Abstract
Lycopus lucidus Turcz has been widely used as a traditional Oriental medicine (TOM) in Korea and China and prescribed for the enhancement of heart function. However, the precise effects have yet to be defined. The purpose of the present study was, therefore, to address whether the ethanol extract of Lycopus lucidus Turcz (ELT) has a positive inotropic effect. ELT-induced changes in atrial mechanical dynamics (pulse pressure, dp/dt, and stroke volume), and cAMP efflux were measured in perfused beating rabbit atria. Three active components, rosmarinic acid, betulinic acid, and oleanolic acid were identified in ELT by high performance liquid chromatography analysis. ELT increased atrial dynamics in a concentration-dependent manner without changes in atrial cAMP levels and cAMP efflux. The ELT-induced positive inotropic effect was blocked by inhibition of the L-type Ca2+ channels and sarcoplasmic reticulum (SR). Inhibitors of β-adrenoceptors had no effect on the ELT-induced positive inotropic effect. The results suggest that ELT exerts a positive inotropic effect via activation of Ca2+ entry through L-type Ca2+ channel and Ca2+ release from the SR in beating rabbit atria.
Key Words: inotropic effect, L-type Ca2+ channel, Lycopus lucidusTurcz, sarcoplasmic reticulum
Introduction
Congestive heart failure is a condition in which the heart's function as a pump is inadequate to meet the body's needs and is commonly caused by myocardial infraction, ischemic heart disease, hypertension, and cardiomyopathy.1 A variety of pharmacological agents are used to potentiate cardiac contractility and augment pumping, thereby improving hemodynamics and increasing exercise tolerance levels. However, these drugs have side effects, including increasing risk for arrhythmias and sudden cardiac death.2 In addition, traditional Oriental medicine (TOM), recognized as one of the numerous complementary and alternative medicine modalities in the West, is very popular in the general population of Eastern world and several special herbal products with low side-effects have great potential for treating heart diseases.3–5
Lycopus lucidus Turcz, a perennial herb known as “Taekran” in Korea and “Zelan” in China, has been widely used for centuries as a TOM. In China, “Zelan” recorded in the most famous original herbal classic “Shennongbencao” has the function of promoting blood circulation and removing blood stasis. Recently, the crude drug has been used for the treatment of menstrual disorder6 and inflammatory disease.7 Moreover, it has been reported that aqueous extract of L. lucidus inhibited vascular inflammatory process induced by high glucose in human umbilical vein endothelial cells8 and decreased mast cell-mediated immediate-type allergic reaction.9 Further, some active components including triterpenoids (ursolic acid [UA], oleanolic acid [OA], and betulinic acid [BA]) and polysaccharides that were found in the leaves of Lycopus lucidus Turcz and have been shown to decrease heart rates in rats and improved the immune system in mice.10,11
In a screening study of cardioactive principles from TOM, we found that the herb L. lucidus has significant cardiotonic activity. Although L. lucidus has been known to be used for the treatment of cardiovascular disease, few studies have investigated its pharmacological activities and mechanisms of actions on cardiac contractility. Thus, the aim of the present study was to define the effect of ethanol extract of L. lucidus (ELT) on cardiac contractility and its mechanism of action.
Materials and Methods
Extraction of L. lucidus
Lycopus lucidus Turcz was purchased from the Herbal Medicine Co-Operative Association (Iksan, Korea) in March 2007. Herbarium voucher specimens of ELT (DH-95) were prepared and deposited in the Herbarium of the Professional Graduate School of Oriental Medicine, Wonkwang University (Iksan, Korea). After cleaning, L. lucidus was air-dried at room temperature. The dried L. lucidus herb (500 g) was extracted with 3000 mL of ethanol (99%) at 24°C for a week. The extract was filtered through Whatman No. 5 filter paper and concentrated using a rotary evaporator and lyophilized. The yield of the ethanol extract (ELT) was 1.49% of the plant powder and dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO was less than 0.1%.
High performance liquid chromatography fingerprinting and nuclear magnetic resonance analysis
ELT (2.5 g) was subjected to octadecyl functionalized silica gel flash column (75 μm particle size, 5 cm×40 cm) chromatography. The column was eluted with a stepwise gradient of MeOH in H2O (from 20% to 100% with 20% increment, five fractions, 500 mL each), followed by 500 mL of 50% MeOH in CH2Cl2, affording Fr. 1 to Fr. 6. The chromatographic fingerprint of ELT was performed on a YOUNGLIN system (YOUNGLIN Instrument, Busan, Korea) equipped with YOUNGLIN UV detector (UV 730D) and Zam3000 Evaporative Light Scattering Detector (Schambeck SFD GmbH, Bad Honnef, Germany). Chromatographic separation was carried out on an Eclipse XDB-C18 column (4.6 mm×150 mm, 5 μm) at room temperature with an injection volume of 50 μL using a gradient elution of 10% methanol in water (0.1% formic acid) to 100% methanol over 60 min, followed by 100% methanol for 20 min. Peaks were simultaneously detected at 210 and 254 nm using UV detection and Evaporative Light Scattering Detection. Nuclear magnetic resonance (NMR) spectra (1D- and 2D-) were recorded using a JEOL JNM ECP-400 spectrometer (400 MHz for 1H and 100 MHz for 13C), and chemical shifts were referenced relative to the corresponding residual solvents signals. Heteronuclear singular quantum correlation and heteronuclear multiple-bond correlation experiments were optimized for 1JCH=140 Hz and nJCH=8 Hz, respectively.
Preparation of beating perfused rabbit atria
All animal procedures were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996) and were approved by the Institutional Animal Care and Utilization Committee for Medical Science of Wonkwang University. Male New Zealand White rabbits (average 2 kg) were obtained from Korean Experimental Animals Company (Daejeon, Korea). An isolated perfused atrial preparation was set up as described previously,12,13 allowing atrial pacing (at 1.3 Hz) and measurements of changes in atrial volume during contraction (stroke volume), pulse pressure, and cAMP efflux. Briefly, the left atrium was rapidly dissected from the heart after anesthesia (Ketamine, 50 mg/kg, i.v.). The atrium was cannulated with a calibrated transparent atrial cannula containing two small catheters. The cannulated atrium was transferred to an organ chamber and perfused with HEPES-buffered solution by means of a peristaltic pump (Gilson, Villiers-le-Bel, France, 1 mL/min) at 34°C. The HEPES buffer contained the following (in mM): 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 10.0 glucose, 10.0 HEPES (adjusted to pH 7.4 with NaOH), and bovine serum albumin (0.1%). As soon as the perfused atrium was set up, transmural electrical field stimulation with a luminal electrode was started at 1.3 Hz (duration, 0.3 ms; voltage, 30–40 V). Isolated beating atria were stabilized up to 60 min in our experiments. When the atria are in a stable state, the levels of stroke volume, pulse pressure, and cAMP efflux show steady values. The changes in pulse pressure, dp/dt, and atrial rates were measured via pressure transducer and Power Lab/8sp (model ML118, AD Instrument, Bella Vista NSW, Sydney, Australia). dP/dt was calculated by Power Lab software. Stroke volume was determined by reading the lowest levels of the water column in calibrated atrial cannula during end diastole.12
Experimental protocols
The beating atria were perfused for 60 min to stabilize stroke volume, pulse pressure, and cAMP efflux. About 30–40 min was required to stabilize atrial dynamics and cAMP efflux. The perfusate was collected at 2-min intervals at 4°C for analyses. Experiments were carried out using 12 groups of atria. The control cycles (three 12-min periods) were followed by an introduction of ELT (group 1, 0.1 mg/mL, n=4; group 2, 0.3 mg/mL, n=6; group 3, 1 mg/mL, n=4; group 4, time-matched control, n=8). Total amount of ELT infused for 24 min (0.1 mg/min) was relevant to the dose prescribed for human subjects. To analyze the effects of ELT on the Ca2+ entry from the extracellular space, diltiazem and verapamil, L-type Ca2+ channel inhibitors, were used. Diltiazem or verapamil was followed by ELT or vehicle in the presence of inhibitor or vehicle (group 5, diltiazem [5 μM]+ELT, n=7; group 6, diltiazem+vehicle, n=6; group 7, verapamil [1 μM]+ELT, n=6; group 8, verapamil+vehicle, n=4). To define the influence of sarcoplasmic reticulum (SR) Ca2+ release, combined treatment with ryanodine, a ryanodine receptor (RyR) antagonist, and thapsigargin, a SR Ca2+-ATPase inhibitor, was for pretreatment before ELT or vehicle in the presence of antagonist. To fully inhibit SR Ca2+ cycling, we treated the atria with ryanodine in combination with thapsigargin (group 9, ryanodine [3 μM]+thapsigargin [1 μM]+ELT, n=6; group 10, ryanodine+thapsigargin+vehicle, n=6). To clarify the roles of the β-adrenoceptor, pretreatment with propranolol was followed by ELT or vehicle (group 11, propranolol [1 μM]+ELT, n=4; group 12, propranolol+vehicle, n=3).
Preparation of perfusates for cAMP radioimmunoassay
For preparation of perfusates, 100 μL of each perfusate was mixed with trichloroacetic acid (100 μL) to a final concentration of 6% for 15 min at room temperature and centrifuged at 4°C. The supernatant (100 μL) was transferred to a polypropylene tube, extracted with water-saturated ether (300 μL) thrice, and dried using a speedVac concentrator (Savant, Farmingdale, NY, USA). The dried samples were resuspended with 50 mM sodium acetate buffer (pH 4.85).
Radioimmunoassay of cAMP
In the present study, cAMP production was measured by equilibrated radioimmunoassay.14 Standards or samples were prepared in a final volume of 100 μL of 50 mM sodium acetate buffer (pH 4.85) containing 8 mM theophylline, and then 100 μL of diluted cAMP antiserum and iodinated 2′-O-monosuccinyl-adenosine 3′,5′-cyclic monophosphate tyrosyl methyl ester (125I-ScAMP-TME, 10,000 counts/min per 100 μL) were added and incubated for 24 h at 4°C. For the acetylation reaction, 5 μL of a mixture of acetic anhydride and triethylamine (1:2 dilution) were added to the assay tube before the addition of antiserum and tracer. The bound form was separated from the free form by charcoal suspension. Radioimmunoassay for cAMP was done on the day of experiments, and all samples from one experiment were analyzed in a single assay. Nonspecific binding was <2.0%. The 50% intercept was at 10.46±0.19 fmol/tube (n=10). The amount of cAMP was expressed as pmol/min/g of atrial tissue.
Statistical analysis
Significant differences were compared using repeated measures one-way analysis of variance followed by Bonferroni's multiple-comparison test. Student's t-test was used for unpaired data. Statistical significance was defined as P<.05. The results are presented as means±standard error.
Results
Identification of rosmarinic acid, BA, and OA
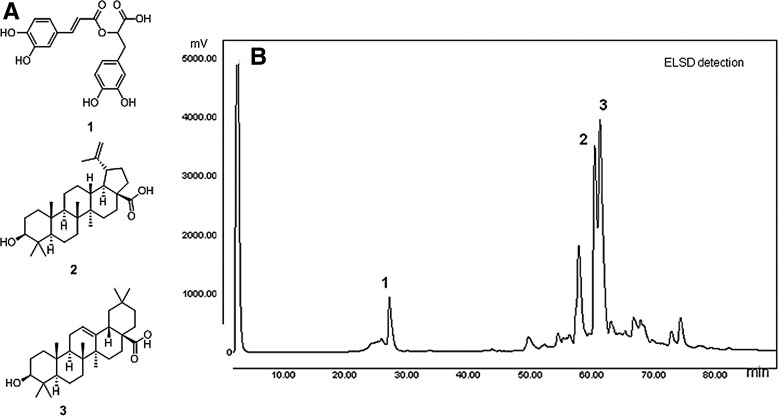
1H NMR analysis of the sub-fractions obtained from the flash column chromatography indicated that the fraction eluted with 60% MeOH in H2O contained pure aromatic compounds, and subsequent detailed analysis of 1D and 2D NMR data revealed the presence of rosmarinic acid (RA) (1). 1H NMR spectra for the fractions eluted with 80% and 100% MeOH in H2O suggested the presence of terpenoids as main components. Eventually, the presence of BA (2) and OA (3) in the extract of L. lucidus was confirmed by co-chromatography with authentic standard compounds on high performance liquid chromatography (HPLC) (Fig. 1).
FIG. 1.
Chemical structures of rosmarinic acid (1), betulinic acid (2), and oleanolic acid (3) (A) and high performance liquid chromatography chromatographic profile of the extract of Lycopus lucidus (B). Peak numbers correspond to structures given in (A) and the identity of the compounds was confirmed by co-elution with authentic samples.
Effects of ELT on atrial dynamics and cAMP levels in beating rabbit atria
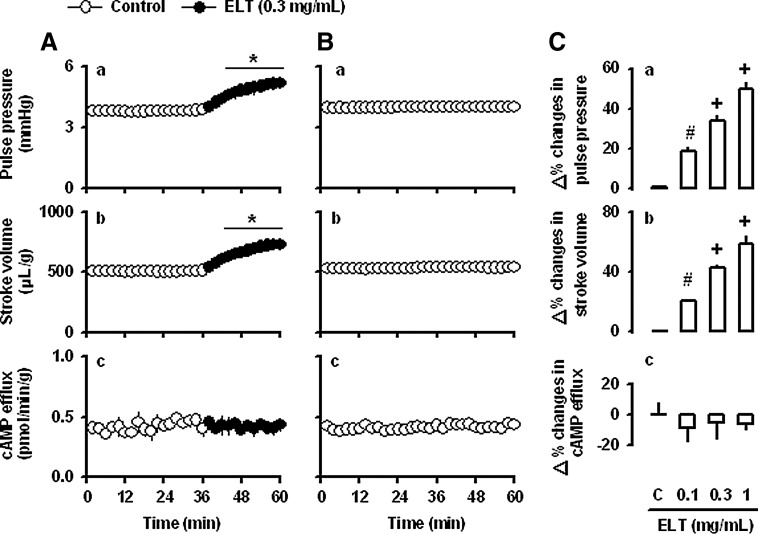
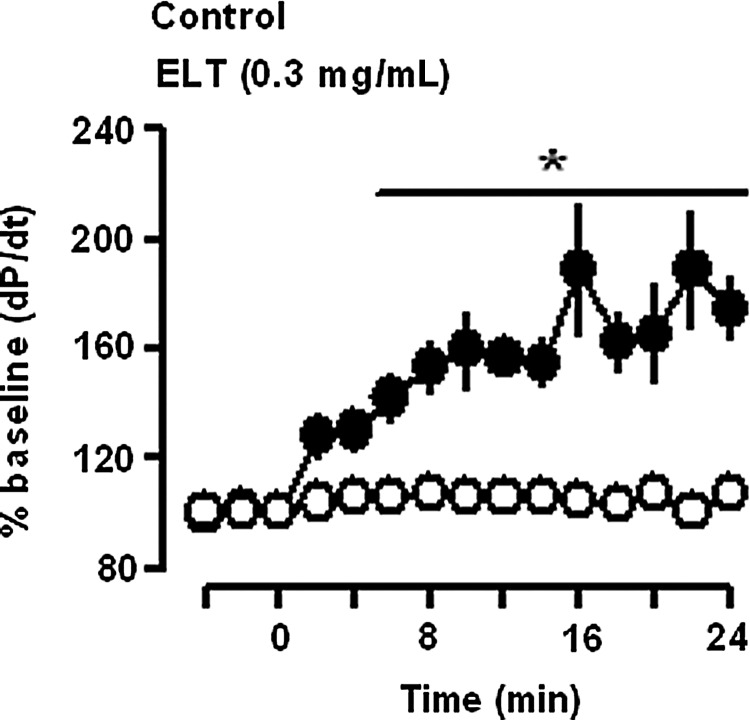
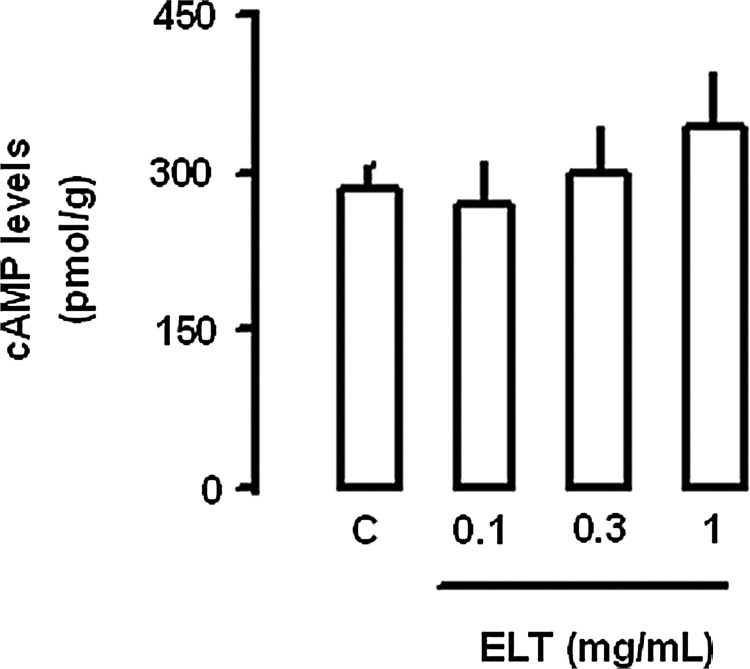
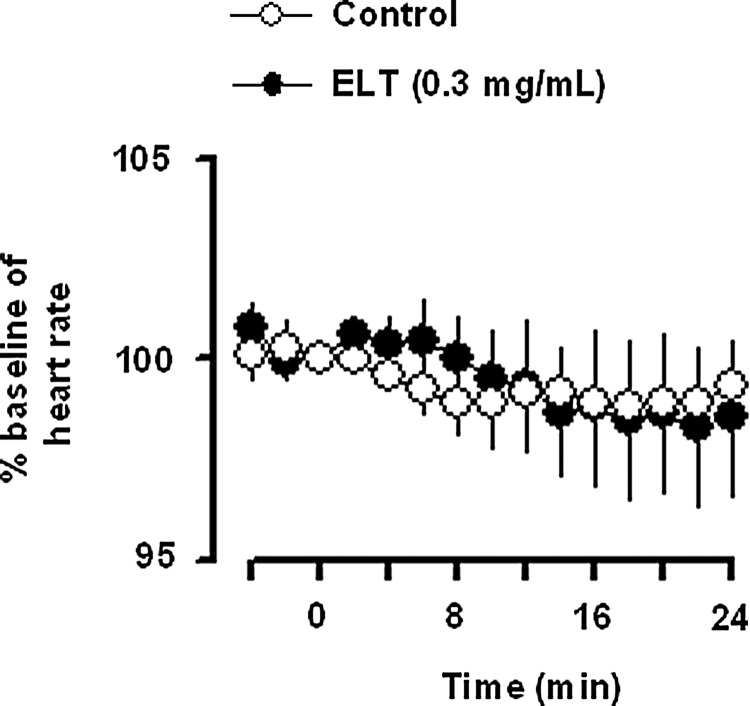
ELT (0.3 mg/mL) increased atrial dynamics, pulse pressure, and stroke volume, without changing cAMP efflux levels in beating rabbit atria (Fig. 2A[a–c]). Atrial dynamics and cAMP efflux were stable during the period observed (Fig. 2B[a–c]). The ELT-induced increase in atrial dynamics was concentration-dependent (Fig. 2C[a,b]). ELT had no effects on the cAMP efflux levels (Fig. 2C[c]). ELT significantly increased the force of contraction, dp/dt (Fig. 3). To determine whether ELT increased intracellular cAMP levels, we measured tissue content of cAMP. ELT had no effects on the tissue contents of cAMP (Fig. 4). In spontaneously beating right atria (baseline beating rates, beats/min; control group 155.0±7.1, n=6; ELT group 154.5±6.7, n=6), ELT showed no significant changes in atrial rate (Fig. 5).
FIG. 2.
Effects of ethanol extract of Lycopus lucidus Turcz (ELT) on atrial dynamics, and cAMP efflux in perfused beating rabbit atria. (A) Effects of ELT on atrial pulse pressure (a), stroke volume (b), and cAMP efflux (c). (B) Time-matched controls for the same parameters (a–c); (C) concentration-dependent responses in atrial pulse pressure (a), stroke volume (b), and cAMP efflux (c). The values (C[a–c]) are expressed as percent difference over the mean value before the addition of ELT or vehicle. X-axis is the time lapsed. Data are the mean±standard error (SE). Number of experiments: control (C), n=8; ELT 0.1 mg/mL, n=4; ELT 0.3 mg/mL, n=6; ELT 1 mg/mL, n=4. *P<.001 vs. control period; #P<.01 vs. control group (C); +P<.01 vs. ELT (0.1 mg/mL).
FIG. 3.
Effect of ELT on the force of contraction (dp/dt) in perfused beating rabbit atria. Data were derived from Figure 2. *P<.01 vs. control group. The empty circle is the corresponding control group and shaded circle is the ELT-treated group.
FIG. 4.
Effect of ELT on the atrial contents of cAMP in perfused beating rabbit atria. Number of experiments: control (C), n=4; ELT 0.1 mg/mL, n=3; ELT 0.3 mg/mL, n=3; ELT 1 mg/mL, n=3.
FIG. 5.
Effect of ELT on heart rates in spontaneously beating rabbit right atria. Number of experiments: control group, n=6; ELT group, n=6.
Effects of β-adrenoceptors inhibition and L-type Ca2+ channel blockade on the ELT-induced increase of atrial dynamics
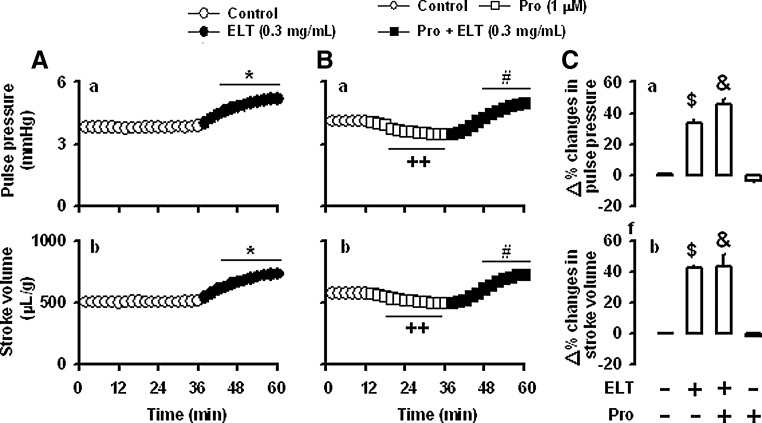
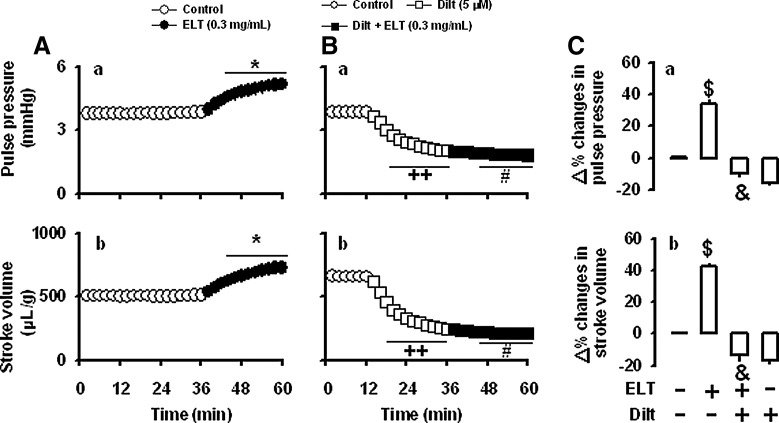
To determine the roles of the β-adrenoceptors, propranolol was used. Propranolol (1 μM) had no effect on the ELT-induced increase in atrial dynamics (Fig. 6B[a, b], C[a, b]). Propranolol slightly, but significantly, decreased atrial dynamics (Fig. 6B[a, b]). The data suggest that the β-adrenoceptors are not involved in the ELT-induced positive inotropic effects. In the next series of experiments, to investigate whether L-type Ca2+ channels are involved in the ELT-induced positive inotropic effect, beating rabbit atria were treated with diltiazem. Diltiazem (5 μM) blocked the ELT-induced increase of atrial stroke volume and pulse pressure (Fig. 7B[a, b], C[a, b]). Treatment with diltiazem markedly decreased stroke volume and pulse pressure (Fig. 7B[a, b]). Verapamil (1 μM) showed very similar effects on the ELT-induced changes in atrial dynamics (data not shown). These data suggest that L-type Ca2+ channels are involved in the ELT-induced positive inotropic effect.
FIG. 6.
Effects of propranolol on the ELT-induced increase in atrial dynamics. (A) Effects of ELT on the atrial pulse pressure (a), and stroke volume (b). Data were from Figure 2A. (B) Effects of ELT in the presence of propranolol on the same parameters (a, b). The values (C[a, b]) are expressed as percent difference over the mean values before the addition of ELT. Number of experiments: ELT (0.3 mg/mL), n=6; propranolol (1 μM)+ELT (0.3 mg/mL), n=4; propranolol control, n=3. *P<.001 vs. control period; #P<.01 vs. the values before the addition of ELT; ++P<.001 vs. control period; $P<.001 vs. control group; &P<.001 vs. control group.
FIG. 7.
Effects of diltiazem on the ELT-induced changes in atrial dynamics in perfused beating rabbit atria. (A) Effects of ELT on atrial pulse pressure (a) and stroke volume (b). Data were from Figure 2A. (B) Effects of diltiazem (Dilt, 5 μM) on the ELT-induced increase of atrial pulse pressure (a) and stroke volume (b); (C) summarized data showing the effects of diltiazem on the ELT-induced increases in atrial pulse pressure (a) and stroke volume (b). The values (C[a, b]) are expressed as percent difference over the mean value before the addition of ELT. Data are the mean±SE. Number of experiments: vehicle+ELT, n=6; diltiazem+ELT, n=7; diltiazem+vehicle, n=6. *P<.001 vs. control period; ++P<.001 vs. control period; #P<.01 vs. values of 32–36 min; $P<.001 vs. control group; &P<.001 vs. ELT.
Effect of inhibiting the SR system on the ELT-induced increase of atrial dynamics
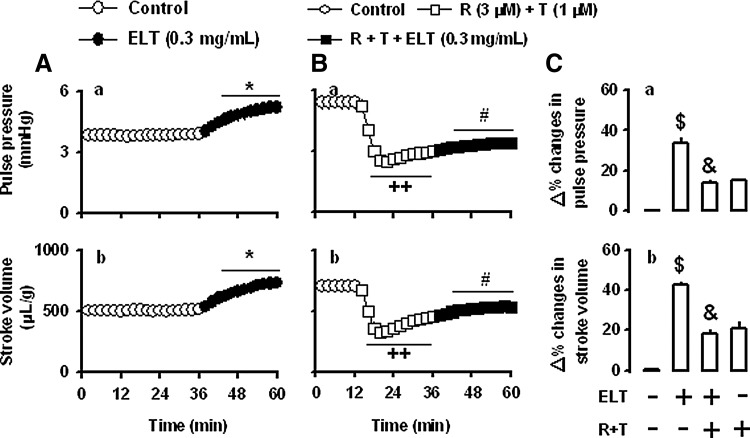
To define the roles of Ca2+ release from the SR in the ELT-induced increase of atrial dynamics, we tested the effects of ryanodine (3 μM) plus thapsigargin (1 μM). Combined treatment with ryanodine and thapsigargin significantly decreased atrial stroke volume and pulse pressure at the first cycle (12 min) of an infusion of agents, and the atrial dynamics gradually recovered toward the control levels up to the fifth cycle (60 min) (data not shown). Ryanodine plus thapsigargin attenuated the ELT-induced increase of atrial dynamics (Fig. 8B[a, b], C[a, b]). These findings indicate that ELT increases atrial dynamics via the SR Ca2+ release pathway.
FIG. 8.
Effects of combined treatment with ryanodine and thapsigargin on the ELT-induced increase in atrial dynamics in beating rabbit atria. (A) Effects of ELT on atrial pulse pressure (a) and stroke volume (b). Data were derived from Figure 2A. (B) Effects of ryanodine (R) plus thapsigargin (T) (R, 3 μM; T, 1 μM) on the ELT-induced increase of atrial pulse pressure (a) and stroke volume (b); (C) summarized data showing the effects of R plus T on the ELT-induced increase in atrial pulse pressure (a) and stroke volume (b). The values (C[a, b]) are expressed as percent difference over the mean values before the addition of ELT or vehicle. Data are the mean±SE. Number of experiments: vehicle+ELT, n=6; R plus T+ELT, n=6; R plus T+vehicle, n=6. *P<.001 vs. control period; ++P<.001 vs. control period; #P<.01 vs. values of 32–36 min; $P<.001 vs. control group; &P<.001 vs. ELT.
Discussion
TOM, including herbal medicine, is popularly considered to be a method of applying medicinal plants and herbs for prevention and treatment of diseases with magical-energetic principles; it encompasses traditional and popular medicines of every country using standardized and titrated herbal extracts.15 However, herbal-based treatments often lack scientific support for their safety and efficacy including information on their active components. Therefore, our present experiments used HPLC fingerprinting and NMR analysis of ELT for further explaining and analyzing its active components. Eventually, it was confirmed that RA, BA, and OA had been separated from ELT by HPLC with authentic standard compounds. Among them, the triterpenoid compounds, BA and OA, had increased atrial dynamics with stimulation of atrial natriuretic peptide secretion in isolated beating rabbit atrium (data not shown). In addition, Somova et al. showed that OA and UA had vasodepressor, antidysrhythmic, and cardiotonic effects in rats,11 and UA, the structural isomer of OA, enhanced cardiac contractility via Na+/K+-ATPase activity.16 These findings suggest that the triterpenoids might be involved in ELT-induced increase in atrial dynamics in beating rabbit atria.
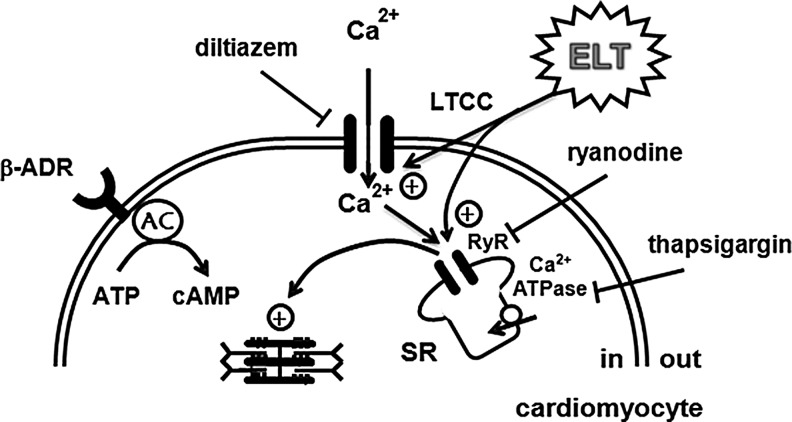
The ubiquitous second messenger Ca2+ plays an essential role in cardiac excitation–contraction coupling (ECC) and Ca2+ influx through sarcolemmal Ca2+ channels is the key event causing myocardial contraction, which triggers Ca2+ release from the SR. In the cardiomyocytes, L-type Ca2+ channels are the primary source of Ca2+ influx to initiate cardiac contractility, and the combination of Ca2+ influx and release from the internal store is responsible for elevation of cytosolic Ca2+ concentration, accelerating the binding of Ca2+ to the myofilament troponin C, which switches on the contractile machinery.17,18 Contraction of cardiomyocytes is mainly controlled by Ca2+ entry via L-type Ca2+ channels and subsequent activation of the RyR, positioned in the SR membrane.19 RyR, as a SR Ca2+ release channel, is an essential component of ECC in cardiac contractility and is modulated by several factors such as Ca2+, Mg2+, and protein kinase A (PKA).20,21 Further, phospholamban is a phosphoprotein modulating Ca2+-ATPase 2a through which Ca2+ is pumped back into the SR system and phosphorylated by several kinases including PKA and Ca2+ calmodulin-dependent protein kinase II.17 In our present study, combined treatment with ryanodine and thapsigargin blocked ELT-induced increases in atrial dynamics, which indicates that SR Ca2+ release is involved in the positive inotropic effect of ELT. To identify the roles of intracellular Ca2+ release on ELT-induced positive inotropic effects, we intended to fully suppress the SR Ca2+ cycling by inhibiting SR Ca2+ pump and simultaneously Ca2+ release from the intracellular Ca2+ store. To this end, a combined treatment with thapsigargin, an inhibitor of SR Ca2+ uptake, and ryanodine, an inhibitor of Ca2+ release, was applied. Combined treatment with ryanodine and thapsigargin fully suppresses SR Ca2+ cycling and elevation of intracellular Ca2+ induced by stimulation in canine sinoatrial node and rabbit ventricular myocytes.22,23 It has been known that the combined treatment with ryanodine and thapsigargin inhibits isoproterenol-induced increase in intracellular Ca2+ levels in the Langendorff-perfused canine sinoatrial node.22 Also, depolarization-induced increase in intracellular Ca2+ was inhibited by combined treatment with ryanodine and thapsigargin in rabbit ventricular myocytes.23 Further, the finding showing that L-type Ca2+ channel inhibitors suppress the ELT-induced positive inotropic effects indicates that Ca2+ entry is also involved in this effect. Therefore, these results suggest that ELT increased atrial dynamics via activation of Ca2+ entry through L-type Ca2+ channels and Ca2+ release from the SR (Fig. 9).
FIG. 9.
Proposed mechanisms for the ELT-induced increase of force of contraction in atrial cardiomyocytes. ELT, ethanol extract of Lycopus lucidus Turcz; β-ADR, β-adrenoceptor; LTCC, L-type Ca2+ channel; SR, sarcoplasmic reticulum; propranolol, a β-adrenoceptor blocker; diltiazem and veraparmil, the selective L-type Ca2+ channel inhibitors; ryanodine, a ryanodine receptor antagonist, thapsigargin, an SR Ca2+-ATPase inhibitor;+, stimulation; ⊺, inhibition.
It is well known that some sympathomimetic agents accentuate cardiac contractility via stimulation of β-adrenoceptors to increase the levels of cAMP participating in the activation of adenylyl cyclase and subsequent increases in the concentration of intracellular Ca2+ through the activation of protein kinase A and phosphorylation of L-type Ca2+ channels and RyR in SR membrane. One of our recent studies showed that Zanthoxylum schinifolium, as a TOM herb, elicited positive inotropic effects via β-adrenoceptor-cAMP-Ca2+ signaling pathway in perfused isolated beating atria.24 However, the present study shows that positive inotropic effects (increase in pulse pressure, dp/dt, and stroke volume) of ELT is not accompanied with the changes in cAMP levels and not influenced by inhibition of β-adrenoceptor activation (propranolol). The findings indicate that the mechanism by which ELT increases mechanical atrial dynamics may not be closely related to the β-adrenoceptor-cAMP signaling pathway (Fig. 9).
In conclusion, the present study shows that ELT increases atrial dynamics without changes in cAMP levels and suggests that ELT accentuates atrial dynamics via activation of Ca2+ entry through L-type Ca2+ channel and Ca2+ release from the SR in beating rabbit atria.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea and funded by the Ministry of Education, Science, and Technology (MEST) (No. 2010-0029467).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Figueroa MS. Peters JI. Congestive heart failure: Diagnosis, pathophysiology, therapy, and implications for respiratory care. Resp Care. 2006;51:403–412. [PubMed] [Google Scholar]
- 2.Saini-Chohan HK. Hatch GM. Biological actions and metabolism of currently used pharmacological agents for the treatment of congestive heart failure. Curr Drug Metab. 2009;10:206–219. doi: 10.2174/138920009787846314. [DOI] [PubMed] [Google Scholar]
- 3.Chang PN. Mao JC. Huang SH. Ning L. Wang ZJ. On T. Duan W. Zhu YZ. Analysis of cardioprotective effects using purified Salvia miltiorrhiza extract on isolated rat heart. J Pharmacol Sci. 2006;101:245–249. doi: 10.1254/jphs.fpj05034x. [DOI] [PubMed] [Google Scholar]
- 4.Guo J. Gan XT. Haist JV. Rajapurohitam V. Zeidan A. Faruq NS. Karmazyn M. Ginseng inhibits cardiomyocyte hypertrophy and heart failure via NHE-1 inhibition and attenuation of calcineurin activation. Circ Heart Fail. 2011;4:79–88. doi: 10.1161/CIRCHEARTFAILURE.110.957969. [DOI] [PubMed] [Google Scholar]
- 5.Zhang WD. Chen H. Zhang C. Liu RH. Li HL. Chen HZ. Astragaloside IV from Astragalus membranaceus shows cardioprotection during myocardial ischemia in vivo and in vitro. Planta Med. 2006;72:4–8. doi: 10.1055/s-2005-873126. [DOI] [PubMed] [Google Scholar]
- 6.Sung CK. Kimura T. But PPH. Guo JX. International Collation of Traditional Folk Medicine. Vol. 3. World Scientific; New Jersey: 1997. p. 137. [Google Scholar]
- 7.Park JH. Medicinal Plants of Korean. Shinil Books; Seoul, Korea: 2004. pp. 1171–1173. [Google Scholar]
- 8.Lee YJ. Kang DG. Kim JS. Lee HS. Lycopus lucidus inhibits high glucose-induced vascular inflammation in human umbilical vein endothelial cells. Vascul Pharmacol. 2008;48:38–46. doi: 10.1016/j.vph.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Shin TY. Kim SH. Suk K. Ha JH. Kim I. Lee MG. Jun CD. Kim SY. Lim JP. Eun JS. Shin HY. Kim HM. Anti-allergic effects of Lycopus lucidus on mast cell-mediated allergy model. Toxicol Appl Pharmacol. 2005;209:255–262. doi: 10.1016/j.taap.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Yang X. Lv Y. Tian L. Zhao Y. Composition and systemic immune activity of the polysaccharides from an herbal tea (Lycopus lucidus Turcz) J Agric Food Chem. 2010;58:6075–6080. doi: 10.1021/jf101061y. [DOI] [PubMed] [Google Scholar]
- 11.Somova LO. Nadar A. Rammanan P. Shode FO. Cardiovascular antihyperlipidemic and antioxidant effects of oleanolic and ursolic acid in experimental hypertension. Phytomedicine. 2003;10:115–121. doi: 10.1078/094471103321659807. [DOI] [PubMed] [Google Scholar]
- 12.Cho KW. Kim SH. Kim CH. Seul KH. Mechanical basis of ANP secretion in beating atria: Atrial stroke volume and ECF translocation. Am J Physiol. 1995;268:1129–1136. doi: 10.1152/ajpregu.1995.268.5.R1129. [DOI] [PubMed] [Google Scholar]
- 13.Choi DH. Kang DG. Cui X. Cho KW. Sohn EJ. Kim JS. Lee HS. The positive inotropic effect of the aqueous extract of Convallaria keiskei in beating rabbit atria. Life Sci. 2006;79:1178–1185. doi: 10.1016/j.lfs.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Cui X. Lee SJ. Kim SZ. Kim SH. Cho KW. Effect of pituitary adenylate cyclase activating polypeptide27 on cyclic AMP efflux and atrial dynamics in perfused beating rabbit atria. Eur J Pharmacol. 2000;402:129–137. doi: 10.1016/s0014-2999(00)00514-8. [DOI] [PubMed] [Google Scholar]
- 15.Firenzuoli F. Gori L. Herbal medicine today: Clinical and research issues. Evid Based Complement Alternat Med. 2007;4:37–40. doi: 10.1093/ecam/nem096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui HZ. Wen JF. Choi HR. Li X. Cho KW. Kang DG. Lee HS. Ursolic acid increase the secretion of atrial natriuretic peptide in isolated perfused beating rabbit atria. Eur J Pharmacol. 2011;653:63–69. doi: 10.1016/j.ejphar.2010.10.098. [DOI] [PubMed] [Google Scholar]
- 17.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 18.Benitah JP. Alvarez JL. Gómez AM. L-type Ca2+ current in ventricular cardiomyocytes. J Mol Cell Cardiol. 2010;48:26–36. doi: 10.1016/j.yjmcc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Pieske B. Maier LS. Schmidt-Schweda S. Sarcoplasmic reticulum Ca2+ load in human heart failure. Basic Res Cardiol. 2002;97:63–71. doi: 10.1007/s003950200032. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Sylvester PL. Copello JA. Voltage-dependent modulation of cardiac ryanodine receptors (RyR2) by protamine. PloS One. 2009;4:e8315. doi: 10.1371/journal.pone.0008315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarma S. Li N. van Oort RJ. Reynolds C. Skapura DG. Wehrens XH. Genetic inhibition of PKA phosphorylation of RyR2 prevents dystrophic cardiomyopathy. Proc Natl Acad Sci USA. 2010;107:13165–13170. doi: 10.1073/pnas.1004509107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joung B. Tang L. Maruyama M. Han S. Chen Z. Stucky M. Jones LR. Fishbein MC. Weiss JN. Chen P-S. Lin S-F. Intracellular calcium dynamics and the acceleration of sinus rhythm by b-adrenergic stimulation. Circulation. 2009;119:788–796. doi: 10.1161/CIRCULATIONAHA.108.817379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldhaber JI. Xie L-H. Duong T. Motter C. Khuu K. Weiss JN. Action potential duration restitution and alternans in rabbit ventricular myocytes: The key role of intracellular calcium cycling. Circ Res. 2005;96:459–466. doi: 10.1161/01.RES.0000156891.66893.83. [DOI] [PubMed] [Google Scholar]
- 24.Cui HZ. Choi HR. Choi DH. Cho KW. Kang DG. Lee HS. Aqueous extract of Zanthoxylum schinifolium elicits contractile and secretory responses via beta1-adrenoceptor activation in beating rabbit atria. J Ethnopharmacol. 2009;126:300–307. doi: 10.1016/j.jep.2009.08.025. [DOI] [PubMed] [Google Scholar]