Abstract
The protective effect of hydroalcoholic extract of hawthorn berries (HBE) on acetic acid (AA)–induced colitis in rats was investigated. Forty-two Wistar rats were divided into seven groups, including control and test groups (n=6). The control animals received saline, and the test animals were treated with saline (sham group), mesalamine (50 mg/kg; M group), atorvastatin (20 mg/kg; A group), HBE (100 mg/kg; H group), mesalamine and HBE (HM group), or atorvastatin plus HBE (HA group), 3 days before and a week after colitis induction. Colitis was induced by administration of 1 mL AA (4%) via a polyethylene catheter intrarectally. High-performance liquid chromatography analyses showed that HBE contained 0.13% and 0.5% oleanolic acid and ursolic acid, respectively. Elevated myeloperoxidase activity and lipid peroxidation were attenuated in the HA group. The H and HM groups showed marked reductions in colitis-induced decreases in total thiol molecules and body weight. The histopathological studies revealed that HBE decreased colitis-induced edema and infiltration of neutrophils. Our data suggest the anti-inflammatory and antioxidant effects of HBE and atorvastatin protect against AA-induced colitis. The anti-inflammatory effect of HBE may be attributable to its ability to decrease myeloperoxidase activity as a biomarker of neutrophil infiltration.
Key Words: acetic acid-induced colitis, antioxidant status, hawthorn berries extract, histopathological examinations, myeloperoxidase activity
Introduction
Ulcerative colitis (UC) is a chronic unspecified inflammatory disease of the gastrointestinal tract, which mostly affects the colon and often is characterized by main clinical symptoms such as diarrhea, rectal bleeding, body weight loss, and abdominal pain.1 Other clinical signs, including fever, anemia, joint pain, damage to mucus membranes, renal calculi, and osteoporosis, have also been reported.2 The major histopathological changes which are observed in UC include ulceration and erosion of the mucosa mostly in the rectum and the distal colon. Other symptoms have been reported in the colons of rats with experimentally induced colitis, such as coarsened epithelium, substantial dilation and thinning of the intestinal walls, hyperemia, edema, friable mucus membranes with hemorrhage, and inflammatory exudates.3 The exact etiology and pathogenesis of UC is not clear yet. There are, however, several factors, such as environmental, genetic, and immunological factors, which play crucial roles in the pathogenesis of UC. The abnormal release of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and prostaglandin D2 are important mediators in the pathogenesis of UC.4,5
In general, the medical treatment of UC starts with 5-aminosalicylic acid as the baseline medication, followed by steroids and immunomodulators. Moreover, to intensify the treatment, infliximab, calcineurin inhibitors (cyclosporine A and tacrolimus), or surgery may be considered as rescue therapy.6 There are some recommended alternative approaches for UC therapy, such as transdermal administration of nicotine, omega-3 fatty acids with anti-inflammatory properties, and biological substances, including TNF-α, interleukin (IL)-2 receptor, and anti-IL-12 and IL-6 antibodies.7,8
Statins or 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors exert pleiotropic effects, such as anti-inflammatory and antioxidant properties, improvement of the endothelial function, and immunomodulatory effects, which may be independent of their basic lipid-lowering properties.9 There are promising data indicating the effectiveness of statin therapy in patients with heart and renal failure, neurological disorders, and infectious diseases.10–12 There is, however, little known about the effects of statins on UC; therefore, the first goal of the current study was to clarify the possible protective effect of atorvastatin on experimentally induced UC in a rat model.
Hawthorn (Crataegus spp.) and hawthorn-derived products, including its extract and dried fruit, are used worldwide. There are various species of hawthorn which are widely used as medicinal and food materials in European countries, China, and other countries. Hawthorn extract is traditionally used for preventing and treating cardiovascular diseases, including angina, hypertension, and congestive heart failure. Moreover, other medical benefits such as lowering plasma cholesterol and triacylglycerols have also been reported for hawthorn.13 Previous studies have demonstrated that hawthorn berry extracts (HBEs) contain various constituents, including ursolic acid (UA), hyperoside, isoquercitrin, epicatechin, chlorogenic acid, quercetin, rutin, and protocatechuic acid, with remarkable biological activities such as free-radical scavenging, antilipoperoxidation, and anti-inflammatory actions.14 Toxicological studies have reported no major side effects from the consumption of hawthorn. Therefore, in this study, we aimed to demonstrate the protective effect of HBEs on experimentally induced UC in a rat model. The protective effect of HBEs was compared with the currently used synthetic compound—mesalamine—in the treatment of UC. Moreover, a combination therapy was also designed to discover any interaction between HBE and two other compounds.
Materials and Methods
Chemicals
5,5′-Dithiobis-2-nitrobenzoic acid, N-(1-naphthyl) ethylenediamine-2HCl (NED), hexadecyltrimethylammonium bromide, and tetramethylbenzidine were purchased from Sigma Chemical Co. Oleanolic acid (OA), UA, and NED were obtained from Sigma-Aldrich. Thiobarbituric acid (TBA), phosphoric acid (85%), dimethyl sulfoxide (DMSO), sodium nitrite, and ethanol were purchased from Merck. N-butanol was obtained from Carl Roth, GmbH Co. Sulfanilamide was purchased from ACROS Organics. All other chemicals were commercial products of analytical grade. A commercially available standard kit was used for the determination of alkaline phosphatase (ALP, 744; Man Inc.). Mesalamine (Pentasa®; FERING GmbH) and atorvastatin (Darou Pakhsh Pharmaceutical Co.) were obtained from a local drug store (Urmia, Iran).
Plant material collection and extraction
Hawthorn berries were collected from east and west Azerbaijan, Iran, between August and September 2011 and after identification by an expert botanist (botanic herbarium, Biology Department, Faculty of Basic Sciences), the samples were air-dried. The voucher sample of hawthorn was kept in the laboratory of H.M. The seeds were removed from berries and the dried berries were ground using a coffee grinder. The powder was extracted using ethanol (70%) and after filtration (sterile gauze), was concentrated using a vacuum evaporator (Heidelberg, Germany). To reach full dryness, the extracts were exposed to a gentle stream of nitrogen gas.
Triterpenic acid determination in HBE
The triterpenic acids were analyzed using reversed-phase high-performance liquid chromatography (HPLC) according to the previously described method with minor modification.15 The HPLC analysis was performed on an Agilent 1200 HPLC system equipped with a Quart pump (G 1311 A), an autosampler (G 1329 A), and an ultraviolet detector (VWD, G 1314 B). The HBE (100 mg) was dissolved in mobile phase and 20 μL of the dissolved solution was injected into the column (Eclipse XDB-C 18, 5 μm, 4.6 mm×15 cm) using an autosampler. The mobile phase consisted of a mixture of 15% H2O, 85% methanol, and 0.15% acetic acid (AA). A total run time of 27.5 min and a flow rate of 1 mL/min were performed. The eluent was detected by an ultraviolet detector at 210 nm. To identify the OA and UA, we used pure standards to prepare calibration curves and compare the OA and UA peaks of the samples with those of the external standards using the same conditions.
Animals, experimental design, and UC induction
Forty-two adult male Wistar rats (200–220 g) were obtained from the animal resource center of the Faculty of Veterinary Medicine, Urmia University. The rats were acclimatized for a week with free access to food and water. The experimental protocols were approved by the ethics committee of Urmia University in accordance with the principles of laboratory animal care (NIH publication no. 85–23, revised 1985). Animals were assigned to the control and test groups (n=6). The control animals received saline and the test animals were pretreated with saline (sham group) and/or test compounds 3 days before and 7 days after the colitis induction.
Animals in the test group were subdivided to the following groups based on received compounds:
Sham group: animals in this group received saline (0.9%, 5 mL/kg)
M group: animals in this group received mesalamine (50 mg/kg body weight [b.w.], orally)
A group: animals in this group received atorvastatin (20 mg/kg b.w., orally)
H group: animals in this group received HBE (100 mg/kg b.w., orally)
HM group: rats in this group received HBE (100 mg/kg b.w., orally) and mesalamine (50 mg/kg, orally)
HA group: animals in this group received HBE (100 mg/kg b.w., orally) and atorvastatin (20 mg/kg)
Control animals without colitis received only saline (0.9%, 5 mL/kg) during the 10-day experimental period. All test compounds were administered through the gastric gavage. Colitis was induced by administration of 1 mL of AA (4%) via polyethylene catheter intrarectally in all test groups.16
Serum preparation and tissue sample collection
On day 12, following light diethyl ether anesthesia, the blood samples were collected directly from the heart. After one hour at room temperature, the samples were centrifuged at 3000 g for 10 min to obtain the serum. The serum samples were then stored at −20°C for further analyses.
The anesthetized animals were euthanized by using CO2 gas in a special device, and immediately, the macroscopically abnormal looking (congested and gaseous) sections of the distal colon were removed and rinsed with chilled saline. The samples were then divided into two parts, the first of which was fixed in 10% formalin in phosphate-buffered saline for pathological examinations and the second part was snap-frozen in liquid nitrogen and kept at −70°C for further biochemical analyses.
Measurement of serum ALP
Serum levels of ALP were measured using a commercial kit (744; Man Inc.) according to the manufacturer's instructions.
Nitric oxide measurement
The total nitrate/nitrite content of the colon section was measured according to the Griess reaction.17 In the Griess reaction, nitric oxide (NO) is rapidly converted into the more stable nitrite, which in an acidic environment nitrite is converted to nitrous acid (HNO2). When reacted with sulfanilamide, HNO2 forms a diazonium salt, which reacts with NED to form an azo dye that can be detected by measuring absorbance at 540 nm. The NO contents of the examined organs were expressed as nmol per mg of protein in samples.
Malondialdehyde determination
The lipid peroxidation rate was estimated by measuring TBA reactive substances (TBARS) of the colon samples as described previously.18 The amount of TBARS was expressed as nmol malondialdehyde (MDA) equivalents per mg of protein. The protein content of the samples was assessed according to Lowry et al.19
Measurement of total thiol molecules
Total sulfhydryl levels in the colon section were measured as described previously.20 The total thiol molecule (TTM) capacity was expressed as nmol per mg of protein in samples. The protein content of the samples was measured according to Lowry et al.19
Myeloperoxidase activity
Myeloperoxidase (MPO), as a biochemical indicator of neutrophil infiltration into the gastrointestinal tissue, was measured in collected colon samples as previously described.21 In brief, the specimens were homogenized in a 10 mM potassium phosphate buffer (pH 7.0), containing 0.5% hexadecyl trimethyl ammonium bromide and centrifuged at 20,000 g for 30 min at 4°C. A solution of 1.6 mM tetramethylbenzidine and 0.1 mM hydrogen peroxide (H2O2) was added and reacted with an aliquot of the supernatant. The rate of change in the optical density was measured at 650 nm spectrophotometerically. One unit of the MPO activity was defined as that degrading 1 μmol of H2O2 per minute at 37°C and was expressed as units per milligram of tissue sample (U/mg tissue).
Histopathological examinations
On day 12 (a day after the last drug administration), animals were euthanized by diethyl ether and the distal colon was removed, cut longitudinally, and rinsed with chilled normal saline. Using a magnifying glass, macroscopic damage scores were assigned by an independent pathologist according to Kuralay et al.22 The following scores were assigned when evaluating the macroscopic damage: 1, an intact epithelium with no damage; 2, patch-type superficial hyperemia; 3, generalized patch-type hyperemic regions; 4, generalized hyperemia and hemorrhage.
For microscopic examinations, the colon samples were fixed in 10% formalin in phosphate-buffered saline, embedded in paraffin, cut into 5–6-μm sections, stained with eosin and hematoxylin, and at least three sections of each colon sample were examined by a pathologist blinded to groups. The following criteria were used to score the microscopic injuries: 0, an intact epithelium, no leukocyte or hemorrhage; 1, <25% disrupted epithelium, focal leukocyte infiltrates, and focal hemorrhage; 2, 25% disrupted epithelium, focal leukocyte infiltrates, and focal hemorrhage; 3, ≤50% disrupted epithelium, widespread leukocytes, and hemorrhage; 4, >50% disrupted epithelium, extensive leukocyte infiltration, and hemorrhage.23
Statistical analyses
Results are expressed as mean±standard deviation. The data were analyzed by one-way analysis of variance followed by the Bonferroni post hoc test for multiple comparisons. For macroscopic and histopathological data, a nonparametric Kruskal–Wallis test was used. A P-value<.05 was considered statistically significant.
Results
OA and UA levels in hawthorn berry ethanolic extract
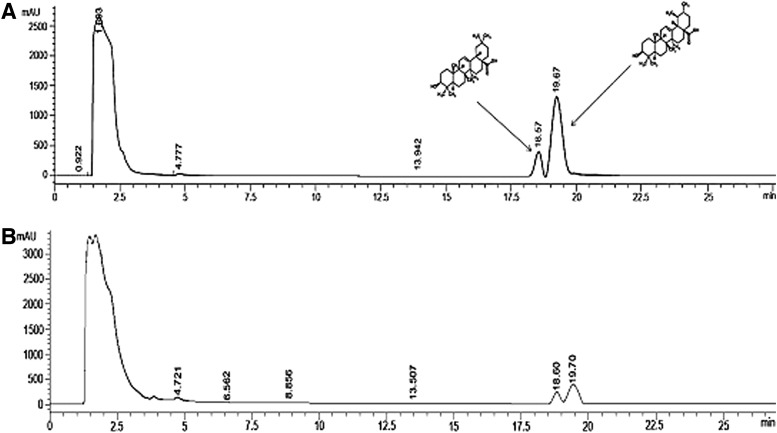
Total contents of the HBE included 0.13% OA and 0.5% UA. The HPLC profile of the triterpenic standards and HBE are shown in Figure 1.
FIG. 1.
High-performance liquid chromatography profile of the oleanolic acid (retention time=18.57 min) and ursolic acid (retention time=19.67 min) in the (A) standard and (B) test sample from hawthorn berry extract.
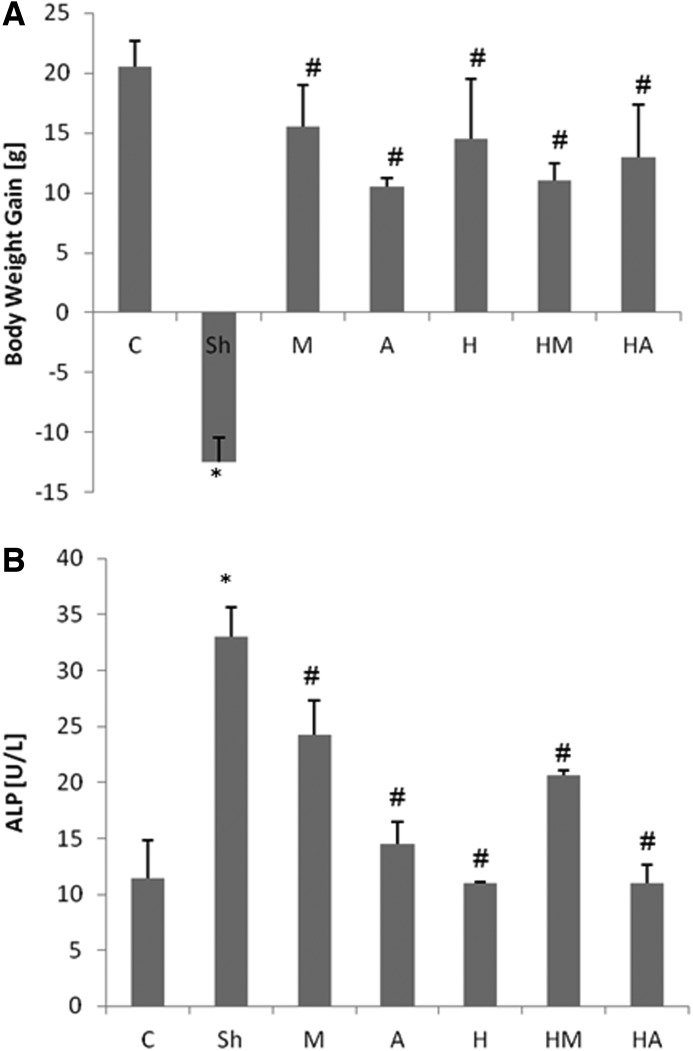
HBE prevented colitis-reduced body weight loss and lowered the colitis-elevated ALP level
The body weight gain in the sham group that received only normal saline before and after colitis induction was significantly less (P<.01) in comparison to the control group, while mesalamine, atorvastatin, and HBE administration in individual and combination forms remarkably prevented the colitis-induced body weight loss (Fig. 2A).
FIG. 2.
Effect of hawthorn berry extract (HBE) and atorvastatin on (A) colitis-decreased body weight gain and (B) serum level of alkaline phosphatase. Bars represent mean±standard deviation; n=6 in each group. Significant differences (P<.05) were found in comparison between the untreated colitis-positive group (sham [Sh]) and *the control group (C) or #the treated colitis-positive groups (M, 50 mg/kg mesalamine; A, 20 mg/kg atorvastatin; H, 100 mg/kg HBE; HM, both mesalamine and HBE; HA, both atorvastatin and HBE).
HBE, atorvastatin, and mesalamine were able to decrease the colitis-elevated levels of ALP. HBE alone exerted the strongest protective effect on ALP elevation, followed by atorvastatin and mesalamine. No synergistic effect between HBE and atorvastatin or mesalamine was observed (Fig. 2B). Although coadministration of HBE along with mesalamine resulted in lower ALP levels in comparison to the sham group, it was statistically lower than when HBE was given alone.
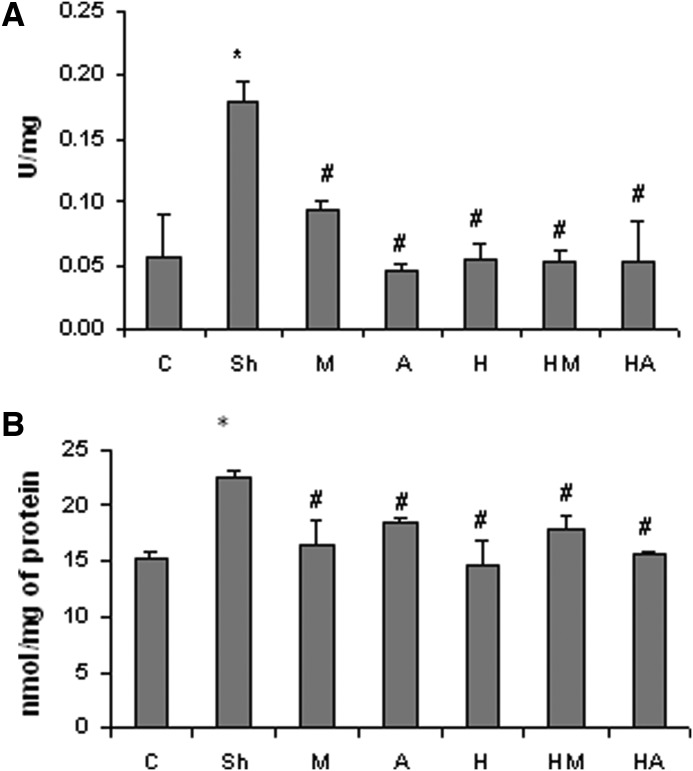
HBE decreased the colitis-enhanced MPO activity and NO level
The AA-induced colitis resulted in significant increases in the MPO activity in the colon tissue, but there were significantly lower MPO activities in the colon sections of treated groups. The maximum decrease in the MPO activity was found in the group of animals, which was treated with atorvastatin. Concurrent administration of HBE with atorvastatin and/or with mesalamine did not result in a synergistic lowering of the MPO activity (Fig. 3A). The NO content of the colon region in the AA-treated animals was significantly elevated compared to the control group. By contrast, the NO levels in all the other treated groups were slightly, but significantly lower and were lowest in the HBE group (Fig. 3B).
FIG. 3.
Effect of HBE and atorvastatin on (A) the colitis-elevated myeloperoxidase activity and (B) the nitric oxide level in the distal colon. Bars represent mean±SD; n=6 in each group. Significant differences (P<.05) were found in comparison between the untreated colitis-positive group and *the control group or #the treated colitis-positive groups.
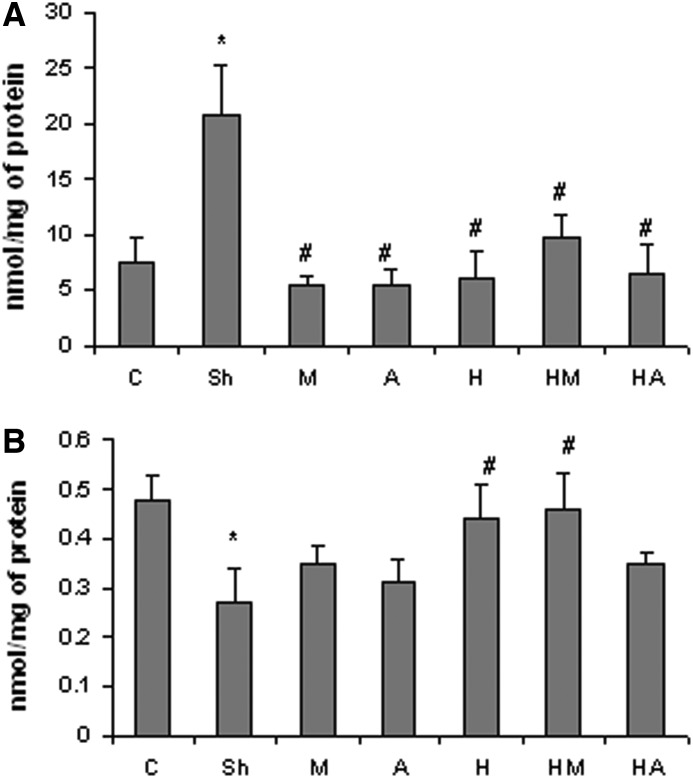
HBE attenuated the colitis-elevated MDA content and protected against the colitis-induced TTM depletion
The TBARS content of the colon region in the AA-administered group was significantly elevated, while all treated groups showed a lower lipid peroxidation rate. The lipid peroxidation lowering potency of HBE was found to be comparable to that of mesalamine and atorvastatin. The lowest level of TBARS was found in the group of animals which received mesalamine after colitis induction (Fig. 4A). The TTM concentration was significantly lower in the untreated colitis-induced animals, while HBE alone and in combination with mesalamine was able to remarkably protect against TTM depletion. Although mesalamine and atorvastatin alone partially prevented the colitis-induced decrease in TTM levels, it was not statistically significant (Fig. 4B).
FIG. 4.
Effect of hawthorn berry extract and atorvastatin on (A) the colitis-elevated malondialdehyde content and (B) the total thiol molecule concentration in the distal colon. Bars represent mean±SD; n=6 in each group. Significant differences (P<.05) were found in comparison between the untreated colitis-positive group and *the control group or #the treated colitis-positive groups.
Effect of HBE on macroscopic features
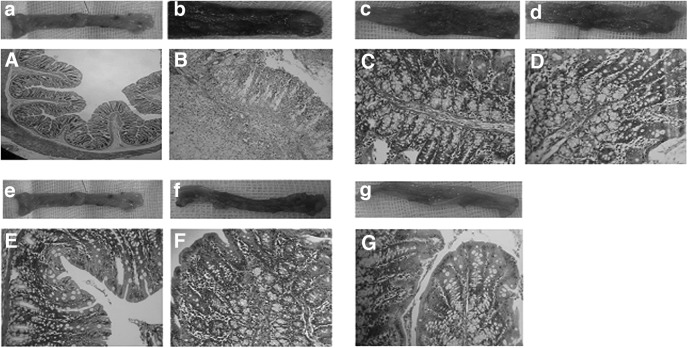
In the macroscopic evaluation, the distal colon in animals that received AA without further treatment exhibited inflamed mucosa with ulcerated and hemorrhagic features compared to the control group (Fig. 5b). However, the test groups had significantly better macroscopic scores, with maximum positive improvement in the group of animals that received HBE. Coadministration of HBE with atorvastatin and mesalamine failed to potentiate the HBE protective effect (Table 1).
FIG. 5.
Macroscopic and microscopic photomicrograph of the rat's colon: (a, A) normal colon tissue from the control group, (b, B) are representing, respectively, the macroscopic ulcerative and hemorrhagic epithelium with edematous and severe infiltration of inflammatory cells in lamina propria of distal colon from untreated colitis-positive animals, (c, C) are showing the macroscopic and microscopic features of the mesalamine-treated rats with remarkable improvement, (d, D) are demonstrating the macroscopic and microscopic appearance of distal colon from the atorvastatin-treated animals, (e, E) are illustrating the macroscopic and microscopic feature of the distal colon from the hawthorn berry extract (HBE)-received animals with an approximately normal epithelium and a submucosal structure, (f, F) are showing the macroscopic and microscopic architecture of the distal colon from the HBE and mesalamine-treated group with a relatively improved structure, and (g, G) are illustrating the macroscopic and microscopic features of the distal colon from HBE and atorvastatin-received rats. Eosin and hematoxylin staining; magnification: (A, B) 100×, (C–G) 400×.
Table 1.
Effect of Hawthorn Berry Extracts and Atorvastatin on Macroscopic and Histological Architecture of the Distal Colon
| Group | Macroscopic damage score | Microscopic injury score |
|---|---|---|
| Control | 1±0.00a | 0±0.00a |
| Sham | 3.6±0.42b | 3.8±0.36b |
| M | 2.4±0.27c | 3.2±1.20b |
| A | 2.1±0.70c | 2.4±0.81c |
| H | 1.8±0.24c | 1.5±0.36c |
| HM | 2.1±0.64c | 2.4±0.32c |
| HA | 2.4±0.42c | 2.7±0.18c |
Treated colitis-positive groups: M, 50 mg/kg mesalamine; A, 20 mg/kg atorvastatin; H, 100 mg/kg hawthorn berry extract (HBE); HM, both mesalamine and HBE; HA, both atorvastatin and HBE.
Values with different letters in the same column are significantly different (P<.05).
Effect of HBE on histopathological features
The distal colonic mucosa of rats in the control group had a normal feature with an intact epithelium (Fig. 5A). Comparing the colitis-induced rats with the untreated control group revealed severe and statistically significant necrosis, hemorrhage, submucosal edema, and inflammatory cell infiltration in the lamina propria (Fig. 5B). In atorvastatin- and/or mesalamine-treated groups, the histopathological changes were significantly attenuated, and declines in edema and inflammatory cell recruitment in lamina propria were observed. Although a few inflammatory cells were observed in the HBE-treated rats, the colon structure was approximately similar to that in the control group. We failed to show any additive or potentiation effect between HBE and atorvastatin or HBE and mesalamine in microscopic features (Table 1). As depicted in Table 1, HBE in both individual and combination therapy with atorvastatin and/or mesalamine could remarkably attenuate the AA-induced macroscopic and microscopic injuries. Mesalamine only was able to significantly decrease the macroscopic damage in the colitis-induced animals.
Discussion
This study showed that a hydroalcoholic extract of hawthorn berries (HBE) containing OA and UA, similar to the currently used mesalamine and/or statins such as atorvastatin, could protect from AA-induced colitis pathologies in rat. The protective effect of HBE on experimentally induced colitis was reflected in its ability to decrease the AA-elevated MPO activity, to lower the elevated NO content and TBARS level, and enhance the colitis-induced decrease in TTM concentrations in the distal colon. Moreover, AA-induced pathological damages were partially prevented by the HBE treatment.
Induction of colitis with AA has been used extensively in many experimental studies.24,25 Therefore, in the current study, we also used AA (4%) for experimental colitis induction. Our preliminary evaluation, including clinical symptoms, such as diarrhea (in some cases bloody) and body weight loss, confirmed that colitis was induced in the animals.
The pathophysiology of inflammatory bowel disease (IBD) and UC has not been fully identified; however, among the other immunological and genetic disorders, stress and dietary factors have been recognized as direct and indirect risk factors of IBD induction.
The most accepted theory about the etiology of IBD suggests that the T cell is inappropriately activated due to a confluence of genetic and environmental factors, which generate an immune imbalance leading to inflammation.26 Following immunological disorders in the gastrointestinal tract and, in particular, in the colon, reactive oxygen species are elevated remarkably and contribute considerably to the development of tissue injury.27,28 Previous studies reported that in IBD cases, the gastrointestinal NO production is increased, which results in the formation of toxic metabolites.29 Our results confirmed the previous report that experimentally induced colitis in rats results in a significant elevation of the NO content and a remarkable reduction in TTM levels in the colon, suggesting a major role of the pro-oxidants in colitis induction. This finding was later supported by the increased colon TBARS levels of animals with colitis induced by 4% AA.
The MPO activity as a biomarker of polymorphonuclear (PMN) leukocyte infiltration into the inflammation site (colon) was elevated by 200% in the sham group. This finding was strongly supported by macroscopic and microscopic examinations, which were characterized by the dramatic inflammatory edema and massive infiltration of PMN in the lamina propria. Previous studies showed the MPO activity elevation in both AA- and trinitrobenzenesulfonic acid-induced experimental colitis in rodents.16,30
The second part of the current study was devoted to examine the gastroprotective effect of Crataegus monogyna berry extract and atorvastatin on the AA-induced colitis. Moreover, any possible interaction between test compounds was also investigated. The potency of test compounds was compared to mesalamine as the currently used medicine in the treatment of colitis and HBE exerted a comparable protective effect to mesalamine. The biomarkers used to evaluate the protective effect of atorvastatin and HBE on the AA-induced colitis revealed anti-inflammatory and antioxidant effects of both compounds. A significant reduction in the colon MPO activity was observed in the M, A, and H groups, suggesting anti-inflammatory effects of HBE and atorvastatin on AA-induced inflammation. Although there is no direct in vivo study to show the anti-inflammatory effect of HBE on AA-induced colitis, its anti-inflammatory and gastroprotective effects have been previously demonstrated in carrageenan-induced rat paw edema and ethanol-induced gastric lesions, respectively.31
Our analysis, on the other hand, showed that HBE contains considerable amounts of the triterpenic acids OA and UA. We found that OA and UA account for as much as 0.13% and 0.5% of the HBE total content. These findings are in agreement with a previous report that the amounts of OA and UA in hawthorn fruit powder were determined to be 0.24% and 0.38%.32 The anti-inflammatory effect of OA and UA on epidermal keratinocytes via the peroxisome proliferator activated receptors-α pathway and on activated T cells, B cells, and macrophages through suppression of nuclear factor kappa B, AP-1, and nuclear factor of activated T cells has been reported.33,34 Moreover, the antitumor activity of OA and UA in the human colon carcinoma cell line, HCT15, has been demonstrated.35 Other biological activities of OA and UA, including hepatoprotective, antiulcer, antimicrobial, and antihyperlipidemic, have also been investigated.36 Therefore, the anti-inflammatory effects of HBE, which were shown in this study may be due to the OA and UA content of the extract.
The AA-induced inflammation in colitis may initiate with a destruction of the colon epithelium, leading to an increase in the influx of bacterial-produced proinflammatory products into the laminal properia and stimulating the activated enterocytes to produce a variety of cytokines. Accordingly, the chemotactic gradient results in a remarkable migration of neutrophils.37,38 The elevated colon MPO activity, which is reported in the present study is consisting with pathogenesis. Moreover, it is worthwhile to conclude that the HBE exerts its anti-inflammatory and gastroprotective effects at least partly by decreasing the MPO activity and preventing further epithelial injuries. Consistent with this finding, the histopathological findings confirmed that in the HBE-treated animals, no pathologic sign of inflammation, edema, and epithelial damages were recordable. The exact mechanism of anti-inflammatory and gastroprotective effects of HBE is not clear. However, the results suggest HBE protective effects that include the antilipid peroxidation effects of HBE. Our findings showed that HBE significantly prevented the AA elevation of TBARS in the colon, suggesting an antilipid peroxidation property of HBE. To this end, we also showed that HBE was more effective than M in the recovery of the TTM content in the colon. Lipid peroxidation, a known biomarker of oxidative stress, and TTM, a good indicator of the antioxidant capacity of cells, are used in the evaluation of oxidant/antioxidant effects. Our results indicate that the HBE exerted a profound antioxidant effect in the AA-induced colitis. Therefore, the diminished MPO activity and pathological manifestations in the HBE-treated group may be attributed to its antioxidant properties. Further, reports indicate that HBE has an appreciable phenol content and a free radical scavenging capability that supports its antioxidant potency in in vivo systems. Previous analytical studies have demonstrated that HBE contains flavonoids (such as quercetin and isoquercetin), oligomeric proanthocyanidins, phenolic acids, triterpene acids, organic acids, and sterols.39,40 Flavonoids and polyphenols are considered to be the active antioxidant groups in hawthorn extracts.41 Additionally, the anti-inflammatory role of quercetin and isoquercetin in HBE in inhibiting inducible nitric oxide synthease and cyclooxygenase II in stimulated macrophages has been reported.42,43
Statins are other anti-inflammatory medicines that may be indicated in the treatment of colitis. The anti-inflammatory and antioxidative effects of atorvastatin were evaluated and compared with HBE for decreasing the colon MPO activity, TTM levels, TBARS content, and AA-induced pathological damage. Our results indeed confirmed that atorvastatin could prevent neutrophil accumulation and TBARS formation, suggesting an anti-inflammatory property of atorvastatin. Atorvastatin, unlike HBE, failed to significantly reverse the AA-induced decrease in TTM levels in the distal colon, indicating a less antioxidative capacity in comparison with HBE. Jahovic et al. also showed anti-inflammatory effects of simvastatin and fluvastatin on experimentally induced colitis in rats, which was characterized by the prevention of lipid peroxidation, superoxide generation, cytokine production, and neutrophil accumulation.44 Moreover, the anti-inflammatory effect of atorvastatin in human patients with Crohn's disease has also been reported.45
Any synergistic or additive effects between HBE and atorvastatin or mesalamine on the attenuation of AA-induced inflammatory reactions and antioxidant status were further investigated. Indeed, we failed to show any synergistic relationship between chemical and HBE. Since all three compounds are administered orally, there is the possibility of chemical antagonism between the agents and/or chelation of atorvastatin and/or mesalamine by HBE in the gastrointestinal tract, which would ultimately lower their bioavailability. Another explanation may be a possible inhibition/stimulation in the biotransformation enzymes of atorvastatin and/or mesalamine by the HBE content. It has been reported that mesalamine and/or statins are mainly metabolized in the intestine and liver.46 At the same time, it is well known that flavonoids interact with cytochrome P450 enzymes, which are involved in the biotransformation of xenobiotics, including drugs. This interaction may raise concerns about the resulting overdose or loss of therapeutic effect due to the inhibition/stimulation of responsible enzymes.47
Thus, our data showed that HBE exerts protective effects against AA-induced colitis similar to the currently used mesalamine. The anti-inflammatory effect of HBE may attribute to its OA and UA content and antioxidative properties. Additionally, the gastroprotective effects of HBE were found to be comparable to that of mesalamine and atorvastatin. However, no synergism between HBE and tested compounds was found.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Du-Zheng G. A clinical analysis of treating ulcerative colitis on 183 cases. Shandong Med J. 2006;46:35. [Google Scholar]
- 3.Upur H. Yunus K. Mijiti Y. Jing HJ. Jie H. Jing LJ. The histologic effects of the Uyghur medicine xipayi kuijiean on ulcerative colitis in rats. JATMS. 2011;17:219–224. [Google Scholar]
- 4.Huang XL. Xu J. Zhang XH. Qiu BY. Peng L. Zhang L. Gan HT. PI3K/Akt signaling pathway is involved in the pathogenesis of ulcerative colitis. Inflamm Res. 2011;60:727–734. doi: 10.1007/s00011-011-0325-6. [DOI] [PubMed] [Google Scholar]
- 5.Hokari R. Kurihara C. Nagata N. Aritake K. Okada Y. Watanabe C. Komoto S. Nakamura M. Kawaguchi A. Nagao S. Urade Y. Miura S. Increased expression of lipocalin-type-prostaglandin D synthase in ulcerative colitis and exacerbating role in murine colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:401–408. doi: 10.1152/ajpgi.00351.2010. [DOI] [PubMed] [Google Scholar]
- 6.Meier J. Sturm A. Current treatment of ulcerative colitis. World J Gastroenterol. 2011;17:3204–3212. doi: 10.3748/wjg.v17.i27.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summers RW. Elliott DE. Urban JF. Thompson RA. Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 8.MacLean CH. Mojica WA. Newberry SJ. Pencharz J. Garland RH. Tu W. Hilton LG. Gralnek IM. Rhodes S. Khanna P. Morton SC. Systematic review of the effects of n-3 fatty acids in inflammatory bowel disease. Am J Clin Nutr. 2005;82:611–619. doi: 10.1093/ajcn.82.3.611. [DOI] [PubMed] [Google Scholar]
- 9.Kwak B. Mulhaupt F. Myit S. Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 10.Campese VM. Park J. HMG-CoA reductase inhibitors and the kidney. Kidney Int. 2007;71:1215–1222. doi: 10.1038/sj.ki.5002174. [DOI] [PubMed] [Google Scholar]
- 11.Heart Protection Study Collaborative Group: MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20.536 high risk individuals: a randomized placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 12.Liappis AP. Kan VL. Rochester CG. Simon GL. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352–1357. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- 13.Wang H. Zhang Z. Guo Y. Sun P. Lv X. Zuo Y. Hawthorn fruit increases the antioxidant capacity and reduces lipid peroxidation in senescence-accelerated mice. Eur Food Res Technol. 2001;232:743–751. [Google Scholar]
- 14.Zhang Z. Chang Q. Zhub M. Huang Y. Hoa WKK. Yu Z. Characterization of antioxidants present in hawthorn fruits. J Nutr Biochem. 2001;12:144–152. doi: 10.1016/s0955-2863(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y. Vermeer MA. Trautwein EA. Triterpenic acids present in hawthorn lower plasma cholesterol by inhibiting intestinal ACAT activity in hamsters. Evid Based Complement Alternat Med. 2010 doi: 10.1093/ecam/nep007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popov SV. Ovodova RG. Markov PA. Nikitina IR. Ovodov YS. Protective effect of Comaruman, a pectin of cinquefoil Comarum palustre L., on acetic acid-induced colitis in mice sergey. Dig Dis Sci. 2006;51:1532–1537. doi: 10.1007/s10620-005-9034-8. [DOI] [PubMed] [Google Scholar]
- 17.Green LC. Wagner AD. Glogowski J. Skipper PL. Wishnok JS. Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 18.Niehaus WG. Samuelsson JRB. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1986;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 19.Lowry OH. Rosebrough NJ. Farr AL. Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Ranjbar A. Khorami S. Safarabadi M. Shahmoradi A. Malekirad AA. Vakilian K. Mandegary A. Abdollahi M. Antioxidant activity of Iranian Echium amoenum Fisch & C.A. Mey flower decoction in humans: a cross-sectional before/after clinical trial. Evid Based Complement Alternat Med. 2006;3:469–473. doi: 10.1093/ecam/nel031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuzzocrea S. Ianaro A. Wayman NS. Mazzon E. Pisano B. Dugo L. Serraino I. Di Paola R. Chatterjee PK. Di Rosa M. Caputi AP. Thiemermann C. The cyclopentenone prostaglandine 15-deoxy-delta (12,14)-PGJ2 attenuates development of colon injury caused by dinitrobenzene sulphonic acid in the rat. Br J Pharmacol. 2003;138:678–688. doi: 10.1038/sj.bjp.0705077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuralay F. Yildiz C. Ozutemiz O. Islekel H. Caliskan S. Bingol B. Ozkal S. Effects of trimetazidine on acetic acid-induced colitis in female Swiss rats. J Toxicol Environ Health. 2003;66:169–179. doi: 10.1080/15287390306402. [DOI] [PubMed] [Google Scholar]
- 23.Noronha-Blob L. Lowe VC. Muhlahauser RO. Burch RM. NPC 15669, an inhibitor of neutrophil recruitment, is efficacious in acetic acid-induced colitis in rats. Gastroenterology. 1993;104:1021–1029. doi: 10.1016/0016-5085(93)90269-i. [DOI] [PubMed] [Google Scholar]
- 24.La JH. Kim TW. Sung TS. Kang WN. Kim HJ. Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9:2791–2795. doi: 10.3748/wjg.v9.i12.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguilar-Nascimento JE. França-da-Silva LR. De-Oliveira AF. Gomes-da-Silva MH. Enhanced mucosal re-epithelialization induced by short chain fatty acids in experimental colitis. Braz J Med Biol Res. 1999;32:961–966. doi: 10.1590/s0100-879x1999000800005. [DOI] [PubMed] [Google Scholar]
- 26.Korzenik JR. Past and current theories of etiology of IBD toothpaste, worms, and refrigerators. J Clin Gastroenterol. 2005;39:59–65. doi: 10.1097/01.mcg.0000155553.28348.fc. [DOI] [PubMed] [Google Scholar]
- 27.Grisham MB. Oxidant and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- 28.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 29.Parner A. Rask-Madsen J. The potential role of nitric oxide in chronic inflammatory bowel disease. Aliment Pharmacol Ther. 1999;2:135–144. doi: 10.1046/j.1365-2036.1999.00453.x. [DOI] [PubMed] [Google Scholar]
- 30.Ancha HR. Kurella RR. Mc Kimmey CC. Lightfoot S. Harty RF. Luminal antioxidants enhance the effects of mesalamine in the treatment of chemically induced colitis in rats. Exp Biol Med. 2008;233:1301–1308. doi: 10.3181/0805-RM-140. [DOI] [PubMed] [Google Scholar]
- 31.Tadic VM. Dobric S. Markovic GM. Dordevic SM. Arsic IA. Menkovic NR. Stevic T. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J Agric Food Chem. 2008;56:7700–7709. doi: 10.1021/jf801668c. [DOI] [PubMed] [Google Scholar]
- 32.Cui T. Li JZ. Kayahara H. Ma L. Wu LX. Nakamura K. Quantification of polyphenols and triterpen acids in Chinese hawthorn fruit by high performance liquid chromatography. J Agric Food Chem. 2006;54:4574–4581. doi: 10.1021/jf060310m. [DOI] [PubMed] [Google Scholar]
- 33.Checher R. Sandur SK. Sharma D. Patwardhan RS. Jayakumar S. Kohli V. Sethi G. Aggarwal BB. Sainis KB. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLos One. 2012;7:e31318. doi: 10.1371/journal.pone.0031318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HK. Nam CW. Kim SH. Lee SH. Phytocomponents of triterpenoids, oleanolic acid and ursolic acid, regulated differently the processing of epidermal keratinocytes via PPAR-alpha pathway. Exp Dermatol. 2006;15:66–73. doi: 10.1111/j.0906-6705.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 35.Li J. Guo WJ. Yang QY. Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. World J Gastroenterol. 2002;8:493–495. doi: 10.3748/wjg.v8.i3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 37.Boismenu R. Chen Y. Insights from mouse models of colitis. J Leukoc Biol. 2000;67:267–278. doi: 10.1002/jlb.67.3.267. [DOI] [PubMed] [Google Scholar]
- 38.Nusrat A. Parkos CA. Liang TW. Carnes DK. Madara JL. Neutrophil migration across model intestinal epithelia: monolayer disruption and subsequent events in epithelial repair. Gastroenterology. 1997;113:1489–1500. doi: 10.1053/gast.1997.v113.pm9352851. [DOI] [PubMed] [Google Scholar]
- 39.Chang Q. Zuo Y. Harrison F. Chow MS. Hawthorns: an overview of chemical, pharmacological and clinical studies. J Clin Pharmacol. 2002;42:605–612. doi: 10.1177/00970002042006003. [DOI] [PubMed] [Google Scholar]
- 40.Rohr GE. Meier B. Sticher O. Quantitative reversed-phase high performance liquid chromatography of procyanidins in Crataegus leaves and flowers. J Chromatogr A. 1999;835:59–65. [Google Scholar]
- 41.Kirakosyan A. Seymour E. Kaufman PB. Warber S. Bolling S. Chang SC. Antioxidant capacity of polyphenolic extract from leaves of Crataegus laevigata and Crataegus monogyna (hawthorn) subjected to drought and cold stress. J Agric Food Chem. 2003;51:3973–3976. doi: 10.1021/jf030096r. [DOI] [PubMed] [Google Scholar]
- 42.Raso GM. Meli R. Di Carlo G. Pacilio M. Di Carlo R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001;68:921–931. doi: 10.1016/s0024-3205(00)00999-1. [DOI] [PubMed] [Google Scholar]
- 43.Rogerio AP. Kanashiro A. Fontanari C. Da Silva EVG. Lucisano-Valim YM. Soares EG. Faccioli LH. Antiinflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res. 2007;56:402–408. doi: 10.1007/s00011-007-7005-6. [DOI] [PubMed] [Google Scholar]
- 44.Jahovic N. Gedik N. Ercan F. Sirvanci S. Yuksel M. Sener G. Alican I. Effects of statins on experimental colitis in normocholesterolemic rats. Scand J Gastroenterol. 2006;41:954–962. doi: 10.1080/00365520600554444. [DOI] [PubMed] [Google Scholar]
- 45.Grip O. Janciauskiene S. Bredberg A. Use of atorvastatin as an anti-inflammatory treatment in Crohn's disease. Br J Pharmacol. 2008;155:1085–1092. doi: 10.1038/bjp.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JE. Kim KB. Bae SK. Moon BS. Liu KH. Shin JG. Contribution of cytochrome P450 3A4 and 3A5 to the metabolism of atorvastatin. Xenobiotica. 2006;38:1240–1251. doi: 10.1080/00498250802334391. [DOI] [PubMed] [Google Scholar]
- 47.Lee Chao PD. Hsiu SL. Hou YC. Flavonoids in herbs: biological fates and potential interactions with xenobiotics. J Food Drug Anal. 2002;10:219–228. [Google Scholar]