported that RASSF2 (isoform A) can function as a tumor suppressor gene in vitro inhibiting the growth of breast cancer cell lines in colony formation and soft agar growth assays, and in vivo inhibiting tumor formation when cells expressing RASSF2A were subcutaneously injected into SCID mice.36 All together and in agreement with the literature, we found that Maitake D-Fraction significantly increased the expression of the RASSF2 gene (4.62-fold) in MCF-7, and thus may be implicated in the suppression of tumor cell growth (Fig. 3).
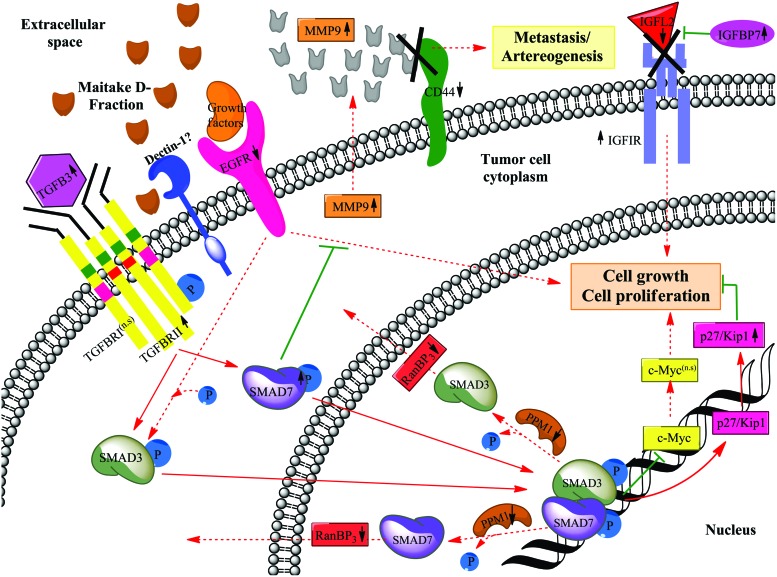
Transforming growth factor-β (TGF-β) is probably the best-characterized antigrowth pathway. Acquired resistance to growth inhibition induced by TGF-β signaling is a characteristic of the early stages of many solid tumors.37 SMAD proteins function as a transcriptional modulator activated by TGF-β and are thought to play a role in the regulation of carcinogenesis.38 SMAD3 target genes in epithelial cells include CDK inhibitors that generate a cytostatic response.39 Chen et al.39 defined how SMAD3 can mediate transcriptional repression of the growth-promoting gene c-Myc. Although it has been suggested that SMAD7 blocks downstream signaling of EGFR, the role of SMAD7 in the EGF signaling pathway has not been fully elucidated. In our experiments, we found that Maitake treatment resulted in significant overexpression of the TGF-β Receptor II (2.80-fold) and its ligand TGF-β3 (3.98-fold) in MCF-7 cells. Also, SMAD7 are upmodulated (5.92-fold) in these cells after Maitake treatment. We did not find SMAD3 significantly upmodulated after treatment. It is well known that the heterodimer SMAD3/SMAD7 is responsible for inducing the expression of the CDK inhibitor (p27/KIP1) directly involved in the arrest of cell cycle, which was detected after Maitake D-Fraction treatment. Also, these heterodimers are able to exert inhibition of c-Myc oncogene expression, a nuclear phosphoprotein that plays a role in cell cycle progression, suggesting a negative regulation of TGF-β in the cell proliferation. Recent work demonstrates that a phosphatase-dependent mechanism operates in the export of dephosphorylated SMAD2 out of the nucleus during termination of TGF-β signaling.40 PPM1A has been identified as a SMAD2 phosphatase, which can promote nuclear export of SMAD2,41 but the exact mechanism has not been elucidated. In our experiments, we found that the phosphatase proteins PPM1E, PPM1H, and PPM1K are downmodulated (−0.95-, −0.79-, and −0.80-fold, respectively), by Maitake D-Fraction treatment in MCF-7 cells; likewise, we observed downmodulation of RanBP3 gene expression (−1.92-fold), a key component that mediates Smad3/7 nuclear export, suggesting that the downstream signaling of TGF-β is not terminated under this stimulus, maintaining thus the tumor cell-growth and proliferation inhibited (Fig. 3). Moreover, other authors reported that RanBP3 directly recognizes dephosphorylated Smad2/3, and mediates nuclear export of Smad2/3 in a Ran-dependent manner.42 Also, it has been reported that increased expression of RanBP3 inhibits TGF-β signaling in mammalian cells and Xenopus embryos, but depletion of RanBP3 expression or dominant negative inhibition of RanBP3 enhances TGF-β–induced antiproliferative and transcriptional responses.42 Altogether, and in agreement with the literature, we can conclude that Maitake D-Fraction significantly inhibits the tumor cell growth and proliferation through activation of TGF-β signaling (Fig. 5).
FIG. 5.
Maitake D-Fraction blocks tumor cells growth and proliferation in MCF-7 cells through activation of p27/Kip1 gene expression. The putative molecular mechanism of cell growth and proliferation inhibition by activation of p27/Kip1 gene expression is shown; this process is stimulated by SMAD3 and SMAD7 proteins, which are activated after TGFBRII interaction. Stimulated (red) and inhibitory (green) pathways are indicated. Discontinuous gray lines represent the genes whose expression has not been changed after Maitake treatment. Color images available online at www.liebertpub.com/jmf
Insulin-like growth factor (IGF)–binding proteins (IGFBPs) are soluble proteins that bind IGFs with high affinity. Their principal functions are to regulate IGF availability in body fluids and tissues and to modulate IGF binding to its receptors. The IGFBP gene family plays a key role in regulating proliferation, differentiation, and apoptosis in different organ systems, including the human mammary gland. The principal mechanism of action of IGFBPs is believed to involve binding to and influencing the actions of IGFs, although IGFBPs may also affect cellular response through an IGF-independent pathway.43 IGFBPs may prevent interaction between IGF and IGF-IR or, when anchored to the extracellular matrix or the cell surface, may act as a reservoir where they may localize and release IGFs to cell-surface receptors, thereby enhancing IGF action.43 In agreement with that, in this study we found that the ligand IGFL2 was downmodulated (−1.61-fold) after D-Fraction treatment in MCF-7 cells. Although IGFIR was upmodulated (4.31-fold), we have also found that IGFBP-7 was upmodulated (3.67-fold), suggesting that the IGFR signaling pathway cannot be triggered after treatment, thus blocking cell growth and proliferation in these tumoral cells (Fig. 5). A study supports the role of IGFBP-1, -3, and -7 as potential tumor suppressor genes in human breast cancer.44 More recently, it has been demonstrated that IGFBP-7 expression is inversely correlated with disease progression in breast cancer and is associated with poor outcome.45 They showed that ectopic overexpression of IGFBP-7 significantly reduced the growth of the IGFBP-7–transfected MDA-MB-468 cells compared to the parental MDA-MB-468 cells. The role of IGFBP-7 on cell migration, a key determinant of malignant progression and metastasis was also checked.45 These results suggest that the growth of breast cancer could be prevented by the forced expression of the IGFBP-7 protein.45 Based upon these evidences and our results, we can suggest that Maitake D-Fraction induces the expression of the putative tumor suppressor gene IGFBP-7 in breast cancer cells to reduce tumor cell proliferation and malignancy (Fig. 5). Also, in this work, we found that Maitake D-Fraction induces the overexpression of the IGFBP-5 gene (5.21-fold) in MCF-7 cells (Fig. 6). Butt and colleagues have shown that IGFBP-5 prevents the in vitro and in vivo growth of human breast cancer cells.46 In their study, stable expression of IGFBP-5 inhibited the growth of tumors derived from MDA-MB-231 cells by elevated BAX and decreased BCL-2 at the mRNA level. Thus, we can suggest that Maitake D-Fraction may be involved in tumor cell growth inhibition.
FIG. 6.
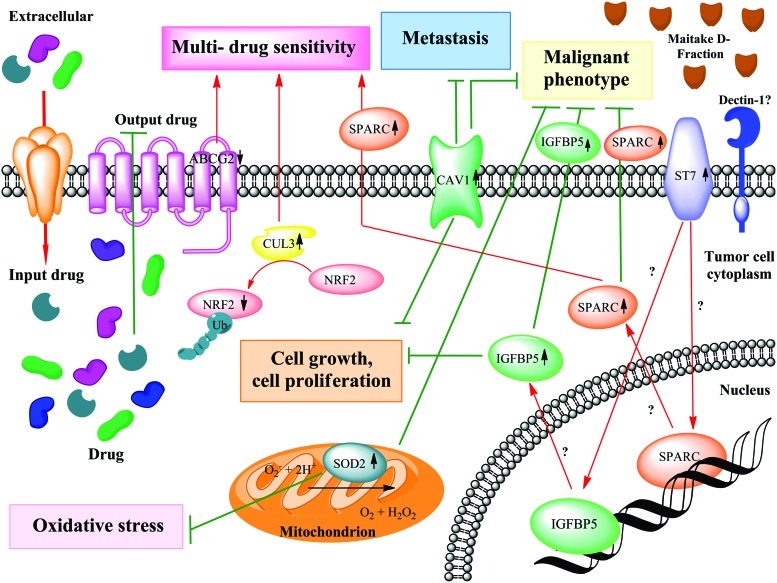
Maitake D-fraction blocks malignant phenotype through activation of SPARC gene expression, and in collaboration with CUL3, they promote multi-drug sensitivity. The putative molecular mechanism of inhibition of malignant phenotype through activation of SPARC gene expression in MCF-7 cells is depicted. Also shown are the means by which SOD2 blocks the oxidative stress in tumoral cells and CUL3 participates in multi-drug sensitivity, blocking the output drug in those cells. Stimulated (red) and inhibitory (green) pathways are indicated. Discontinuous gray lines represent the genes whose expression has not been changed after Maitake treatment. Color images available online at www.liebertpub.com/jmf
Caveolin 1 (CAV1) is greatly reduced in most oncogenically transformed and human cancer cells. It has been shown that the level of CAV1 is correlated with mammary cancer progression in vitro and the overexpression of CAV1 results in substantial growth inhibition of mammary tumor cells, which normally do not express endogenous CAV1.47 More recently, it has been demonstrated that CAV1 expression decreases the MCF-7 cell proliferation rate and markedly reduces their capacity to form colonies in soft agar.48 In our experiments, we found that Maitake D-Fraction induces the expression of CAV1 (2.91-fold) in MCF-7 cells after 24 h of treatment. Thus, and in concordance with the literature, we can suggest that D-Fraction inhibits cell growth and cell proliferation in breast cancer cells (Fig. 6).
Maitake D-Fraction downregulates pathway induced by NF-κB (p105)
NF-κB is a pleiotropic transcription factor that is present in almost all cell types. NF-κB regulates the expression of genes that play a key role in the development and progression of cancer, such as proliferation, migration, and apoptosis. It is well known that aberrant or constitutive NF-κB activation is present in many human malignancies.49 p50 and p52 are DNA-binding members of the NF-κB family. They are synthesized as precursors of p105 and p100, respectively, which are proteolytically processed to generate active DNA-binding p50 and p52 peptides.50 Also, it is well known that NF-κB complexes are held in the cytoplasm as inactive state complexes with members of the NF-κB inhibitor (IκB) family.51 In a conventional activation pathway, IκB is phosphorylated by IκB kinases (IKKs) in response to different activators, subsequently degraded, thus liberating the active NF-κB complex, which translocates to the nucleus.51 In the cytoplasm, NF-κB is inhibited by IκB. The upstream activating signal may cause phosphorylation of IκB by IKK. These trigger the degradation of IκB through the ubiquitin system, where the target molecule is masked by a chain of ubiquitins for degradation by the 26S protesome. The free NF-κB can then translocate to the nucleus and activate transcription. In our experiment, we found that the expression of the NF-κB p105 subunit is induced (4.08-fold) by Maitake D-Fraction in MCF-7 cells; however, we did not find p50 or p52 subunits activated after treatment. Also, we found that Maitake D-Fraction significantly downmodulated (−1.82-fold) IKKγ gene expression, thus suggesting that active NF-κB DNA-binding peptides are not generated after treatment, thus avoiding NF-κB nuclear translocation, DNA binding, and transcription of factors involved in the regulation of a wide variety of biological responses (Fig. 4).
Maitake D-Fraction induces cell cycle arrest in MCF-7 cells
It has been reported that SPARC causes cell cycle arrest at G1 phase in endothelial cells52,53 and in human colorectal cancer (MIP101 cells).54 In the present study, we found that Maitake D-Fraction significantly induces expression of the SPARC gene (5.45-fold) in MCF-7 cells, probably involved in arresting the cell cycle of breast cancer cells (Fig. 4). However, the exact SPARC molecular mechanisms remain unclear. In addition, other studies show that the expression of PTEN induces a marked decrease of cell proliferation due to cell cycle arrest in the G1 phase, which is attributed to a p27/Kip1 increase and cyclin D1 nuclear localization.55 In this work, we have observed that PTEN gene expression is upmodulated (3.27-fold) by Maitake D-Fraction treatment and thus, we can suggest that this compound induces cell cycle arrest through p27/Kip1 activation (Fig. 4).
Maitake D-Fraction blocks tumor cell migration, cell invasion, metastasis, and arteriogenesis
In our work, we found that 187 μg/mL of Maitake D-Fraction increases (4.05-fold) the expression of the α2 integrin (ITGA2) gene in tumoral MCF-7 cells after 24 h of treatment. It has been found that α2 integrin was significantly reduced in breast cancer compared to normal breast tissue in the Richardson cohort (P=.038).56 Not only was the loss of ITGA2 gene expression highly associated with malignant disease, the decrement in α2 integrin expression consistently correlated with disease progression in two different public data sets.57,58 We can postulate that the over expression of α2 integrin may serve as an important biomarker with antimetastasic potential and patient survival (Fig. 4). We also found that ITGA9 was upmodulated (3.35-fold) after 183 μg/mL Maitake treatment. It was shown that ITGA9 is expressed in normal human breast tissue,59 while in breast cancer, ITGA9 expression was down regulated or lost in 44% of tumors. Even more, it has been found by real-time PCR that the downregulation of tumor-suppressor genes RASSF1A and ITGA9 was associated significantly with lung adenocarcinoma progression.60 Based on these findings, we can hypothesize that overexpression of ITGA9 and ITGA2 could be involved in the antitumoral effect of Maitake D-Fraction in MCF-7 breast cancer cells. However, the exact mechanisms of its action are not yet elucidated (Fig. 4).
Likewise, it has been previously demonstrated that MTSS1 plays an important role in cancer metastasis.61 It has been reported that the overexpression of MTSS1 significantly suppresses (P<.01) the invasive, migratory growth and adherence properties of a human breast cancer cell line.61 In this work, we observed MTSS1 gene expression upmodulated (2.28-fold) after Maitake D-Fraction treatment, suggesting its role as a metastasic suppressor in MCF-7 breast cancer cells. Based on these findings, MTSS1 could be a prognostic indicator of disease-free survival in breast cancer patients. However, the biochemical mechanisms by which MTSS1 functions in cells and the physiological role of the MTSS1 protein in humans remain largely unknown (Fig. 4).
The ICAM proteins appear to have a dual role in cancer. Two works found that ICAM molecules (particularly ICAM-1) may target and block tumor progression by stimulation of an immune response. Conversely, other investigations have shown that ICAM molecules are involved in cancer malignancy and their overexpressions are associated with poor diagnosis, lower survival rates, and metastasis in some cancers, including melanoma, breast cancer, and leukemia.62 It has been demonstrated that overexpression of ICAM-3 increased AKT phosphorylation, which caused an increase in cellular migration/invasion and MMP activities. Even more, it has been shown that CREB is involved in ICAM-3–induced cell migration and invasion-intermediating signaling from Akt to MMP proteins.62 In this study, our results suggest that D-Fraction induces downmodulation of ICAM-3 (−1.53-fold) at 91 μg/mL in MCF-7 cells and thus reinforces the decreased activation of the AKT pathway. Also, we found that CREB was downmodulated (Fig. 4). Thus, we suggest that D-Fraction blocks cancer cell migration/invasion via the ICAM-3/AKT/CREB/MMP pathway.
Another gene related to metastasis is CD44. It has been demonstrated that CD44 promotes tumor growth and metastasis by mechanisms that remain poorly understood.63 CD44 was associated with a proteolytic form of the matrix metalloproteinase-9 (MMP-9) on the surface of mouse mammary carcinoma and human melanoma cells, promoting cell-mediated collagen IV degradation in vitro and mediating tumor cell invasion of G8 myoblast monolayers.63 Also, it has been demonstrated that the disruption of CD44/MMP-9 cluster formation, by overexpression of soluble or truncated cell surface CD44, inhibits tumor invasiveness in vivo.64 In agreement with this, in our work, we observed that CD44 gene expression is downmodulated (−1.47-fold) by Maitake D-Fraction treatment, suggesting that the MMP-9, which was found upmodulated (4.47-fold), cannot bind the CD44 receptor in the cell surface. Based upon this, we can postulate that because of CD44 downmodulation after Maitake treatment, the complex CD44/MMP-9 cannot occur, thus blocking tumor cell survival, metastasis, and arteriogenesis in tumoral MCF-7 cells. Moreover, other authors reported severely impaired arteriogenesis in CD44−/− knockout mice, accompanied by reduced leukocyte trafficking to sites of collateral artery growth and reduced expression of FGF2 (Fig. 5).65
Finally, in our experiments, we found that Maitake D-Fraction induces the expression of CAV1 (2.91-fold) in MCF-7 cells after 24 h of treatment. Many in vitro studies have demonstrated that CAV1 expression in cancer epithelial cells controls tumor cell behavior and indicate that CAV1 downregulation drives aggressive tumor growth. Also, exogenous CAV1 expression in a highly metastatic breast cancer cell line suppresses primary tumor growth and spontaneous metastasis formation in an orthotopic mouse model.66 In contrast, it has been reported that many solid tumors upregulate CAV1 in epithelial cells, which were correlated with poor clinical outcome.67 Also, it has been shown that CAV1 expression is significantly associated with a basal-like phenotype and decreased progression-free and overall survival.68 Conversely, an association between CAV1 overexpression and a favorable clinical outcome has been reported by fewer studies. It has been shown as clinical and mechanistic evidence that CAV1 is a critical target for suppression by Stat3 in driving invasion and metastasis of breast cancer cells.69 In our experiments, we found that Maitake D-Fraction induces the expression of CAV1 (2.91-fold) in MCF-7 cells after 24 h of treatment (Fig. 6). However, more research must be done to reconcile these discrepancies between the extensive evidence showing the tumor-suppressor functions of CAV1 and the clinical data showing high CAV1 levels in human tumors.
Maitake D-Fraction induces multidrug sensitivity in MCF-7 cells
Multidrug resistance is a major obstacle to success in cancer treatment. One mechanism by which cells can become resistant to chemotherapy is the expression of ABC transporters that use the energy of ATP hydrolysis to transport a wide variety of substrates across the cell membrane.70 The ATP-binding cassette transporter breast cancer resistance protein (ABCG2 or BCRP) is apparently a major determinant of multidrug resistance phenotype of tumors by extruding chemically diverse cytostatic drugs out of tumor cells. Thus, ABCG2 is a xenobiotic transporter that appears to play a major role in the multidrug resistance phenotype of a specific human breast cancer.71 In our experiments, we found that Maitake D-Fraction treatment reduces the expression of ABCG2 (−0.52-fold) in MCF-7 cells, suggesting that this compound could be a potential agent for multidrug sensitivity in breast cancer cells. In conclusion, cancer cells that do not express multidrug transporters are sensitive to chemotherapy and are eliminated in the course of chemotherapy (Fig. 6).
It has been demonstrated that low expression of SPARC was involved in colorectal cancer resistance to chemotherapy.72 Moreover, treatment of mice with SPARC conferred increased sensitivity to chemotherapy. These authors concluded that SPARC-based gene or protein therapy may ameliorate the emergence of resistant clones and eradicate existing refractory clones and offers a novel approach to treating cancer.72 Recently, it has been identified that an NT-domain of SPARC and its 51-aa peptide are highly efficient in modulating and enhancing apoptosis, thereby conferring greater chemosensitivity to resistant tumors.73 Also, it has been found that SPARC overexpression is a functionally important feature of a subset of triple-negative breast cancer patients. This study suggests that nab-paclitaxel may serve as a therapeutic agent for the subset of triple-negative patients that overexpress SPARC.74 In the present study, we found that Maitake D-Fraction significantly induces the overexpression of the SPARC (5.45-fold) gene in MCF-7 cells to probably increase the sensitivity to chemotherapy in these breast cancer cells (Fig. 6).
Also, overexpression of ICAM-3 was reported to increase the resistance of cancer cells to radiation by the activation of focal adhesion kinase (FAK).75 In this study, we found that Maitake D-Fraction induces downmodulation of ICAM-3 (−1.53-fold) and FAK (−1.40-fold) genes at 91 μg/mL in MCF-7 cells. Thus, we can suggest that these could be the mechanism by which D-Fraction decreases resistance to radiation in MCF-7 cancer cells (Fig. 4).
It has been demonstrated that simultaneous loss of CUL3 and p53 in hepatic progenitors turned these cells into highly malignant tumor-initiating cells, which formed largely undifferentiated tumors in nude mice. In addition, loss of CUL3 and p53 led to the formation of primary hepatocellular carcinomas.76 Importantly, loss of CUL3 expression was also detected in a large series of human liver cancers and correlated directly with tumor dedifferentiation. The overexpression of CUL3 during hepatic differentiation therefore safeguards against transformation to progenitory cells.76 Here we found that Maitake D-Fraction significantly induces the overexpression (4.56-fold) of the CUL3 gene in MCF-7 cells after 24 h of treatment. It has been previously demonstrated that Keap1 and the CUL3 E3 ligase are responsible to maintain NRF2 (GA binding protein transcription factor, alpha subunit 60 kDa) at very low concentrations by proteasomal degradation, a key transcription factor for cytoprotective gene programs.77 Therefore, high levels of CUL3/low NRF2 signature may be the key to cellular sensitivity to both chemical carcinogenic stimuli as well as to cytotoxicity of commonly used chemotherapeutic drugs in established breast cancers.77 Moreover, knocking down CUL3 constitutively upregulates the NRF2 as well as multiple carcinogen-detoxifying genes.77 Altogether, our data show that Maitake D-Fraction induces the overexpression of the CUL3 gene and downmodulates the NRF2 (−0.76-fold) gene, presenting a potential new target for breast cancer chemoprevention and treatment (Fig. 6).78
Maitake D-Fraction reverts the malignant phenotype MCF-7 cells
It has been demonstrated that the suppression of tumorigenicity 7 (ST7) gene functions as a tumor suppressor in both prostate and breast cancer cells.79 A role for ST7 in breast carcinogenesis is further supported by the finding that it is downregulated in a large proportion of primary breast cancers. These data, together with the observation that LOH at 7q31 is common in many tumor types, support the proposal that ST7 may behave as a multitissue tumor suppressor.79 Expression profiling of PC-3 cells revealed that ST7 predominantly induces changes in genes involved in remodeling the extracellular matrix such as SPARC, IGFBP5, and several matrix metalloproteinase.79 In this study, we found that ST7 was upmodulated (3.62-fold) after D-Fraction treatment in MCF-7 cells. The present data indicate that Maitake D-Fraction may mediate tumor suppression through ST7 gene expression (Fig. 6). However, the specific mechanism through which ST7 mediates tumor suppression remains unclear. Further studies of the function of ST7 should be done in the tumor suppression pathway. In relation with ST7, in this work, we found that Maitake D-Fraction induces upmodulation of IGFBP5 and SPARC genes in MCF-7 cells. IGFBPs are traditionally known as carrier proteins that regulate the activity of IGFs by prolonging their half-life and their circulation turnover. Importantly, there is increasing evidence that IGFBP-2, IGFBP-3, and IGFBP-5 are important players in the phenotypes of various cancers.80 Studies have supported the notion that IGFBP-5 overexpression contributes to metastasis and poor prognosis of cancer.81 However, recent studies have demonstrated that IGFBP-5 may regulate the metastatic capacity through protein–protein interactions in downstream signal transduction pathways that are activated in breast cancer: MAPK and PI3K/AKT pathways.80 In this work, we found that Maitake D-Fraction induces the overexpression of the IGFBP5 gene (5.21-fold) in MCF-7 cells, which may be involved in the control of malignant phenotypes (Fig. 6). However, the exact role of this gene is not yet fully understood.
Likewise, it has been reported that in ovarian cancer, lower SPARC expression is noted in advanced tumors,82 and studies using SPARC–/– animals reveal that loss of SPARC enhances growth of tumor xenografts of pancreatic and lung cancers.83,84 The implication that loss of SPARC expression promotes tumor growth is supported by observations that it is downregulated following malignant transformation in ovarian cancers82 and that upregulation of SPARC in these cells results in the development of nontumorigenic clones, while downregulation of SPARC results in more aggressive phenotypes.85 In the present study, we found that Maitake D-Fraction significantly induces the expression of the SPARC gene (5.45-fold) in MCF-7 cells to probably revert the malignant phenotype (Fig. 6).
Also, in our experiments, we found that Maitake D-Fraction significantly increases (3.94-fold) the expression of the SOD2 gene in MCF-7 cells after 24 h of treatment. It has been found that overexpression of SOD2 (Mn superoxide dismutase) in pancreatic tumoral cells can significantly attenuate the malignant phenotype, by decreasing cell growth, plating efficiency, and growth in soft agar.86 Moreover, it has been found that overexpression of SOD2 in melanoma cells can alter the phenotype in culture; the cells lose the ability to form colonies, a trait characteristic of malignant cells.87 Recently, it has been demonstrated that the expression of SOD2 in neoplasm tissues, independent of the clinicopathologic characters, plays a critical role in breast cancer biology.88 Thus, we can suggest that Maitake D-Fraction could be suppressing the malignant phenotype of MCF-7 cells by overexpression of SOD2 (Fig. 6).
Finally, in this work, we have observed that PTEN gene expression is upmodulated (3.27-fold) by Maitake D-Fraction treatment. PTEN is a tumor suppressor gene and was found mutated in multiple cancers. It has been published that PTEN protein functions as a negative regulator of the PI3K/Akt oncogenic pathway. Reduced PTEN expression and the deregulated PI3K/Akt pathway were associated with aggressive breast cancer phenotypes and poor outcome of the disease.89 Furthermore, tumors with reduced PTEN protein expression have been shown to carry a particular gene expression signature that predicts worse outcome and metastasis in breast cancer as well as in prostate and bladder carcinomas.90 In conclusion, here we can hypothesize that Maitake D-Fraction is able to reduce the aggressive breast cancer phenotype by activating the PTEN gene in MCF-7 cells (Fig. 4).
Maitake D-Fraction reduces the oxidative stress in MCF-7 cells
Antioxidant enzymes maintain cellular redox homeostasis. SOD2, an enzyme located in mitochondria, is the key enzyme that protects the energy-generating mitochondria from oxidative damage. It has been found that levels of SOD2 are reduced in many diseases, including cancer, neurodegenerative diseases, and psoriasis.87 Moreover, it has been demonstrated that SOD expression played a critical role in free radical detoxification and it is directly correlated with the cell cycle, defining one of the most important characteristics of tumor cells, namely, cell growth and proliferation.91 Recently, Sotgia et al.92 postulated a possible mechanism by which mitochondrial oxidative stress contributes to tumor initiation and progression: “[M]itochondrial oxidative stress in epithelial cancer cells leads to ROS production and ensuing DNA damage, resulting in an increased mutation rate and tumor evolution, via the positive selection of tumor cell mutations that confer a growth advantage.”92 In support of this notion, in our experiments, we found that Maitake D-Fraction significantly increases (3.94-fold) the expression of the SOD2 gene in MCF-7 cells after 24 h of treatment. These findings are in agreement with the hypothesis that SOD2 could play a role as a putative tumor suppressor gene and negative regulation of oxidative stress in these cells (Fig. 6).
Maitake D-Fraction reduces general pain through inhibition of HCN gene in MCF-7 cells
The nonselective cation channel HCN1 is a novel molecular target for the relief of pain, and has a relatively unexplored pharmacology. Development of a high-throughput compatible functional assay designed to detect antagonists of HCN1 has been demonstrated.93 As such, HCN channels may represent valid targets for novel analgesic agents. In the present work, we found that the HCN1 gene was downmodulated (−0.65-fold) after Maitake D-Fraction treatment in breast cancer cells. The exact molecular mechanisms of HCN1 in pain relief are not yet elucidated. However, screening studies for novel HCN channel blockers may be useful for the treatment of chronic pain in cancer patients. In agreement with that, we also observed in our in vivo experiments that 5 mg/kg/day of Maitake D-Fraction significantly reduces general pain in BALBc mice with mammary tumors after 15 days of i.p. injection (data non shown). To demonstrate if the downmodulation of the HCN1 gene is involved in the oncologic pain reduction in mice after Maitake treatment, we will measure the expression of HCN1 by RT-PCR assay.
In conclusion, it is widely agreed that the tumoral phenotype is characterized to uncontrolled cell proliferation. The growth ability of tumor cells could be related not only to an abnormal signaling proliferative, but also to the inability of cancer cells to activate apoptosis. Here we showed that Maitake D-Fraction treatment blocks tumor cell growth and proliferation pathways in MCF-7 cells (Supplementary Fig. S1). Moreover, the present study showed that Maitake treatment increases proapoptotic gene activation, which will lead to cell death, while suppressing activation of genes that block apoptotic pathways in breast cancer MCF-7 cells. Also, tumor cells are characterized by invasion to surrounding tissues and metastatic spread to distant sites. Our results show that Maitake D-Fraction treatment plays a role in governing the metastatic nature on breast cancer MCF-7 cells by inducing the overexpression of ITGA9, ITGA2, MTSS1, CAV1, and blocking the ICAM-3/AKT/CREB/MMP pathway (Supplementary Fig. S1). The resistance of cancer cells to radiotherapy and chemotherapy is a major problem of these standard protocols; given that a select cell population may carry advantageous mutations, the standard treatments may block tumorigenesis only temporarily, ultimately making tumors no longer responsive to treatment. Our results show that Maitake D-Fraction could be a potential agent for multidrug sensitivity in breast cancer cells, promoting the elimination of the tumor cells during chemotherapy (Supplementary Fig. S1).
Our data support the concept that Maitake D-Fraction can influence the switching on and off of genes expressed in human breast cancer MCF-7 cells, and thus could be able to control the breast cancer phenotype and even could be able to revert the aggressive/malignant phenotype by the molecular mechanisms postulated. Here we have shown how a compound derived from an edible mushroom has the potential to activate expression of certain genes that could affect the malignant cell phenotype, avoiding their aggressiveness and invasion, affecting their sensitivity to chemotherapy, inducing their apoptosis, and probably involved in the reversion of the malignant phenotype (Supplementary Fig. S1). This work leaves a wide-open door to begin investigating each of the pathways shown here one by one and demonstrates how a nutrigenomic agent can be transformed into a therapeutic agent for breast cancer disease.
Supplementary Material
Acknowledgments
This work was supported by the contribution of several colleagues that we would like to thank, especially to Lic. Maria Julia Ferronato. Also, we need to thank Dr. Mike Shirota and Tomoko Nakamura from Mushroom Wisdom, Inc., East Rutherford, NJ, USA, for their contribution in this work and for kindly providing the Maitake D-Fraction to us. Finally, we thank the Argentinian National Research Council, CONICET, for their support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mayell M. Maitake extracts and their therapeutic potential—a review. Altern Med Rev. 2001;6:48–60. [PubMed] [Google Scholar]
- 2.Borchers AT. Stern JS. Hackman RM. Kenn CL. Gershwin ME. Mushrooms, tumors, and immunity. Proc Soc Exp Biol Med. 1999;221:281–293. doi: 10.1046/j.1525-1373.1999.d01-86.x. [DOI] [PubMed] [Google Scholar]
- 3.Nanba H. Kubo K. Antitumor substance extracted from Grifola. U.S.Patent. 1998 Dec 29;5(854):404. issued. [Google Scholar]
- 4.Adachi Y. Okazaki M. Ohno N. Yadomae T. Enhancement of cytokine production by macrophages stimulated with (1→3)-beta-D-glucan, grifolan (GRN), isolated from Grifola frondosa. Biol Pharm Bull. 1994;17:1554–1560. doi: 10.1248/bpb.17.1554. [DOI] [PubMed] [Google Scholar]
- 5.Adachi Y. Ohno N. Yadomae T. Activation of murine kupffer cells by administration with gel-forming (1–3)-beta-D-glucan from Grifola frondosa. Biol Pharm Bull. 1998;21:278–283. doi: 10.1248/bpb.21.278. [DOI] [PubMed] [Google Scholar]
- 6.Ohno N. Egawa Y. Hashimoto T. Adachi Y. Yadomae T. Effect of beta-glucans on the nitric oxide synthesis by peritoneal macrophage in mice. Biol Pharm Bull. 1996;19:608–612. doi: 10.1248/bpb.19.608. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki I. Itani T. Ohno N, et al. Effect of a polysaccharide fraction from Grifola frondosa on immune response in mice. J Pharmacobiodyn. 1985;8:217–226. doi: 10.1248/bpb1978.8.217. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki I. Hashimoto K. Oikawa S. Sato K. Osawa M. Yadomae T. Antitumor and immunomodulating activities of a beta-glucan obtained from liquid-cultured Grifola frondosa. Chem Pharm Bull. 1989;37:410–413. doi: 10.1248/cpb.37.410. [DOI] [PubMed] [Google Scholar]
- 9.Kubo K. Nanba H. Modification of cellular immune responses in experimental autoimmune hepatitis in mice by maitake (Grifola frondosa) Mycoscience. 1998;39:351–360. [Google Scholar]
- 10.Soares R. Meireles M. Rocha A, et al. Maitake (D Fraction) mushroom extract induces apoptosis in breast cancer cells by BAK-1 gene activation. J Med Food. 2011;14:563–572. doi: 10.1089/jmf.2010.0095. [DOI] [PubMed] [Google Scholar]
- 11.Sambrook J. Fritsch EF. Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; New York: 1989. [Google Scholar]
- 12.Gentleman RC. Carey VJ. Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wettenhall JM. Smyth GK. LimmaGUI: a graphical user interface for linear modeling of microarray data. Bioinformatics. 2004;20:3705–3706. doi: 10.1093/bioinformatics/bth449. [DOI] [PubMed] [Google Scholar]
- 14.Smyth GK. Limma: linear models for microarray data. In: Gentleman R, editor; Carey V, editor; Dudoit S, editor; Irizarry R, editor; Huber W, editor. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer; New York: 2005. pp. 397–420. [Google Scholar]
- 15.Benjamini Y. Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B. 1995;57:289–300. [Google Scholar]
- 16.Ritchie ME. Diyagama D. Neilson J, et al. Empirical array quality weights for microarray data. BMC Bioinformatics. 2006;7:261. doi: 10.1186/1471-2105-7-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perelman E. Ploner A. Calza S. Pawitan Y. Detecting differential expression in microarray data: comparison of optimal procedures. BMC Bioinformatics. 2007;8:28. doi: 10.1186/1471-2105-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chittenden T. Harrington EA. O'Connor R, et al. Induction of apoptosis by the Bcl-2 homologue BAK-1. Nature. 1995;374:733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- 19.Kiefer MC. Brauer MJ. Powers VC, et al. Modulation of apoptosis by the widely distributed Bcl-2 homologue BAK-1. Nature. 1995;374:736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- 20.McPherson JP. Sarras H. Lemmers B, et al. Essential role for Bclaf1 in lung development and immune system function. Cell Death Differ. 2009;16:331–339. doi: 10.1038/cdd.2008.167. [DOI] [PubMed] [Google Scholar]
- 21.Kasof GM. Goyal L. White E. BTF, a novel death-promoting transcriptional repressor that interacts with BCL-2-related proteins. Mol Cell Biol. 1999;19:4390–4404. doi: 10.1128/mcb.19.6.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarras H. Alizadeh Azami S. McPherson JP. In search of a function for BCLAF1. Sci World J. 2010;10:1450–1461. doi: 10.1100/tsw.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donninger H. Hesson L. Vos M, et al. The Ras effector RASSF2 controls the PAR-4 tumor suppressor. Mol Cell Biol. 2010;30:2608–2620. doi: 10.1128/MCB.00208-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie J. Guo Q. PAR-4 is involved in regulation of beta-secretase cleavage of the Alzheimer amyloid precursor protein. J Biol Chem. 2005;280:13824–13832. doi: 10.1074/jbc.M411933200. [DOI] [PubMed] [Google Scholar]
- 25.Tang MJ. Tai IT. A novel interaction between procaspase 8 and SPARC enhances apoptosis and potentiates chemotherapy sensitivity in colorectal cancers. J Biol Chem. 2007;282:34457–34467. doi: 10.1074/jbc.M704459200. [DOI] [PubMed] [Google Scholar]
- 26.Kataoka T. Holler N. Micheau O, et al. BCL-rambo, a novel Bcl-2 homologue that induces apoptosis via its unique C-terminal extension. J Biol Chem. 2001;276:19548–19554. doi: 10.1074/jbc.M010520200. [DOI] [PubMed] [Google Scholar]
- 27.Saji M. Ringel MD. The PI3K-AKT-mTOR pathway in initiation and progression of thyroid tumors. Mol Cell Endocrinol. 2010;321:20–28. doi: 10.1016/j.mce.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vivanco I. Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 29.Sherr CJ. Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 30.Said TK. Moraes RC. Singh U. Kittrell FS. Medina D. Cyclin-dependent kinase (cdk) inhibitors/cdk4/cdk2 complexes in early stages of mouse mammary preneoplasia. Cell Growth Differ. 2001;12:285–295. [PubMed] [Google Scholar]
- 31.Stendahl M. Nilsson S. Wigerup C, et al. p27Kip1 is a predictive factor for Tamoxifen treatment response but not a prognostic marker in premenopausal breast cancer patients. Int J Cancer. 2010;127:2851–2858. doi: 10.1002/ijc.25297. [DOI] [PubMed] [Google Scholar]
- 32.Chu IM. Hengst L. Slingerland JM. p27 as Jekyll and Hyde. Regulation of cell cycle and cell motility. Cell Cycle. 2009;8:3455–3461. doi: 10.4161/cc.8.21.9789. [DOI] [PubMed] [Google Scholar]
- 33.Pohl G. Rudas M. Dietze O, et al. High p27Kip1 expression predicts superior relapse-free and overall survival for premenopausal women with early-stage breast cancer receiving adjuvant treatment with tamoxifen plus goserelin. J Clin Oncol. 2003;21:3594–3600. doi: 10.1200/JCO.2003.02.021. [DOI] [PubMed] [Google Scholar]
- 34.RBBP4. Retinoblastoma Binding Protein 4 [Homo sapiens] www.ncbi.nlm.nih.gov/gene/5928 www.ncbi.nlm.nih.gov/gene/5928
- 35.Kim YG. Kim MJ. Lim JS. Lee MS. Kim JS. Yoo YD. ICAM-3-induced cancer cell proliferation through the PI3K/Akt pathway. Cancer Lett. 2006;239:103–110. doi: 10.1016/j.canlet.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Cooper WN. Dickinson RE. Dallol A, et al. Nuclear localization and epigenetic inactivation of the RAS effector/tumour suppressor RASSF2A in multiple human cancers. Oncogene. 2008;27:1805–1811. doi: 10.1038/sj.onc.1210805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott RL. Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 38.Derynck R. Miyazono K. TGF-β and the TGF-β family. In: Dernyck R, editor; Miyazono K, editor. The TGF-β Family. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2008. pp. 29–44. [Google Scholar]
- 39.Chen CR. Kang Y. Siegel PM. Massague J. E2F4/5 and p107 as Smad cofactors linking the TGF-beta receptor to c-myc repression. Cell. 2002;110:19–32. doi: 10.1016/s0092-8674(02)00801-2. [DOI] [PubMed] [Google Scholar]
- 40.Schmierer B. Hill CS. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor beta-dependent nuclear accumulation of Smads. Mol Cell Biol. 2005;25:9845–9858. doi: 10.1128/MCB.25.22.9845-9858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin X. Duan X. Liang YY, et al. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell. 2006;125:915–928. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai F. Lin X. Chang C. Feng XH. Nuclear export of Smad2 and Smad3 by RanBP3 facilitates termination of TGF-β signaling. Dev Cell. 2009;16:345–357. doi: 10.1016/j.devcel.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perks CP. Holly JMP. IGF binding proteins (IGFBPs) and regulation of breast cancer biology. J Mammary Gland Biol Neoplasia. 2008;13:455–469. doi: 10.1007/s10911-008-9106-4. [DOI] [PubMed] [Google Scholar]
- 44.Subramanian A. Sharma AK. Banerjee D. Jiang WG. Mokbel K. Evidence for a tumour suppressive function of IGF1-binding proteins in human breast cancer. Anticancer Res. 2007;27:3513–3518. [PubMed] [Google Scholar]
- 45.Amemiya Y. Yang W. Benatar T, et al. Insulin like growth factor binding protein-7 reduces growth of human breast cancer cells and xenografted tumors. Breast Cancer Res Treat. 2011;126:373–384. doi: 10.1007/s10549-010-0921-0. [DOI] [PubMed] [Google Scholar]
- 46.Butt AJ. Dickson KA. McDougall F. Baxter RC. Insulin-like growth factor binding protein-5 inhibits the growth of human breast cancer cells in vitro and in vivo. J Biol Chem. 2003;278:29676–29685. doi: 10.1074/jbc.M301965200. [DOI] [PubMed] [Google Scholar]
- 47.Lee SW. Reimer CL. Oh P. Campbell DB. Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- 48.Fiucci G. Ravid D. Reich R. Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002;21:2365–2375. doi: 10.1038/sj.onc.1205300. [DOI] [PubMed] [Google Scholar]
- 49.Dolcet X. Llobet D. Pallares J. Matias-Guiu X. NF-κB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 50.Lin R. Gewert D. Hiscott J. Differential transcriptional activation in vitro by NF-kappa B/Rel proteins. J Biol Chem. 1995;270:3123–3131. doi: 10.1074/jbc.270.7.3123. [DOI] [PubMed] [Google Scholar]
- 51.Luo JL. Kamata H. Karin M. IKK/NF-kappaB signaling: balancing life and death-a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Funk SE. Sage EH. The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:2648–2652. doi: 10.1073/pnas.88.7.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Motamed K. Sage EH. SPARC inhibits endothelial cell adhesion but not proliferation through a tyrosine phosphorylation-dependent pathway. J Cell Biochem. 1998;70:543–552. doi: 10.1002/(sici)1097-4644(19980915)70:4<543::aid-jcb10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 54.Tai IT. Dai M. Owen DA. Chen LB. Genome-wide expression analysis of therapy-resistant tumors reveals SPARC as a novel target for cancer therapy. J Clin Invest. 2005;115:1492–1502. doi: 10.1172/JCI23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Georgescu MM. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer. 2010;1:1170–1177. doi: 10.1177/1947601911407325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richardson AL. Wang ZC. De Nicolo A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Van ’t Veer LJ. Dai H. van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 58.Doughtery ER. Jianping H. Bittner ML. Validation of computational methods in genomics. Curr Genomics. 2007;8:1–19. doi: 10.2174/138920207780076956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mostovich LA. Prudnikova TY. Kondratov AG, et al. Integrin alpha9 (ITGA9) expression and epigenetic silencing in human breast tumors. Cell Adh Migr. 2011;5:395–401. doi: 10.4161/cam.5.5.17949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anedchenko EA. Dmitriev AA. Krasnov GS, et al. Down-regulation of RBSP3/CTDSPL, NPRL2/G21, RASSF1A, ITGA9, HYAL1 and HYAL2 genes in non-small cell lung cancer. Mol Biol. 2008;42:965–976. [PubMed] [Google Scholar]
- 61.Parr C. Jiang WG. Metastasis suppressor 1 (MTSS1) demonstrates prognostic value and anti-metastatic properties in breast cancer. Eur J Cancer. 2009;45:1673–1683. doi: 10.1016/j.ejca.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 62.Park JK. Park SH. So K. Bae IH. Yoo YD. Um HD. ICAM-3 enhances the migratory and invasive potential of human non-small cell lung cancer cells by inducing MMP-2 and MMP-9 via AKT and CREB. Int J Oncol. 2010;36:181–192. [PubMed] [Google Scholar]
- 63.Yu Q. Toole BP. Stamenkovic I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med. 1997;186:1985–1996. doi: 10.1084/jem.186.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu Q. Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Royen N. Voskuil M. Hoefer I, et al. CD44 regulates arteriogenesis in mice and is differentially expressed in patients with poor and good collateralization. Circulation. 2004;109:1647–1652. doi: 10.1161/01.CIR.0000124066.35200.18. [DOI] [PubMed] [Google Scholar]
- 66.Sloan EK. Stanley KL. Anderson RL. Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene. 2004;23:7893–7897. doi: 10.1038/sj.onc.1208062. [DOI] [PubMed] [Google Scholar]
- 67.Sotgia F. Martinez-Outschoorn UE. Howell A. Pestell RG. Pavlides S. Lisanti MP. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol Mech Dis. 2012;7:423–467. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- 68.Savage K. Lambros MB. Robertson D, et al. Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin Cancer Res. 2007;13:90–101. doi: 10.1158/1078-0432.CCR-06-1371. [DOI] [PubMed] [Google Scholar]
- 69.Chiu WT. Lee HT. Huang FJ, et al. Caveolin-1 upregulation mediates suppression of primary breast tumor growth and brain metastases by stat3 inhibition. Cancer Res. 2011;71:4932–4943. doi: 10.1158/0008-5472.CAN-10-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robey RW. Polgar O. Deeken J. To KW. Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 71.Doyle LA. Yang W. Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tai IT. Dai M. Owen DA. Chen LB. Genome-wide expression analysis of therapy-resistant tumors reveals SPARC as a novel target for cancer therapy. J Clin Invest. 2005;115:1492–1502. doi: 10.1172/JCI23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rahman M. Chan AP. Tai IT. A peptide of SPARC interferes with the interaction between caspase 8 and Bcl2 to resensitize chemoresistant tumors and enhance their regression in vivo. PLoS One. 2011;6:e26390. doi: 10.1371/journal.pone.0026390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Basu G. Van Vickle G. Ghazalpour A, et al. Frequency distribution of SPARC in triple-negative breast cancer patients [abstract] J Clin Oncol. 2011;29:s27. [Google Scholar]
- 75.Chung YM. Kim BG. Park CS, et al. Increased expression of ICAM3 is associated with radiation resistance in cervical cancer. Int J Cancer. 2005;117:194–201. doi: 10.1002/ijc.21180. [DOI] [PubMed] [Google Scholar]
- 76.Kossatz U. Breuhahn K. Wolf B, et al. The cyclin E regulator cullin 3 prevents mouse hepatic progenitor cells from becoming tumor-initiating cells. J Clin Invest. 2010;120:3820–3833. doi: 10.1172/JCI41959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loignon M. Miao W. Hu L, et al. Cul3 overexpression depletes Nrf2 in breast cancer and is associated with sensitivity to carcinogens, to oxidative stress, and to chemotherapy. Mol Cancer Ther. 2009;8:2432–2440. doi: 10.1158/1535-7163.MCT-08-1186. [DOI] [PubMed] [Google Scholar]
- 78.Miao W. Loignon M. Hu L. Basik M. Batist G. Cullin-3 is a potential novel target for breast cancer chemoprevention and treatment. ASCO Annual Meeting Proceedings Part I. J Clin Oncol. 2006;24(18Suppl):13070. [Google Scholar]
- 79.Hooi CF. Blancher C. Qiu W, et al. ST7-mediated suppression of tumorigenicity of prostate cancer cells is characterized by remodeling of the extracellular matrix. Oncogene. 2006;25:3924–3933. doi: 10.1038/sj.onc.1209418. [DOI] [PubMed] [Google Scholar]
- 80.Akkiprik M. Feng Y. Wang H, et al. Multifunctional roles of insulin-like growth factor binding protein 5 in breast cancer. Breast Cancer Res. 2008;10:212–225. doi: 10.1186/bcr2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li X. Cao X. Li X. Zhang W. Feng Y. Expression level of insulin-like growth factor binding protein 5 mRNA is a prognostic factor for breast cancer. Cancer Sci. 2007;98:1592–1596. doi: 10.1111/j.1349-7006.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yiu GK. Chan WY. Ng SW, et al. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001;159:609–622. doi: 10.1016/S0002-9440(10)61732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brekken RA. Puolakkainen P. Graves DC. Workman G. Lubkin SR. Sage EH. Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest. 2003;111:487–495. doi: 10.1172/JCI16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Puolakkainen PA. Brekken RA. Muneer S. Sage EH. Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res. 2004;2:215–224. [PubMed] [Google Scholar]
- 85.Abeysinghe HR. Cao Q. Xu J, et al. THY1 expression is associated with tumor suppression of human ovarian cancer. Cancer Genet Cytogenet. 2003;143:125–132. doi: 10.1016/s0165-4608(02)00855-5. [DOI] [PubMed] [Google Scholar]
- 86.Weydert C. Roling B. Liu J, et al. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol Cancer Ther. 2003;2:361–369. [PubMed] [Google Scholar]
- 87.Li C. Zhou HM. The role of manganese superoxide dismutase in inflammation defense. Enzyme Res. 2011;2011:6. doi: 10.4061/2011/387176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsai SM. Hou MF. Wu SH, et al. Expression of manganese superoxide dismutase in patients with breast cancer. J Med Sci. 2011;27:167–172. doi: 10.1016/j.kjms.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heikkinen T. Greco D. Pelttari LM, et al. Variants on the promoter region of PTEN affect breast cancer progression and patient survival. Breast Cancer Res. 2011;13:11. doi: 10.1186/bcr3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saal LH. Johansson P. Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA. 2007;104:7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Echiburú-Chau C. Roy D. Calaf GM. Deleterious MnSOD signals lead to abnormal breast cell proliferation by radiation and estrogen exposure. Int J Oncol. 2011;38:1703–1711. doi: 10.3892/ijo.2011.978. [DOI] [PubMed] [Google Scholar]
- 92.Sotgia F. Martinez-Outschoorn UE. Lisanti MP. Mitochondrial oxidative stress drives tumor progression and metastasis: should we use antioxidants as a key component of cancer treatment and prevention? BMC Med. 2011;9:62–66. doi: 10.1186/1741-7015-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maher MP. Wu NT. Guo HQ. Dubin AE. Chaplan SR. Wickenden AD. HCN channels as targets for drug discovery. Comb Chem High Throughput Screen. 2009;12:64–72. doi: 10.2174/138620709787048028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.