Abstract
SUMMARY
This review begins with a discussion of the large family of Staphylococcus aureus and beta-hemolytic streptococcal pyrogenic toxin T lymphocyte superantigens from structural and immunobiological perspectives. With this as background, the review then discusses the major known and possible human disease associations with superantigens, including associations with toxic shock syndromes, atopic dermatitis, pneumonia, infective endocarditis, and autoimmune sequelae to streptococcal illnesses. Finally, the review addresses current and possible novel strategies to prevent superantigen production and passive and active immunization strategies.
INTRODUCTION
Staphylococcus aureus is a Gram-positive, catalase-positive, coagulase-positive, facultative aerobe that is a major cause of many kinds of illnesses throughout the world. In 2007, based on data collected in 2005, the Centers for Disease Control and Prevention (CDC) and their collaborators published a report stating that S. aureus is the most significant cause of serious infectious diseases and infectious disease deaths in the United States (1). S. aureus can cause a wide variety of infections, ranging from relatively benign furuncles and soft tissue abscesses to others that are life-threatening, such as infective endocarditis, necrotizing (hemorrhagic) pneumonia, sepsis, and toxic shock syndrome (TSS) (2–12). The ability of S. aureus to be such a capable pathogen, while at the same time appearing as part of the human normal flora, resides largely in the myriad of cell surface and secreted virulence factors that the organism produces (7). Estimates suggest that 30 to 40% of the human population are asymptomatically colonized at any given time on one or more of their mucosal surfaces; up to 70% of people may be transiently colonized (7, 10). Importantly, people who are colonized by S. aureus have a higher risk of infection than noncolonized persons.
Streptococcus pyogenes (group A streptococcus) is also a Gram-positive coccus, but the organism is a catalase-negative, aerotolerant anaerobe. Like S. aureus, group A streptococci are highly associated with serious infections and deaths in humans, primarily also due to their myriad cell surface and secreted virulence factors (4, 13–17). Group A streptococci are considered primary human pathogens in that initial exposure to the organisms usually results in acute illness, typically manifested as pharyngitis or impetigo (18). There are an estimated 10 million cases of pharyngitis in the United States each year. The organisms can also cause life-threatening illnesses such as TSS with or without necrotizing fasciitis and myositis (19, 20), and the organisms are associated with development of autoimmune diseases such as rheumatic fever (21), acute glomerulonephritis (22), and guttate psoriasis (23). Group A streptococci may be asymptomatically carried by up to 10 to 20% of humans, usually after having overt infections (24).
Other beta-hemolytic streptococci, including group B, C, and G strains, also have the ability to cause serious human illnesses, including streptococcal TSS with or without necrotizing fasciitis and myositis (25–37). Additionally, group B streptococci are well known to cause neonatal sepsis and meningitis, and group C and G strains cause pharyngitis.
This review discusses a highly important family of secreted virulence factors produced by both organisms and additionally by certain strains of group B, C, and G streptococci. This family, referred to as pyrogenic toxin superantigens or more simply superantigens, overstimulates many immune processes that allow these organisms to cause serious human illnesses (4, 8, 15, 38). The review will focus on S. aureus and group A streptococci since more data are available for these organisms, but the key principles are also relevant to the pathogenesis of other beta-hemolytic streptococci that produce superantigens. It is important to note that whereas other beta-hemolytic streptococci secrete superantigens, other staphylococci (coagulase negative) of human origin so far do not secrete detectable superantigens (39). Coagulase-negative staphylococci from other animals may produce superantigens (40, 41). It is not our intent to discuss cell surface virulence factors of these organisms, though we recognize that both organisms rely heavily on production of numerous cell surface, as well as secreted, exoproteins in order to colonize the host and cause serious illnesses (7, 8, 38, 42, 43). The cell surface virulence factors include the large families of microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) and immunoglobulin Fc-binding proteins which are important for host colonization and for the interference with local host immune responses (42, 43). The secreted virulence factors, in addition to superantigens, include multiple cytolysins, proteases, nucleases, and lipases that we will mention only in relation to superantigen involvement in disease processes.
The major goal of this review is to present new information on superantigen disease associations and novel ways to interfere with the production and activities of superantigens. Superantigens are critical to development of TSS and likely other cardiovascular and vascular diseases associated with S. aureus and beta-hemolytic streptococci. We and our clinical colleagues have described 25 novel superantigen-associated illnesses, making it is likely that agents that interfere with superantigen production and activity will greatly impact human medicine (8, 44, 45).
THE SUPERANTIGEN FAMILY
Superantigens are an extraordinary family of nonglycosylated low-molecular-weight exoproteins. They are secreted by all human-pathogenic S. aureus and group A streptococci that we have tested (>8,000), with secretion dependent on a cleavable signal peptide. Superantigens have molecular sizes ranging from 19,000 to 30,000 Da (8). The proteins are unusually resistant to heat (for example, most remain biologically active despite boiling for 1 h), they are generally resistant to proteolysis (for example, by trypsin and pepsin) and acids (such as stomach acid), and they are highly resistant to desiccation (toxic shock syndrome toxin 1 [TSST-1] remains biologically active after being dried on petri dishes for more than one year) (8, 15, 38). Their biological toxicity and environmental stability have resulted in some superantigens being categorized as select agents of bioterrorism.
S. aureus strains secrete from 1 to 23 of at least 24 serologically distinct superantigens, and group A streptococcal strains have the ability to produce up to 11 superantigens (8, 15, 38). For example, we have one S. aureus strain in our collection that produces 23 superantigens, lacking only the ability to produce TSST-1 (46). The only S. aureus strains that we are aware of that do not secrete superantigens are NCTC 8325-4 and its variant strains (RN4220, RN6390, and RN450); this makes the restriction-less strain RN4220 a highly useful organism for cloning superantigen genes. The S. aureus superantigens include TSST-1, the staphylococcal enterotoxins (SEs) (serotypes A, Bn, Cn [where n refers to multiple variants], D, E, and G), and the SE-like (SE-l) superantigens (serotypes H, I, and J to X) (4, 8, 15, 38, 47). It is important to note that there is no SE serotype F (SEF) or SE-l protein serotype F (SE-l F) designation. The name SEF was retired from use as a result of the renaming of staphylococcal pyrogenic exotoxin C (PE C) and SEF as TSST-1 in 1984 (48).
The SE superantigens are defined by emetic activity when ingested by humans or when given orally to monkeys (47). TSST-1 was originally thought to have emetic activity when purified by Bergdoll et al. (3), but that activity was later shown to result from SEA contamination. TSST-1 lacks emetic activity and lacks the cystine loop structure thought to be important for emetic activity of SEs (49). It is also important to note that TSST-1 was so named to recognize its principal association with TSS and to allow for the possibility that TSST variants may arise (for example, TSST-2, etc.). To date, there are no human TSST-1 variants, though there is a protein referred to as TSST-ovine that has 7 amino acid differences from TSST-1 (50). TSST-ovine is biologically inactive when tested against human lymphocytes, but the protein is active against lymphocytes from sheep (50). The SE-like proteins either lack emetic activity or have not been tested (47). Several, including SE-l H, SE-l K, SE-l L, and SE-l Q, have been tested and are nonemetic; the remaining SE-l proteins have not been tested (51–54). Almost all of the staphylococcal superantigens are encoded on variable genetic DNA elements, with the exception of SE-l X, which is encoded on the core chromosome (8, 55). SEA is encoded by the sea gene located on a bacteriophage (56), and SED is encoded by the sed gene found on a plasmid (57), but most staphylococcal superantigens are encoded by genes on S. aureus pathogenicity islands (SaPIs) (8).
Like other exoproteins and cell surface virulence factors, staphylococcal superantigens are under complex regulatory control, including by global regulators such as agr, sae, rot, and srr. It is not our intent to discuss these DNA regulatory elements in detail. Later in this review, we discuss selected regulatory elements as they pertain to novel agents that prevent superantigen production. Readers are encouraged to read important reviews that discuss global regulatory pathways in detail (58–60).
Group A streptococci also may produce large numbers of superantigens. These were originally known as scarlet fever toxins or erythrogenic toxins due to their abilities to cause the scarlet fever rash (discussed in detail later in this review) but have more recently been referred to as streptococcal pyrogenic exotoxins (SPEs) (8, 15, 61, 62). Group A streptococci can produce up to 11 serologically distinct superantigens (8, 15, 63–67). The streptococcal superantigens include SPE (serotypes A, C, and G to M), streptococcal superantigen (SSA), and streptococcal mitogenic exotoxin Zn (SMEZn) (8, 15). All of the streptococcal superantigens are encoded by genes located within bacteriophages, excluding SPE G and SMEZ, which are encoded on the core chromosome (8, 15, 18). There is another SPE, designated SPE B, whose gene speB is also encoded in the core chromosome (68). Although shown to have superantigen activity, this protein is clearly a cysteine protease based on activities and structure determination (62, 69, 70). Its superantigenic activity has also been controversial, with observed superantigen activity possibly being the result of contaminants (71). However, it is also possible that the molecule has superantigen activity due to regions of the protein not involved in protease activity. There is precedent for this to occur, as another protein, streptococcal M protein, has both recognized antiphagocytic and superantigen activities (72, 73). It thus seems likely that the protein is both a cysteine protease and an atypical superantigen. SPE B will not be discussed further in this review except as related to poststreptococcal acute glomerulonephritis.
As noted above, pyrogenic toxin superantigens are not limited to S. aureus and group A streptococci. In fact, a number of other organisms produce superantigens. Recent reports of coagulase-negative staphylococci of animal origin (40, 41) and other beta-hemolytic streptococci, namely, groups B, C, and G (4, 8, 15, 25, 31, 33, 74–76), have been published. Superantigens of group B streptococci have not been purified. Some superantigens of group C streptococci appear to be unique, whereas others, and those from coagulase-negative staphylococci and group G streptococci, are identical or nearly identical to those from S. aureus and group A streptococcal strains. Superantigens have also been reported to occur in Mycoplasma arthritidis, Yersinia enterocolitica, Yersinia pseudotuberculosis, Plasmodium falciparum, Clostridium perfringens, Candida albicans, and Toxoplasma gondii (77–82). Interestingly, a superantigen has even been found in the rhizomes of the stinging nettle, Urtica dioica (83).
The global regulation of SPEs has not been extensively studied. As noted above, the majority are encoded on bacteriophages. However, SPEs that have been studied, such as SPE A and SPE C, are produced primarily in the post-exponential phase/early stationary phase of streptococcal growth. This corresponds to the time of maximal production of major staphylococcal superantigens such as TSST-1, SEB, and SEC (84, 85) by S. aureus. In unpublished studies, we have shown that the gene speA, encoding SPE A, is regulated by endogenous S. aureus global toxin regulators, such as agr and srrAB, when speA is cloned into S. aureus. Additionally, the SPE A protein, which shares highly significant primary sequence similarity to SEB and SEC, is highly resistant to S. aureus proteases when the protein is produced in S. aureus. In contrast, neither the SPE B nor the SPE C protein is stably produced when their genes are cloned into S. aureus, with both proteins being quickly proteolyzed to small fragments. These data suggest that group A streptococci may have acquired the SPE A gene from bacteriophage transduction from S. aureus or a common ancestral organism. The SPE B and SPE C genes do not appear to have originated from S. aureus or a recent ancestor.
SUPERANTIGEN BENEFITS TO MICROBES
S. aureus and group A streptococci are highly successful pathogens. This almost certainly is because these two organisms are multidimensional in disease causation, producing a myriad of cell surface and secreted virulence factors, among these being superantigens. It is interesting to ask what the potential microbial survival benefit from superantigen production by S. aureus and group A streptococci is. We hypothesize the following. Both S. aureus and group A streptococci colonize and cause infections in the presence of immune systems; neither organism resides in other environments in nature. Thus, it is most likely that superantigens, and the majority of other virulence factors, are selected because they interfere with normal immune function, increasing the chances of survival and transmission for both organisms.
It can be envisioned that there are three levels of protection afforded to both organisms by their virulence factors. First, superantigens interfere with immune system function systemically (globally), while both organisms initiate disease from initial infection sites. It is well recognized that women who develop menstrual TSS (mTSS) do not produce protective antibodies against TSST-1 or other staphylococcal products during or upon recovery from their illnesses (86). The continued use of tampons by such women places them at high risk of recurrent illnesses (86). It has also been shown that production of multiple superantigens by strains is more toxic than production of a single superantigen (87, 88), presumably through overactivation of multiple T cell populations. It has been shown that the suppression of antibody production by superantigens results at least in part from gamma interferon (IFN-γ) production by overactivated CD4 T cells (4, 89). Additionally, it has been shown that the massive production of tumor necrosis factor (TNF), a proinflammatory cytokine, by immune cells exposed to superantigens counterintuitively suppresses normal phagocytic cell infiltration into infection sites (90). This suppression of phagocytic cell infiltration has been noted in both staphylococcal and streptococcal TSS cases (90–93). The major mechanism of immunity to both pathogens is thought to be antibody neutralization of toxins combined with antibody-complement opsonization and microbial killing by phagocytic cells; the production of superantigens interferes with both activities by interfering with antibody production and phagocytic cell chemotaxis. Second, both organisms also produce cytotoxins that act locally to kill immune cells that have come into infection sites in spite of the presence of superantigens. Third, the organisms produce cell surface virulence factors that provide the last line of defense against the immune system. The combination of these mechanisms effectively allows the organisms to persist in their hosts and increase the likelihood of transmission.
BIOCHEMISTRY AND STRUCTURES OF SUPERANTIGENS
Superantigens were initially known by their first described biological activities (4, 94). Thus, SEs were known as enterotoxins due to their causation of staphylococcal food poisoning (95, 96). SPEs were defined as erythrogenic toxin (SPE A) and scarlet fever toxins due to their abilities to induce the scarlet fever rash (61, 94, 97). Unfortunately, the scientific and medical communities adopted these as the only activities of the SEs and SPEs. Thus, when SPEs were first identified as a family of pyrogenic exotoxins, it was thought they were different from erythrogenic toxin and scarlet fever toxins (94). In 1979, Schlievert et al. showed that the rash of scarlet fever, as caused by erythrogenic toxin, depended on lymphocyte activation (now known as superantigenicity) superimposed on preexistent delayed hypersensitivity (61). Thus, it was unlikely that humans or animal models of human illnesses would show a scarlet fever rash upon initial SPE or other superantigen exposure. This is consistent with scarlet fever being more prevalent in certain geographic areas of the United States, with regions with more cases of group A streptococcal infections having more cases of scarlet fever (98). Additionally, it is consistent with the fact that many women who develop menstrual TSS have had at least one episode of TSS in which the rash, a defining criterion of TSS, is absent, prior to the full-blown episode in which the rash is present. Lastly, this is also consistent with the observations that many individuals with streptococcal TSS or persons with nonmenstrual staphylococcal TSS, with only single exposures to TSS organisms, do not show rashes (99). In contrast, women who have menstrual periods with continuous vaginal colonization by S. aureus are likely to have monthly repeated exposures to TSST-1.
For many years, the superantigens TSST-1, SEs, SE-l proteins, and SPEs were known as pyrogenic toxins, since pyrogenicity was a shared activity of all of the proteins (4). Additionally, all of the proteins shared the unusual activity of enhancement of susceptibility to lethal shock caused by lipopolysaccharide (LPS) by up to 106-fold (100, 101). These became the defining properties of the family. It had been known since the 1970s that pyrogenic toxins were highly potent stimulators of T lymphocyte proliferation, independent of antigen specificity, and that this property was important in human illness (9, 61, 87, 102, 103).
The term superantigen was coined by Marrack and Kappler to emphasize the novel way that pyrogenic toxins stimulate T cell proliferation (104). Superantigens cross bridge T cell receptors (TCRs) with major histocompatibility complex class II (MHC II) molecules on antigen-presenting cells (APCs) in a relatively nonspecific manner, inducing highly significant proliferation of T cells and activation of APCs such as macrophages (8, 105, 106). Typically, antigens stimulate approximately 1 in 10,000 T cells through their specific interaction with MHC II and processed peptides displayed on the surface of APCs. In contrast, superantigens stimulate up to 50% of T cells, inducing in T cells and macrophages what is often referred to as a “cytokine storm” that defines many symptoms of TSS (discussed below). There are four properties that define staphylococcal and streptococcal superantigens and distinguish them from conventional peptide antigens (107): (i) superantigens elicit strong primary responses which are not seen with conventional peptide antigens; (ii) the variable part of the β-chain of the TCR (Vβ-TCR) is sufficient for recognition of a superantigen, which is not the case with peptide antigens; (iii) MHC II proteins are required for superantigen stimulation of T cells, but the interactions are not class II restricted; and (iv) superantigens interact with MHC II as unprocessed molecules. It is possible for strong conventional antigens to appear as weak superantigens, so it is necessary to demonstrate that putative superantigens have these four activities.
Numerous structural and mutational analyses have provided an impressive amount of information regarding the three-dimensional structures and host cell interactions of superantigens. Studies that have examined the crystal structures of superantigens determined that superantigens contain a conserved overall structure made of two major protein domains: an amino-terminal oligosaccharide/oligonucleotide binding (O/B) fold, comprised of a β-barrel, and a carboxy-terminal β-grasp domain made of antiparallel β-strands, with domains connected by a central, diagonal α-helix (108, 109). Based on small variations in this common core structure, superantigens can be categorized into 5 major groups (Table 1; representatives are shown in Fig. 1 and 2).
Table 1.
Structural features of staphylococcal and streptococcal superantigens
| Group | Superantigens | MHC II binding | Cystine loop (length in amino acids) | Other feature |
|---|---|---|---|---|
| I | TSST-1, SE-l X, TSST-ovine | Low-affinity site α-chain | No | Unique amino acid sequence |
| II | SEB, SEC, SEG, SE-l U, SE-l W, SPE A, SSA | Low-affinity site α-chain | Yes (10–19) | |
| III | SEA, SED, SEE, SE-I H, SE-l J, SE-l N to SE-l P | Low-affinity site α-chain, high-affinity site β-chain | Yes (9) | |
| IV | SPE C, SPE G, SPE, J, SMEZ | Low-affinity site α-chain, high-affinity site β-chain | No | |
| V | SE-l I, SE-l K to SE-l M SE-l Q to SE-l T, SE-l V, SPE H | Low-affinity site α-chain, high-affinity site β-chain | No | 15-amino acid loop insertion |
Fig 1.
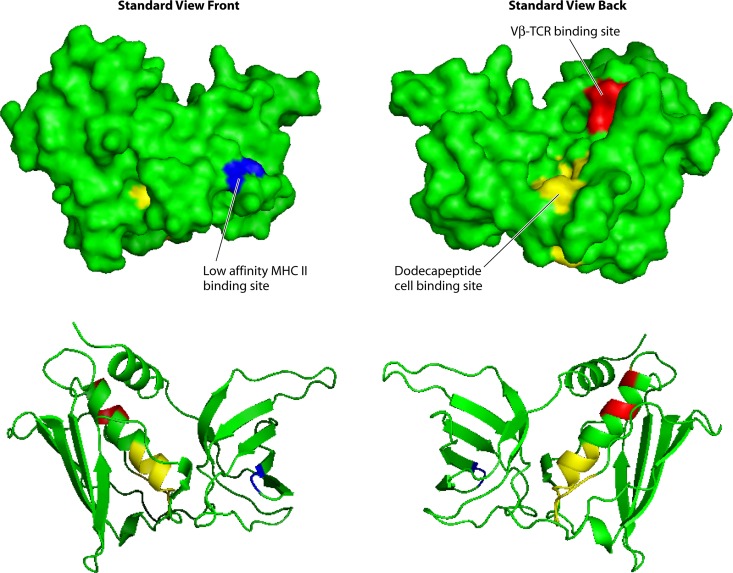
Three-dimensional structure of the group I superantigen TSST-1. Light blue, low-affinity MHC II binding site; red, Vβ-TCR binding site; yellow, epithelial/endothelial/CD40/CD28 binding site.
Fig 2.
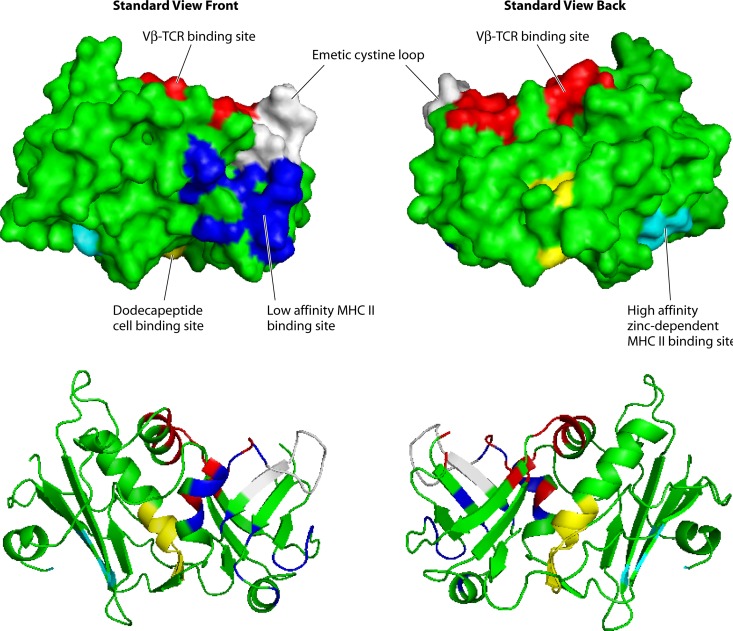
Three-dimensional structure of the group III superantigen SEA. Light blue, low-affinity MHC II binding site; dark blue, high-affinity MHC II binding site; red, Vβ-TCR binding site; white, emetic cystine loop; yellow, epithelial/endothelial/CD40/CD28 binding site.
Group I superantigens are characterized by TSST-1 (Fig. 1), but the group also includes TSST-ovine and the recently described SE-l X (Table 1) (8, 55, 110, 111). Group I superantigens have unique primary amino acid sequences compared to those of the other superantigens. These superantigens have only the core structure, lacking the emetic cystine loop of SEs and the extra loop of 15 amino acids in group V SE-l superantigens (8). Group I superantigens contain only one MHC II binding site, the low-affinity MHC II binding site in their O/B folds that interacts with the α-chains of MHC II molecules (Fig. 1) (112). In its binding to MHC II, TSST-1 also interacts with the antigenic peptide located in the peptide-binding groove of the molecule. The Vβ-TCR binding site of TSST-1 is known; this binding site is located on the top back of the molecule in the standard view, in a groove formed between the O/B fold and β-grasp domains (Fig. 1) (113, 114). At this time, we do not know the location of either the MHC II or Vβ-TCR site of SE-l X.
The group II superantigens are characterized primarily by SEBn, SECn, and SPE A1-4, in addition to others (Table 1) (8, 49). These superantigens contain the core superantigen structure plus a cystine loop that has a varying 10- to 19-amino-acid sequence separating the cysteine residues (49, 115). Importantly, though the cystine loop is required for emetic activity, its presence does not guarantee emesis. Indeed, SPE A contains a cystine loop, like other superantigens in this group; however, it has no emetic activity (49, 115). The lack of emesis of SPE A has been suggested to be due to the presence of a third cysteine in the loop that results in an abnormal cystine loop being formed (115, 116). Additionally, it has been shown in SEC that changing the two cysteines to alanine results in loss of emetic activity, but changing the residues to serine does not (49). Thus, it appears that the conformation of the SE structure, as regulated by cystine loop amino acids, may be more important in determining emesis than the actual presence of the cystine loop itself. Similar to group I superantigens, group II superantigens contain only one MHC II site, the low-affinity, α-chain MHC II binding site, and this interaction does not depend on interaction with the antigenic peptide within the MHC II peptide-binding groove (109, 117, 118). The Vβ-TCR binding site of the group II superantigens is located on the top front of the superantigens (109, 119–121). Even though group I and group II superantigens have only one MHC II site, the low-affinity site, their interaction with Vβ-TCRs is on opposite sides of the superantigens. This makes group I tricomplex interactions appear as three beads on a string, whereas group II tricomplex interactions appear as the superantigen forming a wedge between MHC II and Vβ-TCR. This means that MHC II molecules on APCs cannot simultaneously contact group I superantigens and TCRs, unlike the standard simultaneous interaction of MHC II molecules with antigenic peptides and TCRs.
Group III superantigens include SEA (Fig. 2), SED, and SEE, among others (Table 1) (8). The group III superantigens contain a cystine loop like the group II superantigens, and thus those tested have been shown to have emetic activity; however the loop of the group III superantigens is always nine amino acids long (8). Importantly, the group III superantigens contain the low-affinity α-chain MHC II binding site on their O/B folds, but they also contain a second, high-affinity site, referred to as a Zn2+-dependent MHC II binding site, in their β-grasp domains (Fig. 2) (8, 122). The Zn2+-dependent high-affinity site interacts with the β-chains of MHC II molecules (8, 122). The presence of two MHC II sites allows the superantigens to cross bridge MHC II molecules on adjacent APCs and increase superantigen activity (123). The presence of the Zn2+-dependent high-affinity site on these superantigens makes them 10- to 100-fold more active overall in causing cytokine production from T cells and APCs than those superantigens that have only the low-affinity MHC II site. Group III superantigens bind Vβ-TCRs in the site comparable to that for group II superantigens (Fig. 2, top front in the groove between the O/B fold and β-grasp domains) (123, 124).
The group IV superantigens are produced by group A streptococci but not S. aureus and are characterized by SPE C, SPE G, SPE J, and SMEZ (Table 1) (8). These superantigens contain both low (α-chain)- and high (β-chain)-affinity MHC II binding sites; detailed studies have shown that the SPE C high-affinity MHC II site is similar to the site found in SEA (124–126). It has been suggested that the superantigen SMEZ is the most potent superantigen (127); however, this group IV superantigen, which contains both low-affinity and Zn2+-dependent high-affinity sites, shares expected activity comparable to that of other superantigens that have both MHC II sites (8, 15). Group IV superantigens lack the cystine loop required for emetic activity (8). Their interaction with Vβ-TCRs is similar to that of group II and III superantigens (8).
Group V superantigens are the last described group and contain many of the recently discovered superantigens (Table 1). These include SE-l K to SE-l M, SE-l Q, SE-l V, and SPE H (52–54). Group V contains both the low- and high-affinity MHC II binding sites and lacks the emetic cystine loop. SE-l K, L, and Q have been tested for emetic activity, and all lack activity (52–54). The group V superantigens, however, all have an additional 15-amino-acid insert that is unique to this group. The insert exists between the third α-helix and the β-strand 8 and was hence named the α3-β8 loop. This loop appears to be critical for the specificity of the interaction of the superantigens with their respective Vβ-TCRs (128). Studies have shown that for both SPE I and SE-l K, the loop is required for interaction with T cells (128, 129).
Because group III to V superantigens have a preferred Zn2+-dependent high-affinity β-chain MHC II site and the Vβ-TCR binding sites on the top front, the tricomplex appears as beads on a string like the tricomplex of group I superantigens with MHC II and Vβ-TCR. It is also important to note that even though group III to V staphylococcal superantigens have the Zn2+-dependent high-affinity MHC II site, increasing their activities by 10- to 100-fold compared to that of group I and II superantigens, the group III to V staphylococcal superantigens typically are produced in minute amounts (pg/ml) compared to group I and II superantigens (μg/ml). Thus, group I and II staphylococcal superantigens are associated with serious human illnesses, whereas group III to V staphylococcal superantigens either have no disease association or are causes of staphylococcal food poisoning. SEA, for example, is considered the leading cause of staphylococcal food poisoning worldwide (130, 131).
In addition to the presence of up to two MHC II binding sites, a site for Vβ-TCR binding, and a cystine loop required for emesis, all superantigens also contain an additional host cell binding site, referred to as the dodecapeptide binding region (Fig. 1 and 2) (109, 132, 133). This site is important for interaction with epithelial cells (133), possibly endothelial cells, and the immune costimulatory molecules CD40 (134) and CD28 (135). It has been known since the 1980s that TSST-1 has the ability to bind to both epithelial and endothelial cells (136–139). However, the importance of these interactions has only recently been appreciated. As discussed in a later section of this review, studies indicate that the interaction of superantigens with epithelial and endothelial cells most likely initiates many disease processes (109). The dodecapeptide binding region is fairly conserved among superantigens, with the greatest difference being between TSST-1 and other superantigens (109, 132, 133). This difference in the dodecapeptide binding region has been suggested to explain the greater mucosal epithelium penetration of TSST-1 than of other superantigens (109, 133, 140) and thus TSST-1's unique association with menstrual, vaginal TSS (115). The dodecapeptide binding region is located at the base of the central, diagonal α-helix (Fig. 1 and 2) (109).
ROLE OF SUPERANTIGENS IN HUMAN DISEASES
Introduction
While superantigens are classically defined clinically by their abilities to cause staphylococcal and streptococcal TSS, they have been associated with many other illnesses caused by S. aureus and group A streptococci (Table 2). All serious illnesses caused by S. aureus and group A streptococci have significant vascular-cardiovascular effects on the host, including capillary leak syndromes, in the form of TSS, sepsis, infective endocarditis, pneumonia, and osteomyelitis. Since superantigens are produced by all pathogenic S. aureus and group A streptococcal strains, these toxins should be considered to be contributory to all such serious illnesses. We will discuss selected superantigen-associated illnesses in this review. Readers are encouraged to use Table 2 as a source of references that discuss other possible disease associations.
Table 2.
Human illnesses caused by or associated with superantigens
| Human illnesses | Associated superantigen(s) (reference[s]) |
|---|---|
| Staphylococcal menstrual TSS | TSST-1 (3, 9, 48) |
| Staphylococcal nonmenstrual TSS | Primarily TSST-1, SEB, SEC (145, 200, 327) |
| Soft tissue infection associated | Primarily TSST-1, SEB, SEC (145, 200, 327) |
| Postrespiratory viral | Primarily TSST-1 but also SEB, SEC, and SE-l X (5, 55, 152, 154) |
| Purpura fulminans | Primarily TSST-1, SEB, SEC (154, 155) |
| Extreme pyrexia | Any (156) |
| Recalcitrant erythematous desquamating syndrome in AIDS | Any (153) |
| Anaphylactic | TSST-1 |
| Kawasaki-like | TSST-1, SEB, SEC (328–332) |
| Scleroderma-like | |
| Acute-onset rheumatoid arthritis | |
| Neonatal exanthematous | TSST-1 (333, 334) |
| Streptococcal illnesses | |
| Scarlatina and scarlet fever | SPEs (97) |
| Erysipleas | SPEs |
| Severe invasive disease without hypotension | SPEs (199) |
| TSS with or without necrotizing fasciitis/myositis | SPE A and C (19, 20, 64, 92, 211, 213, 218, 219, 224, 335), SPE B (218, 224, 335, 336), others (67, 199) |
| Purpura fulminans | Any (337) |
| Kawasaki-like | Any (338) |
| Staphylococcal food poisoning | SEs (95, 339) |
| Guttate psoriasis | SPEs (23, 284) |
| Atopic dermatitis | Any (46) |
| Severe nasal polyposis | Any staphylococcal (340) |
| Obsessive compulsive disorder and other nervous system disorders | SPEs (286) |
| Acute rheumatic fever | SPEs (67, 88, 221) |
| Acute glomerulonephritis | SPE B (88, 288–290, 341) |
| Perineal erythema | Any staphylococcal (342) |
| Desquamative inflammatory vaginitis | Any staphylococcal (326) |
| Sudden infant death syndrome | Any (343, 344) |
Staphylococcal Menstrual TSS
The most well-recognized illness caused by superantigens is TSS, which is a potentially life-threatening illness usually caused by S. aureus or group A streptococci. However, group B, C, and G beta-hemolytic streptococci also cause TSS cases. Staphylococcal TSS, as originally described, was most often seen in women during their menstrual periods and is referred to as menstrual TSS (mTSS) (93, 141). mTSS refers only to the association with menstruation and does not refer to the site of S. aureus isolation; approximately 95% of mTSS cases are menstrual with vaginal colonization, whereas the remaining 5% are menstrual with nonvaginal colonization, occurring with almost any other type of infection. Most menstrual, vaginal staphylococcal TSS cases are associated with tampon use (93, 141), with higher incidence associated with higher tampon absorbency (86, 93, 141). The incidence of mTSS peaked in the early 1980s, when the incidence was as high as 10/100,000 women (86). The incidence of this illness is lower today, at approximately 1 to 3/100,000 women (142–145), but importantly, the illness continues to occur; it is alarming that the illness is considerably less recognized today since there has not been media attention for many years. mTSS is defined as occurring within 2 to 3 days of onset of menstruation, during menstruation, and within 2 to 3 days after menstruation. S. aureus reaches its highest numbers on days 2 to 3 of menstruation, which precedes by 1 day the peak onset of TSS (days 3 to 4) (143). On day 2 to 3 of menstruation, S. aureus counts vaginally as measured by numbers on tampons may exceed 1011 per tampon (143). TSST-1 amounts of as high as 100 μg may be present on soiled tampons (146). Counterintuitively, most TSST-1 in tampons is present in regions of tampons that lack menstrual blood (146). Studies have shown that blood prevents production of TSST-1 by S. aureus strains (147). Today, the highest incidence of mTSS is in adolescents of ages 12 to 15, whereas in the early 1980s, the highest incidence was in women of ages 15 to 21 (144). The reason for this change to younger mTSS patients is unknown.
Staphylococcal TSS is defined by the following criteria (93, 141, 148, 149): high fever, hypotension, erythematous (scarlet fever-like) rash, peeling of the skin often during recovery, and any three multiorgan components (often seen as flu-like symptoms, including vomiting and diarrhea), i.e., blood, central nervous system, gastrointestinal system, liver, mucous membranes, muscular system, and renal system. Serology tests for measles, leptospirosis, and Rocky Mountain spotted fever are negative, as are blood and cerebrospinal fluid tests for organisms other than S. aureus. It is now recognized that often one of the defining criteria is not present; this illness is considered probable staphylococcal TSS (99). The defining criterion most often missing is the scarlet fever-like rash that occurs in association with preexistent delayed hypersensitivity to staphylococcal superantigens (61, 150). However, cases of probable TSS in which each defining criterion has been absent have been described. If more than one criterion is absent and other causes ruled out, the illness can be considered toxin-mediated disease (151).
As soon as definitive mTSS was described, it was recognized that the majority of patients with the illness did not meet the full criteria for TSS that had been established to perform epidemiologic studies by the CDC (141). Additionally, it is now recognized that the majority of staphylococcal TSS cases are not menstrual and vaginally associated. Multiple additional nonmenstrual categories of staphylococcal TSS are recognized, including the more commonly seen postrespiratory viral pneumonia-associated TSS (Table 2) (152). This illness may progress exceptionally rapidly, being lethal in a matter of hours, and in children this illness is associated with an extremely high case-fatality rate (up to 90%) (152). Other categories of staphylococcal TSS include (i) illness associated with any type of soft tissue infection; (ii) recalcitrant erythematous desquamating syndrome, an unrelenting TSS in AIDS patients that results in death after as many as 70 days of illness (153); (iii) purpura fulminans, a rapidly progressing TSS illness associated with clotting abnormalities and purpuric rash (154, 155); (iv) extreme pyrexia syndrome, an illness with a 100% case-fatality rate and fevers in excess of 108°F (156); and (v) anaphylactic TSS, an acute illness that has a 100% case-fatality rate and high cardiac eosinophilia. The last illness may be a severe form of chronic TSS episodes in patients who develop atopic dermatitis (AD) rashes instead of the characteristic scarlet fever-like rash. Importantly, each year new categories of staphylococcal TSS are identified, including, most recently, a rediscovered, severe enterocolitis TSS illness (157, 158). It is interesting that staphylococcal superantigens were once considered to be common causes of enterocolitis. With the recognition and association of enterocolitis with Clostridium difficile infection, the staphylococcal superantigen association quickly disappeared; however, it is now increasingly rerecognized that enterocolitis cases can be associated with staphylococcal superantigens in the absence of C. difficile (157, 158).
Staphylococcal superantigens are not evenly distributed among TSS cases. Nearly all mTSS is caused by TSST-1-producing S. aureus strains (3, 9, 145). Rare mTSS cases appear to be associated with production of SE-l G and SEI (159). The reason for the high association of TSST-1 with mTSS is not completely clear, but it likely depends on at least three factors: (i) TSST-1 is produced in high concentrations relative to the majority of other superantigens, (ii) TSST-1 has greater mucosal surface-penetrating ability than other superantigens (115, 140), and (iii) large numbers of mucosal S. aureus strains produce TSST-1 (160). It is important to recognize that when first identified as causing mTSS, S. aureus strains highly associated with illness belonged to the bacteriophage type 29/52 complex (161, 162). These strains with the ability to produce TSST-1 emerged as major clones in 1972 (161, 162), such that today essentially 100% of these organisms produce TSST-1. As many as 25% of persons colonized on mucosal surfaces with S. aureus may be colonized with TSST-1-producing strains (160). We recognize these strains today by other, newer typing mechanisms, including pulsed-field gel electrophoresis (PFGE), as primarily type USA200 and staphylococcal protein A type and/or multilocus sequence type as primarily clonal complex 30. In this review, we use the CDC USA200 designation to refer to these strains. USA200 strains appear to be highly adapted to mucosal surface colonization, with infections resulting from TSST-1 production and/or microbial spread from those surfaces. For example, >95% of USA200 strains have a mutation in the alpha-toxin gene that greatly reduces production of the cytolysin (163–165). Alpha-toxin is highly toxic to humans and is the most inflammatory protein produced by S. aureus strains (163). While alpha-toxin is critical for production of furuncles and soft tissue abscesses originating from skin infections, production of high levels of the cytolysin would be expected to result in highly lethal infections if produced on mucosal surfaces; it is thus likely that the reduction in alpha-toxin production by USA200 strains has allowed them to colonize mucosal surfaces effectively. This mucosal niche location of USA200 strains, with consequent diseases originating from those mucosal surfaces, has been underappreciated, as evidenced by a recent publication suggesting that USA200 strains are less virulent than historic skin-adapted strains referred to as bacteriophage type 80/81 (166).
USA200 strains secrete large amounts of TSST-1 that allows them to cause mTSS (and nonmenstrual TSS) (3, 9). Strains may produce 3 to 20 μg/ml in vitro in broth cultures but up to 16,000 μg/ml in vitro in tampons as biofilms (167). The environmental factors that allow USA200 strains to produce TSST-1 have been established; these are similar for most other superantigens. These conditions include growth in complex media containing animal protein with low glucose (glucose functions as a catabolite repressor of exotoxin production), neutral pH (as expected vaginally during menstruation), temperature of 37°C to 40°C, and oxygen balanced with CO2 (84, 168). As noted previously, blood components, and more specifically hemoglobin peptides, negatively affect production of TSST-1 (147). The introduction of oxygen into the human vagina, a typically anaerobic environment, by tampons is now considered the major reason for the tampon association with mTSS (84, 168, 169). The introduction of oxygen would also explain the major association of mTSS with higher-absorbency tampons in that those tampons introduce more oxygen. Studies of regulation of TSST-1 production by oxygen led to the identification of a global regulator of TSST-1 production called staphylococcal respiratory response (Srr) A/B (170). This important two-component system functions as a repressor of TSST-1 production (and that of other exotoxins) when oxygen levels are low. The repressor function under anaerobic conditions appears to be dominant over all other global regulators of exotoxin production (170). One other factor that increases TSST-1 production has been identified, the surfactant pluronic L-92, which was present in one tampon in the early 1980s (171, 172). It is hypothesized that pluronic L-92 alters staphylococcal two-component system signaling that upregulates TSST-1 production by as much as 8-fold. It has also been suggested that tampons composed of all cotton reduce TSST-1 production compared to that with tampons composed of cotton-rayon blends or all rayon (173). These studies are refuted by multiple, carefully performed studies that fail to find a reduction in TSST-1 production by all-cotton tampons (174–177).
Outside-In Signaling Mechanism Results in Staphylococcal mTSS
S. aureus typically colonizes the vaginal mucosal surface, resulting in TSST-1 production and penetration through the mucosa. TSST-1-producing S. aureus strains accomplish this feat by a mechanism called “outside-in signaling,” where initial interactions with epithelial cells promote TSST-1 penetration and recruitment and stimulation of immune cells (109). As noted previously in this review, TSST-1 exhibits enhanced mucosal surface penetration compared to other superantigens (115, 140). In a rabbit model of vaginal TSS, TSST-1 was the most lethal, compared to SEC and the streptococcal superantigen SPE A (115). Porcine vaginal ex vivo models, which nearly completely mimic the human vaginal mucosa, have shown that TSST-1 alone is able to penetrate the vaginal mucosa (178, 179), but low levels of the cytolysin alpha-toxin greatly enhance penetration.
Human and porcine vaginal mucosae are composed of nonkeratinized, stratified squamous epithelium with a thickness of 10 to 20 cell layers. The cell layers at the top are flattened and relatively senescent, while the deeper layers are more cuboidal and are metabolically active. It appears that TSST-1, alone and as enhanced by cytolysin production, penetrates these mucosae through stimulation of chemokine production by epithelial cells (109, 180, 181). The combined effects of TSST-1, cytolysin-induced inflammation, and cytolysin toxicity likely contribute to destabilization of the stratified squamous epithelial barrier, allowing TSST-1 access to the deeper layers of the mucosal epithelium where the superantigen can directly interact with epithelial cells close to the basement membrane (109). Approximately 104 TSST-1 receptor sites per cell have been demonstrated on primary human epithelial cells and immortalized human vaginal epithelial cells (137, 180). Of these, CD40 and possibly an additional, unknown receptor bind TSST-1 (134, 182). TSST-1 induces the production of proinflammatory chemokines interleukin-8 (IL-8) (CCL8) and MIP-3α (CCL20) in human vaginal epithelial cells in vitro by a mechanism dependent on signaling via ADAM17 and epithelial growth factor receptor (180, 182). These chemokines attract neutrophils and other immune cells, including T cells and macrophages, to infection sites. In line with this, immune cell recruitment to the subepithelial mucosa has been shown to occur in the ex vivo porcine vaginal model (178, 180). Hence, the concerted action of TSST-1 and low levels of cytolysins, such as alpha-toxin, results in mucosal epithelium inflammation and increased permeability, followed by TSST-1 penetration and induction of chemokines by metabolically active epithelial cells and finally recruitment of immune cells to the submucosa. This outside-in signaling mechanism provides TSST-1 accessibility to a sufficient pool of T cells and macrophages to elicit the cytokine storm characteristic of mTSS, IL-1β and TNF-α (produced by macrophages) and TNF-β, IL-2, and IFN-γ (produced by T cells). It is noteworthy that TSST-1 has the ability to induce TSS from other mucosal surfaces such as intestinal and airway surfaces; it is likely that the same outside-in signaling mechanism contributes to TSST-1 production of TSS from those surfaces. Additionally, it is likely that similar outside-in signaling mechanisms explain the production of other microbial infections from mucosal surfaces, such as human immunodeficiency virus (HIV) infections, as we recently proposed (183). In the simian immunodeficiency virus (SIV) model of HIV infection, SIV is proinflammatory to epithelial cells, leading to barrier disruption and recruitment of T cells that become infected.
TSST-1 Production of TSS
Once TSST-1 is produced and T cells and macrophages become activated to secrete a cytokine storm, the cascade of events visibly seen as TSS begins. Fever depends on TSST-1 and/or cytokine stimulation of the hypothalamic fever response control center (101, 184, 185). The most severe symptom associated with TSS is hypotension, which may progress to shock and death, resulting from capillary leakage. TSST-1 induces vascular injury in part by the combined effect of toxin-induced systemic release of vasoactive mediators such as TNF-α and TNF-β (8, 105), synergy with other molecules such as LPS (186–188), and direct toxic interaction with the vascular endothelium (138, 139). The identity of the endothelial cell receptor for TSST-1 is currently unknown and under investigation. However, it is clear that fluid replacement to offset capillary leakage is required for management of TSS cases in humans (93, 141) and rabbit models (189).
An interesting and potentially important property of superantigens is their ability to enhance the lethality of LPS by up to 106-fold. This mechanism depends on synergistic TNF production in the presence of both superantigens and LPS (188) and on the impaired LPS clearance function of the liver in the presence of superantigens (190). Although the LPS enhancement mechanism is not universally accepted as contributing to TSS, it is intriguing for many reasons. Humans and rabbits are approximately equally susceptible to TSST-1 and TSS, and both have high numbers of LPS-containing Gram-negative intestinal and vaginal flora (191). In contrast, mice are approximately 1011 times more resistant to TSST-1 lethality on a per-gram basis than rabbits and humans (191), and mice are less colonized by LPS-containing Gram-negative bacteria. Rabbits become approximately 1,000 times more susceptible to TSST-1 at 8 months of age than young rabbits, and this corresponds to the same time as rabbits become 1,000 times more susceptible to the lethal effects of LPS. Typically in cases of mTSS, S. aureus is cultured vaginally together with Escherichia coli (143, 146, 192), and these E. coli organisms may provide LPS that penetrates into the circulation. Once together in the circulation, the combination of TSST-1 and LPS may synergize to cause enhanced TNF production and consequent enhanced capillary leakage associated with TSS (188). E. coli vaginally may also provide tryptophan or tryptophan precursors needed by the majority of TSS S. aureus organisms, 75% of which are tryptophan auxotrophs because their operons encoding proteins for tryptophan production are disrupted by insertion of the pathogenicity island (SaPI-2) carrying the TSST-1 gene (193–195).
The ability of S. aureus to cause mTSS depends also on the immune status and genetics of colonized women. It has been long appreciated that women with low levels of or no antibodies to TSST-1 are serosusceptible to mTSS, whereas those individuals with antibodies, particularly the IgG4 subclass, appear to be protected (3, 196–198). There is an age-dependent appearance of antibodies to TSST-1 in humans, with 80% of humans having antibodies to the superantigen by 12 years of age (196, 197). The remaining 20% of serosusceptible individuals are among those who develop TSS. Importantly, these 20% appear not to be able to produce protective antibodies to TSST-1 and thus remain susceptible to TSS recurrences (86). The failure to develop antibodies in serosusceptible women likely results from the TSS cytokine storm preventing B cell function (87, 199). The same lack of antibody production has been seen in approximately 50% of rabbits tested in a model of human TSS (134). In contrast, 100% of rabbits respond with production of neutralizing antibodies when challenged with a nontoxic mutant of TSST-1, referred to as TSST-1(G31S/S32P). These studies indicate that the failure to develop antibodies is not due to genetic nonresponsiveness but rather is due to native TSST-1 effects on the immune system. A final important point to mention relative to mTSS is that some women who have TSST-1 present in tampons and who lack antibodies to TSST-1 do not develop mTSS (146). This indicates that these women may lack an epithelial cell receptor for TSST-1, which leads to a failure in the outside-in signaling mechanism. If all known factors are taken into account, such as the percentage of women using tampons, the percentage of women who fail to make antibodies to TSST-1, the percentage of women with TSST-1-producing S. aureus vaginally, and the percentage of women lacking a needed epithelial cell receptor for TSST-1 penetration, the predicted incidence of mTSS should be approximately 5/100,000, which is the approximate incidence seen.
Nonmenstrual Staphylococcal TSS
As noted above, nonmenstrual staphylococcal TSS occurs in association with nearly any type of staphylococcal infection. As with mTSS, not all superantigens are equally associated with nonmenstrual TSS. Studies indicate that 50% of nonmenstrual TSS cases are caused by USA200 and related strains producing TSST-1 (145, 200). The remaining 50% of strains nearly always produce the superantigen SEB or SEC (145, 200). The reason for the association of these three superantigens with most TSS cases, whether mTSS or nonmenstrual TSS, is their high level of production compared to that of other superantigens. SEB and SEC are produced in greater concentrations than even TSST-1 by strains, i.e., 25 to 100 μg/ml in vitro in broth cultures and up to 20,000 μg/ml in vitro in tampon biofilm cultures. However, it is important to remember the greater mucosa-penetrating ability of TSST-1 than of SEB and SEC (115).
Whereas TSST-1 is restricted to USA200 and related strains of S. aureus, SEB and SEC may be produced by both USA200 and USA400 strains. As many as 15 to 30% of mTSS, USA200 strains of S. aureus coproduce TSST-1 and SEC; it is highly unusual, however, to isolate strains that coproduce TSST-1 and SEB, though rare strains have been identified (46). Interestingly, the strains producing both TSST-1 and SEC do not appear to be more lethal in mTSS than strains that only produce TSST-1. This is almost certainly because (i) critical medical intervention prevents lethality and (ii) TSS strains may produce TSST-1 amounts alone or in the presence of SEC in excess of 100,000 TSS-inducing doses per tampon (146, 167). Thus, just production of TSST-1 alone appears to be in vast excess of that needed to cause illness. It has been shown that amounts of superantigens as low as 0.1 μg may induce TSS symptoms in humans (201).
USA400 strains are the major clones of S. aureus that produce either SEB or SEC; rare strains may coproduce SEB and SEC. In the 1990s, community-associated methicillin-resistant strains of S. aureus (CA-MRSA) were first identified in children (6). Many of these strains were USA400 CA-MRSA, as evidenced by the description of children in the Upper Midwest who succumbed to such infections and through characterization of many other infections associated with such strains (5, 154, 202). CA-MRSA USA400 strains, as well as their methicillin-sensitive S. aureus (MSSA) counterparts, are primarily causes of skin and soft tissue infections, but these organisms cause a highly fatal TSS-like illness when present in the lungs and bloodstream. Additionally, these strains are common causes of all forms of nonmenstrual TSS (145, 200), accounting for up to 50% of cases.
Additional comments need to be made regarding TSS strains associated with nonmenstrual illness. Nearly one-half of cases are associated with TSST-1 production. In the United States, the majority, but not all, of these TSST-1-producing organisms are MSSA. However, in other countries, TSST-1-positive MRSA strains appear to be more common (203–205). Given their increased presence on mucosal surfaces today as opposed to 1980 (160), it is possible that TSST-1-positive MRSA strains will continue to increase in numbers in the United States. USA400 strains were the initially identified causes of CA-MRSA necrotizing (hemorrhagic) pneumonia and sepsis. Pneumonia and sepsis do not preclude the patients from also simultaneously having TSS, the symptoms of which are usually present. USA400 strains were more recently displaced in many, but not all, regions of the United States by USA300 CA-MRSA with ability to cause necrotizing pneumonia with TSS-like symptoms (206, 207). These strains most often fail to produce TSST-1, SEB, or SEC (207, 208). However, the strains produce a newly recognized superantigen, SE-l X, which has been linked to necrotizing pneumonia in rabbit model studies (55). Additionally, these strains appear to produce a deletion derivative of TSST-1, as associated with extreme pyrexia syndrome (156). The possible involvement of staphylococcal superantigens in pneumonia will be discussed further in a later section of this review.
Streptococcal TSS
In 1987 (19) and in 1989 in the most definitive clinical study (20), streptococcal TSS was described. This illness is most often associated with group A streptococcal infection associated with breaks in the skin, such as minor cuts (19, 20, 91) or chicken pox lesions in children (209, 210), but may be associated with nearly any type of group A streptococcal infection. It may be important that in a streptococcal TSS outbreak in southeastern Minnesota, as many as 35% of children had pharyngitis caused by M3 streptococci, whereas patients with streptococcal TSS caused by the same organism primarily had infections associated with breaks in the skin (91). It remains unclear why this difference in infectious processes occurs, but it may be related to reduced streptococcal superantigen penetration of mucosal barriers (115).
Streptococcal TSS is defined by the following criteria: isolation of group A streptococci (either from a sterile site, indicating a definitive case, or from a nonsterile site, indicating a probable case), hypotension, and two or more of the conditions adult respiratory distress syndrome, coagulopathy, erythematous macular rash, liver complications, renal dysfunction, and soft tissue necrosis. These criteria are similar to those for staphylococcal TSS (though simplified), except for three major differences: (i) streptococcal TSS with necrotizing fasciitis and myositis is often seen with accompanying bloodstream sepsis in which the causative organisms localize in deep tissue sites of preexistent damage, such as bruises (19, 20, 211–214), whereas staphylococcal TSS is most often associated with localized nonbloodstream infections/colonizations such as of the vaginal mucosa in mTSS (86, 93, 141, 152); (ii) streptococcal TSS with necrotizing fasciitis and myositis is associated with severe pain of the primary site of localized infection (bruises or apparent muscle tears), and this may be masked by use of nonsteroidal anti-inflammatory agents (20, 211–214); and (iii) streptococcal TSS is typically associated with necrotizing fasciitis and myositis, even though cases also occur in the absence of necrotizing fasciitis and myositis, and as such streptococcal TSS may have a case-fatality rate of 50 to 100% (20, 211–214). Until recently, necrotizing fasciitis and myositis were not seen or were uncommon with staphylococcal TSS; it is noteworthy that recent studies now suggest that S. aureus also has acquired the ability to cause TSS that includes necrotizing fasciitis and myositis (215, 216). Soon after the recognition of group A streptococcal TSS, studies recognized that other beta-hemolytic streptococci could cause the same illness, primarily group B, C, and G streptococci.
Recent studies of streptococcal TSS indicate that multiple subsets of illness may develop, with a continuum from mild scarlatina to life-threatening illness. In the early 1900s, scarlet fever was recognized as a potentially life-threatening illness (217). Indeed, many hospitals had isolation wings to sequester patients with the illness. In the 1950s, severe scarlet fever was no longer a serious health threat in the United States, with the illness taking on forms of milder scarlet fever without hypotension and even milder scarlatina. In the mid-1980s, severe scarlet fever returned with the appearance of streptococcal TSS, described initially in patients from the Rocky Mountain West and then becoming recognized worldwide (19, 20). Today, we recognize that streptococcal TSS may occur with or without necrotizing fasciitis and/or myositis, but it is also recognized that severe invasive streptococcal disease (for example, sepsis) may occur without hypotension but with or without necrotizing fasciitis and/or myositis (148). A recent study has shown that the spectrum of these acute streptococcal diseases results in part from the degree of cytokine storm provoked by the causative organisms, with stronger responses leading to TSS, intermediate responses leading to invasive diseases without TSS, and mild responses leading to pharyngitis and mild scarlatinal illnesses (199).
As with staphylococcal TSS, not all superantigens produced by group A streptococci are equally associated with streptococcal TSS. As originally described, streptococcal TSS was associated primarily with M1 and M3 streptococci, and these two M types continue to dominate (19, 20, 91, 211, 213, 214, 218, 219). However, other M types also are clearly associated, including M type 18 strains that are also highly associated with development of rheumatic fever (220–223).
Just as certain M types are highly associated with streptococcal TSS, certain superantigens are more often associated than others. Initially, SPE A was the leading superantigen associated because of its production by causative M1, M3, and M18 strains (19, 20, 91, 92, 210, 214, 218, 219, 224). However, other major SPEs, such as SPE C, and streptococcal mitogenic exotoxin Z are also associated with cases (67, 92, 199, 220, 221, 224–226). Because SPE B (cysteine protease) is in the group A streptococcal core genome, this exoprotein is also associated with streptococcal TSS, and particularly its importance in M1 strains has been thoroughly investigated (226, 227).
Although group A streptococcal TSS is often associated with breaks in the skin, many cases have body site origins that are unknown (148). Some of these are almost certain to originate from mucosal surfaces where group A streptococci often cause diseases, for example, pharyngitis. Outside signaling mechanisms similar to those seen in mTSS may take place to induce streptococcal TSS. In these cases, infection of the oral mucosa initiates a cascade of events that allows penetration not only of the streptococcal superantigens but also of the bacteria, leading to sepsis (109, 181). Proinflammatory cytokine/chemokine induction of human vaginal epithelial cells and mucosal surface penetration studies (used as models for the nonkeratinized, stratified squamous epithelium of the oral mucosa) have also been done on SPE A and the group A streptococcal cytolysin streptolysin O (SLO), with outcomes similar to those obtained with S. aureus TSST-1 and alpha-toxin (109, 181). The major difference is that SLO directly damages the top layers of the mucosal tissue without provoking as strong an inflammatory response as alpha-toxin. The effect is 2-fold: (i) it allows penetration of SPEs and direct interaction with epithelial cells to elicit production of proinflammatory mediators, immune cell recruitment, and induction of TSS (analogous to the case for TSST-1), and (ii) it enhances bacterial penetration and systemic dissemination, which might explain the presence of the organisms in the bloodstream during streptococcal TSS.
Animal Models of TSS
Superantigens clearly cause TSS. This statement is supported by the association of superantigens and causative bacteria with human illnesses. Additionally, superantigens cause TSS symptoms in animal models. For example, studies have shown that administration of staphylococcal and streptococcal superantigens in subcutaneously implanted miniosmotic pumps duplicates the symptoms of TSS in rabbits (228, 229). It is important to remember that mice are highly resistant to superantigens unless the liver is damaged first with d-galactosamine (230), which causes liver necrosis, a feature not seen in cases of human TSS. Additionally, superantigens cause lethal TSS in rabbits as applied intrapulmonarily (231). Studies with the use of isogenic strains that differ only in production of superantigens (232) and the use of active and passive immunization against specific superantigens to protect rabbits (44, 134, 231) conclusively establish that superantigens cause TSS. Recent studies have begun using HLA humanized mice, but the usefulness of these animals remains unclear (233, 234). The studies do, however, demonstrate that HLA class II molecules strongly control superantigenic responses. Finally, superantigens directly injected into humans cause TSS symptoms (201).
Staphylococcal Superantigen Food Poisoning
We discussed the presence of the emetic loop in some superantigens (SEs) in a previous section. Not all superantigens are emetic, but SEs, including most commonly SEA, cause 24- to 48-h episodes of retching, vomiting, and diarrhea every 15 to 30 min, without fever, after human or nonhuman primate ingestion of nanogram quantities of SEs (95, 96). The lack of fever with staphylococcal food poisoning likely results from combinations of nonpyrogenic quantities required to cause emesis and failure of SEs to exhibit high mucosa penetration. Emetic activity has been shown in studies to be independent of superantigenicity (51, 115). Through their emetic activity, S. aureus SEs are primary causes of toxin-mediated food-borne illness and the second leading cause of food-borne illness overall (235). Domestic cats and nonhuman primates have been used extensively in studies of the SE causation of food poisoning; a house musk shrew model is a newly developed animal model used to investigate SE emetic activity (236, 237).
While much of the mechanism of the ability of SEs to induce food poisoning remains unknown, recent studies to examine the effects of SEA on human intestinal epithelial cells demonstrated that SEA induces increases in the intracellular calcium concentration of these cells (238). Using the house musk shrew model, Hu et al. demonstrated that SEA induces the release of serotonin in the intestine to cause emesis (239). Other work suggests that the vagus nerve is involved, where SEs stimulate the vagus nerve, thereby activating the sympathetic nervous system (240). For further information on mechanisms and outbreaks of staphylococcal food poisoning, readers are referred to other reviews (4, 241).
Staphylococcal food poisoning is a self-limiting illness that is rarely if ever fatal. However, this incapacitating activity of SEs may have been primarily responsible for SEs, such as SEB, being listed as select agents of bioterrorism. It is noteworthy that during the 1950s and 1960s, the United States stockpiled tons of SEB yearly as its major bioweapon. Additionally, because it is difficult to denature superantigens and unnecessary to weaponize them for them to be taken orally or intrapulmonarily, SEs pose a significant hazard by these two routes. Likewise, there is no evidence to indicate that humans can be vaccinated against this activity.
Staphylococcal Pneumonia and Superantigens
There are an estimated 70,000 cases of S. aureus pneumonia in the United States each year. Because all pathogenic S. aureus strains produce high levels of superantigens, these toxins contribute to severe pneumonia, as demonstrated in animal models of human disease (44, 134, 231). Even though pneumonia is an illness that describes the primary infection site, the illness does not exclude pneumonia-associated staphylococcal TSS, since TSS is defined as a collection of symptoms rather than a body site of infection.
In 1987, MacDonald et al. (152) described postinfluenza TSS associated with the superantigens TSST-1 and SEB. The illness does not require influenza virus infection, in that cases of pneumonia-associated TSS occur in association with many other upper respiratory viral infections and even asthma. This illness occurs each year, usually during the winter months, throughout the world. Some investigators propose that postinfluenza TSS is the same as Thucydides syndrome, recognized as the plague of Athens in 430 BC (242). In the study by MacDonald et al., there was a 90% case-fatality rate in children, all associated with TSST-1-producing USA200 strains. One strain from the sole surviving child was a USA400 strain producing SEB. One of us (P.M.S.) has tested large numbers of other strains from children with postinfluenza TSS, and the majority of the S. aureus strains belonged to the USA200 clonal group and produced TSST-1.
In 1999, the CDC and colleagues published a report on four fatal cases of S. aureus necrotizing (hemorrhagic) pneumonia in children associated with the recently emergent USA400 clonal group of CA-MRSA (5). Two of these isolates produced SEB and two produced SEC, the expected superantigens produced by USA400 strains. Subsequent studies have shown that these two superantigens are nearly always present in USA400 CA-MRSA strains (202).
More recently, investigators have studied the ability of the USA300 clonal group of CA-MRSA to cause necrotizing pneumonia (206, 243–246). Studies performed in mice have suggested a leading role for alpha-toxin (166, 243–246). This toxin is highly inflammatory, leading to significant lung congestion. However, these prior studies have not evaluated the role of superantigens, since mice are not susceptible to the lethal effects of superantigens (191).
A rabbit model of necrotizing pneumonia conclusively showed that superantigens are critical for the development of lethal pulmonary illness associated with staphylococcal infection (231). Purified SEB and SEC, when installed intrapulmonarily, induce hemorrhagic lung tissue, respiratory distress, and lethal TSS (231). Intrabroncheal administration of CA-MRSA strains of the USA200 (TSST-1-positive) and USA400 (SEB- and SEC-positive) clonal types in rabbits induces lung pathology and lethality similar to those seen with individual administration of superantigens (231). The newly discovered SE-like protein serotype X superantigen (55), encoded by at least USA300, and as well USA100 and USA400 clonal types, appears to be critical for the development of necrotizing pneumonia and lethal TSS in rabbits. Furthermore, vaccination against TSST-1, SEB, or SEC provides complete protection against highly lethal doses of S. aureus strains producing the respective superantigens (231). While cytolysins contribute to lung pathology in rabbits, evidence suggests that they are not the cause of the fatal outcomes associated with staphylococcal pneumonia. Vaccination against alpha-toxin alone does not protect rabbits from lethal pulmonary illness, and CA-MRSA USA200 strains that do not produce alpha-toxin still cause fatal disease (134).
We believe that the outside-in signaling mechanism also applies for TSS developed from lung infections. However, during pneumonia, superantigens encounter only a single cell layer, typically the bronchial epithelium. Primary human bronchial epithelial cells stimulated with TSST-1 express high levels of the proinflammatory molecules TNF-α and IL-8 (247). The simplicity of these tissues, compared to the stratified structure of vaginal mucosal surfaces, would allow for penetration of superantigens that usually do not exhibit great mucosal penetration, which is consistent with the association of S. aureus SEB and SEC with one-half of nonmenstrual TSS cases associated with the organism. Furthermore, direct superantigen cytotoxic effects on the pulmonary endothelial cells could contribute to pulmonary edema, necrosis, and respiratory distress (139).
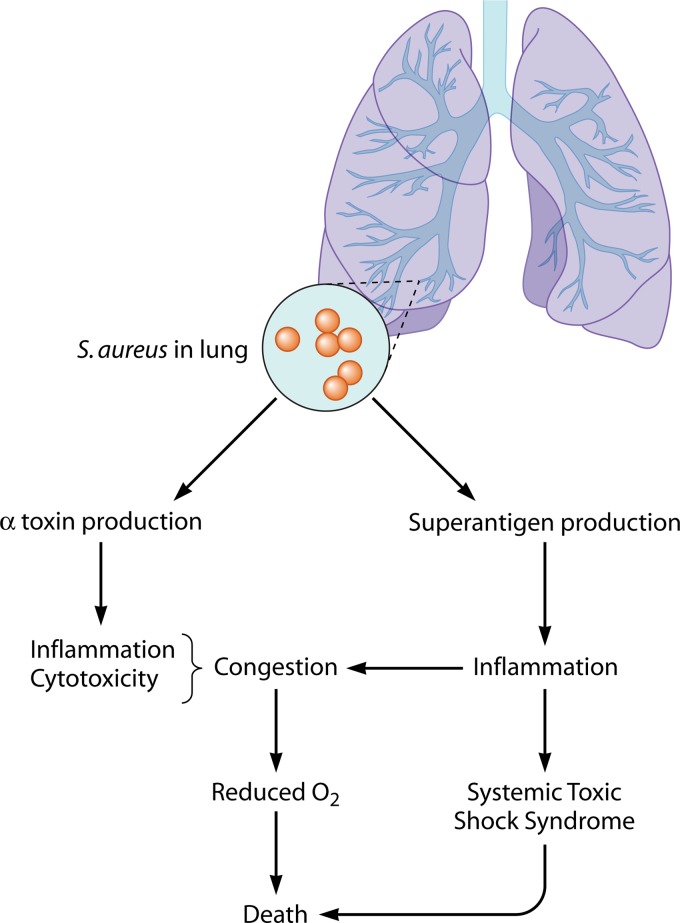
Collectively, the above data indicate there are two pathways to fatal pneumonia, one in which alpha-toxin (and likely other cytolysins) causes high-level intrapulmonary inflammation and one in which superantigens cause pneumonia TSS (Fig. 3). It is likely that both mechanisms contribute.
Fig 3.
Model for S. aureus production of pneumonia. The presence of S. aureus in the lungs leads to inflammation due to alpha-toxin and superantigens and to TSS due to superantigens, both leading to anoxia, hypotension, and possibly death.
Staphylococcal Infective Endocarditis and Superantigens
Infective endocarditis is a serious, life-threatening infection of the heart endothelium characterized by the formation on heart valves of “cauliflower-like” vegetations composed of microorganisms and host factors (248). S. aureus is a major cause of infective endocarditis worldwide (249). Infective endocarditis occurs most often at sites of preexisting heart damage, usually involving valves. Damaged sites become coated with platelets and other host factors, such that in regions of turbulence, S. aureus can adhere and grow. As many as 50% of infective endocarditis patients may succumb, and up to one-half of the patients may develop strokes and metastatic abscesses. Valve failure, strokes, and sepsis associated with metastatic abscesses may lead to death in patients.
Recent studies have shown that as many as 90% of infective endocarditis isolates of S. aureus belong to the USA200 clonal group, all of which produce TSST-1, among other virulence factors (11, 250). Pragman et al. showed that TSST-1 is critical for development of infective endocarditis as tested in rabbits with the use of isogenic strains, one strain positive for TSST-1 and the other strain negative for TSST-1 (251). Only one rabbit administered the TSST-1-negative strain developed a vegetation, which contained only 100 CFU. In contrast, the TSST-1-positive strain caused nearly 7 logs more CFU in cardiac vegetations. In a recent study with the use of passive neutralization of SEC in CA-MRSA USA400 strain MW2, administration of Vβ-TCRs specific for SEC prevented development of cardiac vegetations (252). Finally, active immunization studies targeting superantigens and cytolysins effectively prevented USA200 S. aureus infective endocarditis (134).
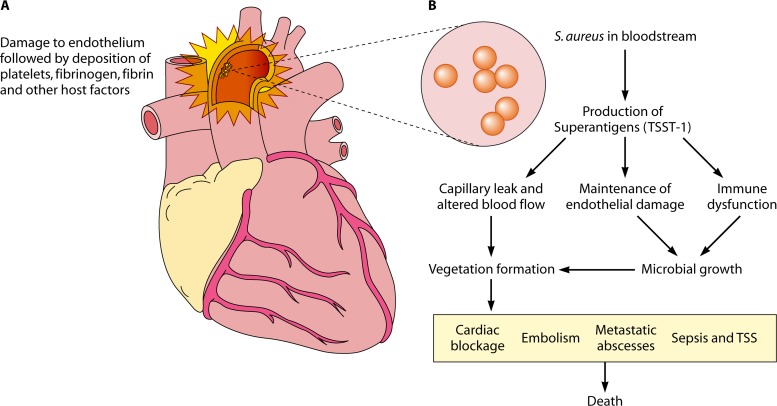
There are multiple possible explanations for superantigen roles in infective endocarditis (Fig. 4). Staphylococci initially colonize damaged endothelium to initiate infective endocarditis (Fig. 4A). Staphylococcal superantigens (Fig. 4B) may then interact with host cells to prevent endothelium wound healing through direct effects on endothelial cells (139). Studies have shown that TSST-1 is toxic to porcine aortic endothelial cells (139). Superantigens may also systemically dysregulate the immune system and prevent clearance of the growing S. aureus valve infections. Finally, superantigens cause mild or major capillary leakage dependent on superantigen concentration. Fluid and electrolyte replacement can be used in humans and rabbit models (189) to prevent the lethal effects of capillary leakage. It is possible that mild hypotension or mild capillary leakage not even manifested as hypotension can alter blood flow in sites of preexisting heart damage to enhance vegetation formation.
Fig 4.
Model of possible roles of S. aureus superantigens in infective endocarditis. S. aureus may colonize damaged endothelium of heart valves (A) and, through combinations of superantigenicity (immune dysfunction), superantigen-induced failure of endothelium healing (maintenance of endothelial damage), and induction of capillary leakage, may facilitate vegetation formation (B).
Staphylococcal Sepsis and Superantigens
Both staphylococcal pneumonia and infective endocarditis patients develop sepsis as a consequence of their infections, and significant numbers of patients develop sepsis following surgery. It is likely that many systemic effects in these patients result from superantigen activities on the host, since the infecting strains are likely to produce the toxins. Investigators have shown that certain superantigens, notably SEA, TSST-1, and SEC, are overrepresented in sepsis cases compared to nonsepsis cases (253–255). However, it is unlikely that superantigens are directly produced in the bloodstream of patients, since hemoglobin peptides inhibit superantigen production (147). It is more likely that superantigens are produced in focal sites of infection protected from hemoglobin peptides and then secreted into the bloodstream.
Superantigens in Atopic Dermatitis
Atopic dermatitis (AD), is a chronic relapsing, highly pruritic, inflammatory skin disease that is often the prelude to development of food allergy, asthma, and allergic rhinitis (256, 257). S. aureus skin infections exacerbate skin disease in patients with AD and modify the host response to environmental allergens and viral pathogens (258, 259). Recent studies suggest that host-pathogen interactions stemming from the production of S. aureus-derived virulence factors, such as superantigens and alpha-toxin, contribute greatly to the skin inflammation seen in AD (260, 261).
Pathobiology Underlying AD
AD patients have various systemic and skin immune abnormalities, including elevated total serum IgE and sensitization to allergens, elevated T helper (Th2)-type cytokine expression in acute lesions, and increased numbers of T cells expressing the skin homing receptor cutaneous lymphocyte-associated antigen (256, 257). These patients also have a defect in terminal differentiation of their keratinocytes leading to reduced expression of barrier proteins such as filaggrin, as well as decreased expression of epidermal antimicrobial peptides needed for innate immune responses against invading bacteria and viruses (262, 263). The reduction in barrier proteins may be due to a combination of mutations in genes encoding skin barrier proteins such as filaggrin (264, 265), as well as downregulation of epithelial differentiation protein levels by Th2-type cytokines and IL-22 (266, 267). A critical link between the barrier defect in AD patients with filaggrin gene (FLG) mutations and Th2 polarization could be explained in part by enhanced allergen penetration through damaged epidermis accompanied by increased production of thymic stromal lymphopoietin (TSLP) by keratinocytes, leading to a Th2-type milieu (268, 269). Loss of filaggrin has been linked to enhanced antigen penetration into the skin and increased S. aureus and viral growth in the skin, as well as susceptibility to the cytotoxic effects of staphylococcal toxins, e.g., alpha-toxin (270–272). AD patients with more polarized Th2-type disease with allergies and asthma and increased biomarkers, including serum IgE and TSLP, have been reported to have more severe skin disease complicated by microbial infections (273). These observations suggest that FLG mutations and Th2 responses enhance S. aureus colonization and create a niche for S. aureus infection in AD skin. Once S. aureus has colonized AD skin, it perpetuates its survival by increasing Th2-mediated responses that suppress antimicrobial responses in atopic skin (274).
Mechanisms by Which Superantigens Drive AD Inflammation
The mechanisms by which S. aureus induces skin inflammation in AD are under active investigation. A number of staphylococcal products, including superantigens, alpha-toxin, peptidoglycan, and lipoteichoic acid have been observed to activate cells involved in the pathogenesis of AD (10). Due to their highly potent immunostimulatory activity, much of the focus has been on the role of superantigens in the pathogenesis of AD (105). Support for a role of superantigens in AD includes the following. (i) The severity of AD correlates with the number of superantigen-secreting S. aureus organisms colonizing the skin (275). Utilizing a murine model of skin inflammation, S. aureus superantigens plus allergens have been shown to have an additive effect in driving cutaneous inflammation (276). (ii) Most AD patients make IgE antibodies directed against superantigens found on their skin (277), and the presence of these IgE antibodies to superantigens correlates with skin disease severity (278). Basophils and skin mast cells from patients with antisuperantigen IgE release histamine upon exposure to the relevant superantigen but not in response to superantigens to which they have no specific IgE. (iii) The superantigen SEB, applied to the skin, can induce eczematoid skin changes (260). The ability of superantigens to induce eczema is likely related to their capacities to bind to MHC II molecules on APCs or to stimulate T cells to produce proinflammatory cytokines (105). (iv) We have demonstrated that superantigens induce T cell expression of the skin homing receptor via stimulation of IL-12 production (279). (v) After stimulation by the superantigen SEB, T regulatory cells lose their immunosuppressive activity, suggesting a novel mechanism by which superantigens could augment T cell activation in patients with AD (280). (vi) Superantigens induce corticosteroid resistance, suggesting that several mechanisms exist by which superantigens increase AD severity (281). Interestingly, superantigens also selectively induce T cells to secrete IL-31, a highly pruritogenic cytokine that regulates filaggrin expression and is produced by Th2 cells (282). Thus, superantigens may contribute to the pathogenesis of AD via multiple immune pathways leading to skin inflammation.
Management of AD
The treatment of AD requires a multipronged approach that includes improvement in skin barrier function, reduction of the skin inflammatory response, and control of S. aureus infection with judicious use of antibiotics (283). Overuse of antibiotics in managing AD can result in MRSA infection with organisms that complicate control of skin disease. Given the key role that superantigens and alpha-toxin play in contributing to skin inflammation in AD, the development of vaccines directed against these staphylococcal toxins would be a welcome addition to the treatment armamentarium for these patients.
Streptococcal Superantigens and Delayed Sequelae
Group A streptococci are well recognized for the ability to cause delayed-sequela autoimmune diseases. We will provide a brief discussion of the possible association of streptococcal superantigens with three autoimmune diseases. In addition to causing TSS and related illnesses, streptococcal superantigens are associated with the autoimmune disease guttate psoriasis (23, 284). This illness follows group A streptococcal illnesses, with susceptible patients developing characteristic lesions at 7 to 15 days postinfection. Patients exhibit T cell receptor skewing that is typical of the superantigen SPE C; T cell skewing is caused by superantigen overactivation of selected Vβ-containing T cells such that these cells are overrepresented (skewed). The mechanism of superantigen involvement in guttate and possibly other forms of psoriasis merits further study.
Throughout history, M18 group A streptococci have caused rheumatic fever. In the United States today, these M18 strains are the most common causes of this autoimmune disease (222, 223). Features of rheumatic fever include evidence of multiple prior group A streptococcal infections, fever, heart abnormalities, arthritis, and Sydenham's chorea (222). There is a clear association of rheumatic fever with immunologic cross-reactivity to streptococcal antigens, with M protein most often cited (21). Studies have shown that all rheumatogenic streptococci produce the superantigen SPE C, including all M18 strains (67, 88, 221). Additionally, it has been shown that SPE C has the ability to penetrate the blood-brain barrier, with possible central nervous system effects resembling Sydenham's chorea (285). Although not definitively established, it has been suggested that SPE C and other M18-associated superantigens may enhance immune cross-reactivity to M proteins and in this way be associated with the autoimmune disease (67, 285) and even other illnesses such as pediatric autoimmune neuropsychiatric disorders associated with streptococci (PANDAS) (286). It has also been demonstrated that superantigens have the ability to induce arthritis in an animal model of rheumatoid arthritis (287).
In 1979, Schlievert et al. (88) noted the association of SPE B (cysteine protease) production by group A streptococcal strains and the development of acute glomerulonephritis. Recent studies have shown that SPE B localizes in kidney deposits associated with the illness (288–290). Our unpublished studies with animals indicate that systemic treatment of rabbits with purified SPE B leads to proteinuria and hematuria, clinical features of acute glomerulonephritis. The role of other superantigens in this autoimmune disease is unknown.
MECHANISMS TO INTERFERE WITH SUPERANTIGEN ACTIONS AND PRODUCTION
There are multiple ways to interfere with superantigen-associated illnesses. These include (i) treatment of infections with antibiotics and supportive care to allow clearance of bacteria and kidney elimination of superantigens, (ii) preventing superantigen production by mucosal microbicides added to tampons and surfaces, (iii) passive immunization against superantigens, and (iv) active vaccination against S. aureus and superantigens.
Treatment of Infections with Antibiotics and Supportive Care To Allow Clearance of Bacteria and Kidney Elimination of Superantigens
The standard of care for treatment of superantigen illnesses begins with initial evaluation for potential sources of infection. For example, it is necessary to perform a vaginal and throat examination, remove tampons or other devices (such as contraceptive diaphragms or nasal packing following surgery), and culture for S. aureus and beta-hemolytic streptococci, most often group A. Wounds should be evaluated for infection, noting that many times these infection sites may be difficult to find, as TSS microbes often do not cause significant inflammation (90–92, 291). For example, we are aware of postsurgical TSS cases where the incision sites have completely healed over, without signs of infection, yet having abscesses of nearly 300 ml. These abscesses, although often thought to be anaerobic, are not; instead they have 60% of the oxygen content of air. Thus, they serve as excellent potential sites for superantigen production. Thorough examination of skin and soft tissues should be performed, paying special attention to painful areas such as bruises. Cultures of blood and other sites should be obtained. Early surgical intervention is extremely important, particularly in cases of streptococcal TSS (20, 45, 91, 92, 211, 213, 219, 292) and cases of staphylococcal necrotizing fasciitis (215, 216). Magnetic resonance imaging (MRI) may be useful to identify deep soft tissue damage and instruct on surgical procedures. Supportive care for hypotension is also of major importance. Patients may require large amounts of intravenous fluids, vasopressors, and management of acute renal failure, acute respiratory distress syndrome, disseminated intravascular coagulation, or myocardial suppression. It is of critical importance to maintain kidney function, since this appears to be the major way for humans to eliminate superantigens.
The standard of care for treatment of all superantigen diseases also includes the use of antibiotics to treat acute infections and reduce the incidence of recurrences. Nearly all S. aureus strains in the United States are resistant to penicillin and ampicillin, but many strains (MSSA) are susceptible to most other β-lactam antibiotics as well as other antibiotics. The antibiotic clindamycin has been shown to reduce production of superantigens by S. aureus and group A streptococci at antibiotic concentrations below those necessary to kill the pathogens (293–296). The percentages of methicillin-resistant S. aureus (MRSA) strains are increasing, and we now recognize both hospital-associated MRSA (HA-MRSA) and CA-MRSA, though the boundary between these groups is blurring. HA-MRSA strains typically exhibit broad antimicrobial resistance based on the presence of a large DNA element, referred to as staphylococcal cassette chromosome mec type II (SCCmec II), which encodes methicillin and other resistances. In contrast, CA-MRSA strains, as originally described, contain smaller SCCmec DNAs, such as SCCmec IV. CA-MRSA strains, although resistant to β-lactam antibiotics, are often susceptible to other antibiotics. Small numbers of vancomycin-resistant S. aureus strains have been identified (297).
It is important in mTSS cases, particularly, to remind patients that they will not develop neutralizing antibodies upon recovery from illness (165). Thus, they remain susceptible to recurrences; we know of women who have had six recurrent, severe mTSS episodes. These recurrences most often occur in women who continue to use tampons, but they also occur in milder forms in women who discontinue tampon use (86). To reduce the incidence of mTSS recurrence, patients should be advised not to use tampons. It should be recognized that approximately 1% of menstruating women have group A streptococci vaginally, and thus these individuals are at risk of streptococcal TSS from that infection site. Such cases are not tampon associated, since group A streptococci grow independent of oxygen. It is interesting to note that some women develop mTSS prior to the appearance of menstrual blood yet within the two-day window of time to be classified as mTSS. Tests of tampons indicate that their vaginal pH has already risen to near neutrality, suggesting that the role of hormone changes may be to bring mucosal pH to the neutrality required for superantigen production. Pregnant women appear to be at risk of streptococcal TSS as well, with a high case-fatality rate for both mother and fetus (292). All persons should be advised to receive yearly influenza vaccination to prevent the highly fatal postinfluenza TSS.
Preventing Superantigen Production by Mucosal Microbicides Added to Tampons and Surfaces
With the occurrence of the mTSS epidemic in the 1980s, there have been many efforts made to develop tampons and other devices used on mucosal surfaces that prevent production of superantigens, particularly TSST-1. For example, Kotex tampons contain a surfactant, cetiol, that reduces TSST-1 production (298). In 1992, Schlievert et al. published that the potential tampon additive glycerol monolaurate (GML) reduces TSST-1 production at concentrations that do not inhibit bacterial growth but kills S. aureus and most other vaginal pathogens at higher concentrations (299). These studies resulted in additional work that demonstrates that GML inhibits TSST-1 production through interference with bacterial signal transduction (300), reduces S. aureus and group B streptococci vaginally while selecting for the normal flora lactobacilli, and reduces vaginal inflammation as measured by reductions in IL-8 production (301); IL-8 is a chemokine that specifically attracts neutrophils to infection sites. In more recent studies, it has been shown that GML stabilizes and is anti-inflammatory to mucosal epithelial cells as well as other immune cells (302, 303). The studies suggest that GML, in addition to preventing TSST-1 production, stabilize the host mucosa to prevent outside-in signaling (109). GML is safe as used vaginally in 5% (50-mg/ml) gels for periods of up to 6 months, as tested in rhesus macaques (304). Studies suggest that GML can also be used to reduce simultaneously bacterial vaginosis pathogens and Candida infections vaginally (305). GML has been used effectively to prevent SIV transmission with a high-dose challenge in a rhesus macaque model (183). Finally, it appears that GML both interferes with bacterial signal transduction to prevent exotoxin production and dissipates the potential across plasma membranes to kill pathogens (167); the reason for the resistance of lactobacilli to GML appears to be that they use GML as a cross-reactive quorum-sensing mechanism that actually stimulates growth (167). Collectively, these and other studies have led to GML being incorporated into o.b. optiBalance tampons in Europe. Other molecules that could be applied to mucosal surfaces and devices to reduce the risk of staphylococcal and streptococcal illnesses have also been identified (306–309), including agents that prevent superantigen production and those that stabilize mucosal surfaces.
Passive Vaccination against Superantigens
It has been known for many years that intravenous immunoglobulin (IVIG) preparations usually have antibodies against all staphylococcal and streptococcal superantigens (310, 311). This has been confirmed by numerous investigators (45, 312, 313). A definitive study performed by Kaul et al. (45) demonstrated a highly significant reduction in the case-fatality rate of streptococcal TSS with use of IVIG. A similar study has not been performed for treating staphylococcal TSS with IVIG, but there are many examples reported to P.M.S. of individual patients responding to such therapy. It is now standard practice in some hospitals to administer vancomycin and IVIG to children with S. aureus pneumonia. A note of caution in regard to the use of IVIG is merited. We are aware of a case of streptococcal TSS where the patient was treated with IVIG and responded so quickly that the decision was made to discontinue additional IVIG treatment. The patient immediately relapsed, and IVIG treatment was reinstated. In calculations of the relative amounts of antibodies in IVIG against major superantigens, knowing that enzyme-linked immunosorbent assay (ELISA) antibody titers of ≥40 in humans appear to be required for protection from superantigens and knowing the blood volume of humans, it appears that IVIG has just sufficient antibodies to superantigens to provide protection. Because of the high cost of IVIG and the occasional shortages that have occurred, there is a need to develop additional antibody and T cell receptor-based therapies.
Studies have been initiated to develop monoclonal antibodies against certain superantigens that can be humanized (314, 315). These studies are in their infancy, but it is expected that in the future such antibody cocktails may replace the need for the costly IVIG. Multiple studies have also been performed that show that engineered high-affinity (picomolar affinity) Vβ-TCRs, which are uniquely specific to binding and inactivating superantigens, prevent fever and lethality due to concurrent treatment or pretreatment with superantigens or staphylococci and streptococci that produce them, as tested in rabbit models (44, 231, 252, 316, 317). These reagents have also been shown in multiple rabbit studies to be important in preventing lethality due to staphylococcal pneumonia (231) and in preventing vegetation formation in infective endocarditis (252).
Active Vaccination against S. aureus and Superantigens
There have been multiple trials of human vaccines against S. aureus, and all of these have failed. The primary antigens used for vaccination have been staphylococcal cell surface antigens. Our recent study suggests that organisms such as those causing infective endocarditis aggregate to facilitate disease causation (318, 319). The same studies suggest that antibodies against cell surface antigens of the organisms further aggregate the organisms and may facilitate their abilities to cause disease (318, 319). Thus, it is possible that continued emphasis on development of cell surface-based vaccines will result in continued failures. We have recently taken a different approach, noting that our prior studies (231) indicate that combinations of secreted superantigens and alpha-toxin are required for S. aureus to cause illnesses as tested in rabbits. With that in mind, we have prepared vaccine toxoids and shown that immunization of rabbits protects against high-dose S. aureus intrapulmonary and intravenous challenge in an infective endocarditis model (134). The data suggest that S. aureus requires its secreted virulence factors to initiate diseases and may require them for colonization.
Similar studies suggest that toxoid vaccines may be produced to vaccinate against group A streptococcal superantigens (320, 321). However, major studies to vaccinate against group A streptococci, like for S. aureus, are directed against surface antigens, particularly M protein (322–324) and C5a peptidase (325). These studies are also in their infancy.
FOR THE FUTURE
As noted above, we continue each year to identify new superantigen-associated illnesses. For example, we have just identified cases of desquamative inflammatory vaginitis that are associated with superantigen production by S. aureus (326). It appears likely that the full extent of superantigen-associated illnesses will be recognized only when effective vaccines against S. aureus and group A streptococci are developed and with their use we observe what illnesses disappear. With the recognition that rabbits can be completely protected from challenge with all major PFGE clonal groups of S. aureus by superantigen and cytolysin toxoids, we may have a path now to develop a safe vaccine for human use. Toxoids are expected to stimulate neutralizing antibodies that prevent serious diseases without concerns about microbial aggregation. These studies should continue.
Additionally, it is useful to develop novel strategies to neutralize superantigens or prevent their formation. It is clear that IVIG can reduce the case-fatality rate of streptococcal TSS and likely staphylococcal TSS. If less expensive reagents such as monoclonal antibodies and Vβ-TCRs that are effective and inexpensive can be developed, they may be applicable to diseases, including nonserious illnesses.
Finally, although many superantigen-associated illnesses are linked with superantigenicity, there are many symptoms of TSS and other illnesses, where the mechanisms of toxicity remain unclear. These should be explored in detail.
ACKNOWLEDGMENTS
We gratefully acknowledge Jeffrey Kavanaugh for his help with preparing Fig. 1 and 2.
This research was supported by U.S. Public Health Service grants R01 AI074283, R01 AR41256, and U54 AI57153. P.M.S. is a member of the Great Lakes Regional Center of Excellence in Biodefense and Emerging Infectious Diseases.
Biographies

Adam R. Spaulding received his Ph.D. from the Department of Microbiology at the University of Iowa Carver College of Medicine. He received his B.S. degree in microbiology from the University of Georgia in 2007. Mr. Spaulding entered graduate school in the fall of 2008, when he joined Dr. Patrick Schlievert's laboratory. He is studying Staphylococcus aureus, superantigens, and their role in virulence, with a focus on the development of a vaccine.

Wilmara Salgado-Pabón obtained her Ph.D. from the University of Wisconsin—Madison in the laboratory of Dr. Joseph P. Dillard. Her studies focused on the molecular mechanisms of DNA secretion in the bacterial pathogen Neisseria gonorrhoeae. After completing her degree, Dr. Salgado-Pabón received a 3-year fellowship to perform research at Institut Pasteur, France, in the laboratory of Dr. Philippe J. Sansonetti. There, her research focused on the in vivo visualization of host-pathogen interactions by two-photon microscopy, specifically, the manipulation of the immune response by the bacterial pathogen Shigella flexneri. Dr. Salgado-Pabón is currently a postdoctoral associate at the University of Iowa in the laboratory of Dr. Patrick M. Schlievert, studying the pathogenic mechanisms of staphylococcal superantigens in important human illnesses such as infective endocarditis.

Petra L. Kohler earned her undergraduate degree with a major in molecular biology in 2003 at the University of Wisconsin—Madison. She then continued her studies at the University of Wisconsin, earning a Ph.D. in microbiology in 2008 with a thesis focused on bacterial pathogenesis. After completing graduate studies, Dr. Kohler carried out postdoctoral studies on Gram-positive pathogens at the University of Minnesota Medical School in the laboratory of Dr. Patrick Schlievert. Dr. Kohler is working as a senior microbiologist in the Corporate Research Laboratories at 3M Company in St. Paul, MN, where she has been since 2010.

Alexander R. Horswill was born in 1973 in Rochester, MN. He studied bacteriology at the University of Wisconsin—Madison as an undergraduate and continued on as a graduate student in the laboratory of Dr. Jorge Escalante-Semerena. He completed his Ph.D. in 2001, researching short-chain fatty acid catabolism in Salmonella. For postdoctoral studies, he joined the group of Dr. Stephen J. Benkovic at Pennsylvania State University and studied enzyme engineering and peptide cyclization with inteins. In 2005, he joined the Microbiology Department at the University of Iowa as an Assistant Professor, and he was promoted to Associate Professor in 2010. His research program focuses on Staphylococcus aureus, with an emphasis on peptide quorum-sensing mechanisms and the characterization of quorum-quenching agents. His group also investigates S. aureus biofilm maturation and dispersal, as well as host-pathogen interactions.

Donald Y. M. Leung is Professor of Pediatrics at the University of Colorado Denver School of Medicine. He received his Ph.D. (1975) and M.D. (1977) degrees from the University of Chicago. He completed a pediatric internship/residency at The Children's Hospital Medical Center in Boston and was a fellow in allergy-immunology at Boston Children's Hospital from 1979 to 1981. He joined the faculty at Harvard Medical School with appointments as Assistant Professor (1983) and Associate Professor (1987) in pediatrics. Since 1989, Dr. Leung has been Head of Pediatric Allergy-Immunology at National Jewish Health, and since 1994 he has been Director of the University of Colorado Clinical and Translation Research Center Satellite at National Jewish Health. From 1995 to 2005, he was the recipient of an NIH/NHLBI merit award for Kawasaki syndrome studies. In 2010, he was appointed principal investigator of a multicenter NIH/NIAID atopic dermatitis research network to study mechanisms underlying infections in atopic dermatitis.

Patrick M. Schlievert received his bachelor's (geology/general science, 1971) and Ph.D. (microbiology, 1976) degrees from the University of Iowa. He was a postdoctoral associate at the University of Minnesota from 1976 to 1979, studying streptococcal pyrogenic toxins. In 1979, Dr. Schlievert accepted a faculty position at UCLA, where he discovered toxic shock syndrome (TSS) toxin 1 and demonstrated why tampons are associated with TSS. Dr. Schlievert returned to the University of Minnesota, where he became a full professor in 1990. In 2011, he accepted the position of Chair of Microbiology at the University of Iowa. Dr. Schlievert and colleagues have described multiple superantigen-associated illnesses, including streptococcal TSS in 1987. His research continues in the description of new diseases, drug discovery, and vaccine development. He received the University of Minnesota's highest honor for teaching microbiology and immunology to medical students and was a member of the Academic Health Center Academy of Excellence in Medical Research.
REFERENCES
- 1. Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 2. Que YA, Moreillon P. 2011. Infective endocarditis. Nat. Rev. Cardiol. 8:322–336 [DOI] [PubMed] [Google Scholar]
- 3. Bergdoll MS, Crass BA, Reiser RF, Robbins RN, Davis JP. 1981. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet i:1017–1021 [DOI] [PubMed] [Google Scholar]
- 4. Bohach GA, Fast DJ, Nelson RD, Schlievert PM. 1990. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit. Rev. Microbiol. 17:251–272 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997–1999. JAMA 282:1123–1125 [PubMed] [Google Scholar]
- 6. Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, Leitch CD, Daum RS. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593–598 [DOI] [PubMed] [Google Scholar]
- 7. Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 [DOI] [PubMed] [Google Scholar]
- 8. McCormick JK, Yarwood JM, Schlievert PM. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77–104 [DOI] [PubMed] [Google Scholar]
- 9. Schlievert PM, Shands KN, Dan BB, Schmid GP, Nishimura RD. 1981. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J. Infect. Dis. 143:509–516 [DOI] [PubMed] [Google Scholar]
- 10. Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY. 2010. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J. Allergy Clin. Immunol. 125:39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiong YQ, Fowler VG, Yeaman MR, Perdreau-Remington F, Kreiswirth BN, Bayer AS. 2009. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J. Infect. Dis. 199:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navarre WW, Schneewind O. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cole JN, Barnett TC, Nizet V, Walker MJ. 2011. Molecular insight into invasive group A streptococcal disease. Nat. Rev. Microbiol. 9:724–736 [DOI] [PubMed] [Google Scholar]
- 14. Kreikemeyer B, McIver KS, Podbielski A. 2003. Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen-host interactions. Trends Microbiol. 11:224–232 [DOI] [PubMed] [Google Scholar]
- 15. McCormick J, Peterson ML, Schlievert PM. 2006. Toxins and superantigens of group A streptococci, p 47–58 In Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI. (ed), Gram-positive pathogens. ASM Press, Washington, DC [Google Scholar]
- 16. Molloy EM, Cotter PD, Hill C, Mitchell DA, Ross RP. 2011. Streptolysin S-like virulence factors: the continuing sagA. Nat. Rev. Microbiol. 9:670–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Navarre WW, Schneewind O. 1994. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol. Microbiol. 14:115–121 [DOI] [PubMed] [Google Scholar]
- 18. Schlievert PM, Bohach GA. 2007. Staphyoloccocal and streptococcal superantigens: an update, p 21–36 In Kotb MA, Fraseer JD. (ed), Superantigens: molecular basis for their role in human diseases. ASM Press, Washington DC [Google Scholar]
- 19. Cone LA, Woodard DR, Schlievert PM, Tomory GS. 1987. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N. Engl. J. Med. 317:146–149 [DOI] [PubMed] [Google Scholar]
- 20. Stevens DL, Tanner MH, Winship J, Swarts R, Ries KM, Schlievert PM, Kaplan E. 1989. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N. Engl. J. Med. 321:1–7 [DOI] [PubMed] [Google Scholar]
- 21. Cunningham MW. 2012. Streptococcus and rheumatic fever. Curr. Opin. Rheumatol 24:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marshall CS, Cheng AC, Markey PG, Towers RJ, Richardson LJ, Fagan PK, Scott L, Krause VL, Currie BJ. 2011. Acute post-streptococcal glomerulonephritis in the Northern Territory of Australia: a review of 16 years data and comparison with the literature. Am. J. Trop. Med. Hyg. 85:703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leung DY, Travers JB, Giorno R, Norris DA, Skinner R, Aelion J, Kazemi LV, Kim MH, Trumble AE, Kotb M, et al. 1995. Evidence for a streptococcal superantigen-driven process in acute guttate psoriasis. J. Clin. Invest. 96:2106–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberts AL, Connolly KL, Kirse DJ, Evans AK, Poehling KA, Peters TR, Reid SD. 2012. Detection of group A streptococcus in tonsils from pediatric patients reveals high rate of asymptomatic streptococcal carriage. BMC Pediatr. 12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimomura Y, Okumura K, Murayama SY, Yagi J, Ubukata K, Kirikae T, Miyoshi-Akiyama T. 2011. Complete genome sequencing and analysis of a Lancefield group G Streptococcus dysgalactiae subsp. equisimilis strain causing streptococcal toxic shock syndrome (STSS). BMC Genomics 12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sendi P, Johansson L, Norrby-Teglund A. 2008. Invasive group B streptococcal disease in non-pregnant adults: a review with emphasis on skin and soft-tissue infections. Infection 36:100–111 [DOI] [PubMed] [Google Scholar]
- 27. Sendi P, Graber P, Johansson L, Norrby-Teglund A, Zimmerli W. 2007. Streptococcus agalactiae in relapsing cellulitis. Clin. Infect. Dis. 44:1141–1142 [DOI] [PubMed] [Google Scholar]
- 28. Ekelund K, Skinhoj P, Madsen J, Konradsen HB. 2005. Invasive group A, B, C and G streptococcal infections in Denmark 1999-2002: epidemiological and clinical aspects. Clin. Microbiol. Infect. 11:569–576 [DOI] [PubMed] [Google Scholar]
- 29. Gardam MA, Low DE, Saginur R, Miller MA. 1998. Group B streptococcal necrotizing fasciitis and streptococcal toxic shock-like syndrome in adults. Arch. Intern. Med. 158:1704–1708 [DOI] [PubMed] [Google Scholar]
- 30. Hussain SM, Luedtke GS, Baker CJ, Schlievert PM, Leggiadro RJ. 1995. Invasive group B streptococcal disease in children beyond early infancy. Pediatr. Infect. Dis. J. 14:278–281 [DOI] [PubMed] [Google Scholar]
- 31. Schlievert PM, Gocke JE, Deringer JR. 1993. Group B streptococcal toxic shock-like syndrome: report of a case and purification of an associated pyrogenic toxin. Clin. Infect. Dis. 17:26–31 [DOI] [PubMed] [Google Scholar]
- 32. Schlievert PM, Varner MW, Galask RP. 1983. Endotoxin enhancement as a possible etiology of early-onset group B beta-hemolytic streptococcal sepsis in the newborn. Obstet. Gynecol. 61:588–592 [PubMed] [Google Scholar]
- 33. Wagner JG, Schlievert PM, Assimacopoulos AP, Stoehr JA, Carson PJ, Komadina K. 1996. Acute group G streptococcal myositis associated with streptococcal toxic shock syndrome: case report and review. Clin. Infect. Dis. 23:1159–1161 [DOI] [PubMed] [Google Scholar]
- 34. Kittang BR, Bruun T, Langeland N, Mylvaganam H, Glambek M, Skrede S. 2011. Invasive group A, C and G streptococcal disease in western Norway: virulence gene profiles, clinical features and outcomes. Clin. Microbiol. Infect. 17:358–364 [DOI] [PubMed] [Google Scholar]
- 35. Kittang BR, Langeland N, Skrede S, Mylvaganam H. 2010. Two unusual cases of severe soft tissue infection caused by Streptococcus dysgalactiae subsp. equisimilis. J. Clin. Microbiol. 48:1484–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Igwe EI, Shewmaker PL, Facklam RR, Farley MM, van Beneden C, Beall B. 2003. Identification of superantigen genes speM, ssa, and smeZ in invasive strains of beta-hemolytic group C and G streptococci recovered from humans. FEMS Microbiol. Lett. 229:259–264 [DOI] [PubMed] [Google Scholar]
- 37. Proft T, Webb PD, Handley V, Fraser JD. 2003. Two novel superantigens found in both group A and group C Streptococcus. Infect. Immun. 71:1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dinges MM, Orwin PM, Schlievert PM. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kreiswirth BN, Schlievert PM, Novick RP. 1987. Evaluation of coagulase-negative staphylococci for ability to produce toxic shock syndrome toxin 1. J. Clin. Microbiol. 25:2028–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Madhusoodanan J, Seo KS, Remortel B, Park JY, Hwang SY, Fox LK, Park YH, Deobald CF, Wang D, Liu S, Daugherty SC, Gill AL, Bohach GA, Gill SR. 2011. An enterotoxin-bearing pathogenicity island in Staphylococcus epidermidis. J. Bacteriol. 193:1854–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park JY, Fox LK, Seo KS, McGuire MA, Park YH, Rurangirwa FR, Sischo WM, Bohach GA. 2011. Detection of classical and newly described staphylococcal superantigen genes in coagulase-negative staphylococci isolated from bovine intramammary infections. Vet. Microbiol. 147:149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Foster TJ, Hook M. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484–488 [DOI] [PubMed] [Google Scholar]
- 43. Patti JM, Allen BL, McGavin MJ, Hook M. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585–617 [DOI] [PubMed] [Google Scholar]
- 44. Buonpane RA, Churchill HR, Moza B, Sundberg EJ, Peterson ML, Schlievert PM, Kranz DM. 2007. Neutralization of staphylococcal enterotoxin B by soluble, high-affinity receptor antagonists. Nat. Med. 13:725–729 [DOI] [PubMed] [Google Scholar]
- 45. Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Schwartz B, O'Rourke K, Talbot J, Low DE. 1999. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. The Canadian Streptococcal Study Group. Clin. Infect. Dis. 28:800–807 [DOI] [PubMed] [Google Scholar]
- 46. Schlievert PM, Case LC, Strandberg KL, Abrams BB, Leung DY. 2008. Superantigen profile of Staphylococcus aureus isolates from patients with steroid-resistant atopic dermatitis. Clin. Infect. Dis. 46:1562–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lina G, Bohach GA, Nair SP, Hiramatsu K, Jouvin-Marche E, Mariuzza R. 2004. Standard nomenclature for the superantigens expressed by Staphylococcus. J. Infect. Dis. 189:2334–2336 [DOI] [PubMed] [Google Scholar]
- 48. Bergdoll MS, Schlievert PM. 1984. Toxic-shock syndrome toxin. Lancet ii:691 [Google Scholar]
- 49. Hovde CJ, Marr JC, Hoffmann ML, Hackett SP, Chi YI, Crum KK, Stevens DL, Stauffacher CV, Bohach GA. 1994. Investigation of the role of the disulphide bond in the activity and structure of staphylococcal enterotoxin C1. Mol. Microbiol. 13:897–909 [DOI] [PubMed] [Google Scholar]
- 50. Lee PK, Kreiswirth BN, Deringer JR, Projan SJ, Eisner W, Smith BL, Carlson E, Novick RP, Schlievert PM. 1992. Nucleotide sequences and biologic properties of toxic shock syndrome toxin 1 from ovine- and bovine-associated Staphylococcus aureus. J. Infect. Dis. 165:1056–1063 [DOI] [PubMed] [Google Scholar]
- 51. Harris TO, Grossman D, Kappler JW, Marrack P, Rich RR, Betley MJ. 1993. Lack of complete correlation between emetic and T-cell-stimulatory activities of staphylococcal enterotoxins. Infect. Immun. 61:3175–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Orwin PM, Fitzgerald JR, Leung DY, Gutierrez JA, Bohach GA, Schlievert PM. 2003. Characterization of Staphylococcus aureus enterotoxin L. Infect. Immun. 71:2916–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Orwin PM, Leung DY, Donahue HL, Novick RP, Schlievert PM. 2001. Biochemical and biological properties of staphylococcal enterotoxin K. Infect. Immun. 69:360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Orwin PM, Leung DY, Tripp TJ, Bohach GA, Earhart CA, Ohlendorf DH, Schlievert PM. 2002. Characterization of a novel staphylococcal enterotoxin-like superantigen, a member of the group V subfamily of pyrogenic toxins. Biochemistry 41:14033–14040 [DOI] [PubMed] [Google Scholar]
- 55. Wilson GJ, Seo KS, Cartwright RA, Connelley T, Chuang-Smith ON, Merriman JA, Guinane CM, Park JY, Bohach GA, Schlievert PM, Morrison WI, Fitzgerald JR. 2011. A novel core genome-encoded superantigen contributes to lethality of community-associated MRSA necrotizing pneumonia. PLoS Pathog. 7:e1002271. 10.1371/journal.ppat.1002271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Betley MJ, Lofdahl S, Kreiswirth BN, Bergdoll MS, Novick RP. 1984. Staphylococcal enterotoxin A gene is associated with a variable genetic element. Proc. Natl. Acad. Sci. U. S. A. 81:5179–5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bayles KW, Iandolo JJ. 1989. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J. Bacteriol. 171:4799–4806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Novick RP. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48:1429–1449 [DOI] [PubMed] [Google Scholar]
- 59. Pragman AA, Schlievert PM. 2004. Virulence regulation in Staphylococcus aureus: the need for in vivo analysis of virulence factor regulation. FEMS Immunol. Med. Microbiol. 42:147–154 [DOI] [PubMed] [Google Scholar]
- 60. Yarwood JM, Schlievert PM. 2003. Quorum sensing in Staphylococcus infections. J. Clin. Invest. 112:1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schlievert PM, Bettin KM, Watson DW. 1979. Reinterpretation of the Dick test: role of group A streptococcal pyrogenic exotoxin. Infect. Immun. 26:467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tomai MA, Schlievert PM, Kotb M. 1992. Distinct T-cell receptor V beta gene usage by human T lymphocytes stimulated with the streptococcal pyrogenic exotoxins and pep M5 protein. Infect. Immun. 60:701–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Banks DJ, Porcella SF, Barbian KD, Beres SB, Philips LE, Voyich JM, DeLeo FR, Martin JM, Somerville GA, Musser JM. 2004. Progress toward characterization of the group A streptococcus metagenome: complete genome sequence of a macrolide-resistant serotype M6 strain. J. Infect. Dis. 190:727–738 [DOI] [PubMed] [Google Scholar]
- 64. Beres SB, Sylva GL, Barbian KD, Lei B, Hoff JS, Mammarella ND, Liu MY, Smoot JC, Porcella SF, Parkins LD, Campbell DS, Smith TM, McCormick JK, Leung DY, Schlievert PM, Musser JM. 2002. Genome sequence of a serotype M3 strain of group A streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. U. S. A. 99:10078–10083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ferretti JJ, McShan WM, Ajdic D, Savic DJ, Savic G, Lyon K, Primeaux C, Sezate S, Suvorov AN, Kenton S, Lai HS, Lin SP, Qian Y, Jia HG, Najar FZ, Ren Q, Zhu H, Song L, White J, Yuan X, Clifton SW, Roe BA, McLaughlin R. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. U. S. A. 98:4658–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nakagawa I, Kurokawa K, Yamashita A, Nakata M, Tomiyasu Y, Okahashi N, Kawabata S, Yamazaki K, Shiba T, Yasunaga T, Hayashi H, Hattori M, Hamada S. 2003. Genome sequence of an M3 strain of Streptococcus pyogenes reveals a large-scale genomic rearrangement in invasive strains and new insights into phage evolution. Genome Res. 13:1042–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Smoot LM, McCormick JK, Smoot JC, Hoe NP, Strickland I, Cole RL, Barbian KD, Earhart CA, Ohlendorf DH, Veasy LG, Hill HR, Leung DY, Schlievert PM, Musser JM. 2002. Characterization of two novel pyrogenic toxin superantigens made by an acute rheumatic fever clone of Streptococcus pyogenes associated with multiple disease outbreaks. Infect. Immun. 70:7095–7104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bohach GA, Hauser AR, Schlievert PM. 1988. Cloning of the gene, speB, for streptococcal pyrogenic exotoxin type B in Escherichia coli. Infect. Immun. 56:1665–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hauser AR, Schlievert PM. 1990. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J. Bacteriol. 172:4536–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kagawa TF, Cooney JC, Baker HM, McSweeney S, Liu M, Gubba S, Musser JM, Baker EN. 2000. Crystal structure of the zymogen form of the group A Streptococcus virulence factor SpeB: an integrin-binding cysteine protease. Proc. Natl. Acad. Sci. U. S. A. 97:2235–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gerlach D, Reichardt W, Fleischer B, Schmidt KH. 1994. Separation of mitogenic and pyrogenic activities from so-called erythrogenic toxin type B (streptococcal proteinase). Zentralbl Bakteriol. 280:507–514 [DOI] [PubMed] [Google Scholar]
- 72. Tomai M, Kotb M, Majumdar G, Beachey EH. 1990. Superantigenicity of streptococcal M protein. J. Exp. Med. 172:359–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pahlman LI, Olin AI, Darenberg J, Morgelin M, Kotb M, Herwald H, Norrby-Teglund A. 2008. Soluble M1 protein of Streptococcus pyogenes triggers potent T cell activation. Cell. Microbiol. 10:404–414 [DOI] [PubMed] [Google Scholar]
- 74. Assimacopoulos AP, Stoehr JA, Schlievert PM. 1997. Mitogenic factors from group G streptococci associated with scarlet fever and streptococcal toxic shock syndrome. Adv. Exp. Med. Biol. 418:109–114 [DOI] [PubMed] [Google Scholar]
- 75. Paillot R, Darby AC, Robinson C, Wright NL, Steward KF, Anderson E, Webb K, Holden MT, Efstratiou A, Broughton K, Jolley KA, Priestnall SL, Marotti Campi MC, Hughes MA, Radford A, Erles K, Waller AS. 2010. Identification of three novel superantigen-encoding genes in Streptococcus equi subsp. zooepidemicus, szeF, szeN, and szeP. Infect. Immun. 78:4817–4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zell C, Resch M, Rosenstein R, Albrecht T, Hertel C, Gotz F. 2008. Characterization of toxin production of coagulase-negative staphylococci isolated from food and starter cultures. Int. J. Food Microbiol. 127:246–251 [DOI] [PubMed] [Google Scholar]
- 77. Boubou MI, Collette A, Voegtle D, Mazier D, Cazenave PA, Pied S. 1999. T cell response in malaria pathogenesis: selective increase in T cells carrying the TCR V(beta)8 during experimental cerebral malaria. Int. Immunol. 11:1553–1562 [DOI] [PubMed] [Google Scholar]
- 78. Bowness P, Moss PA, Tranter H, Bell JI, McMichael AJ. 1992. Clostridium perfringens enterotoxin is a superantigen reactive with human T cell receptors V beta 6.9 and V beta 22. J. Exp. Med. 176:893–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Denkers EY, Caspar P, Sher A. 1994. Toxoplasma gondii possesses a superantigen activity that selectively expands murine T cell receptor V beta 5-bearing CD8+ lymphocytes. J. Exp. Med. 180:985–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rink L, Kirchner H. 1992. Mycoplasma arthritidis-derived superantigen. Chem. Immunol. 55:137–145 [PubMed] [Google Scholar]
- 81. Ito Y, Abe J, Yoshino K, Takeda T, Kohsaka T. 1995. Sequence analysis of the gene for a novel superantigen produced by Yersinia pseudotuberculosis and expression of the recombinant protein. J. Immunol. 154:5896–5906 [PubMed] [Google Scholar]
- 82. Stuart PM, Munn RK, DeMoll E, Woodward JG. 1995. Characterization of human T-cell responses to Yersinia enterocolitica superantigen. Hum. Immunol. 43:269–275 [DOI] [PubMed] [Google Scholar]
- 83. Galelli A, Truffa-Bachi P. 1993. Urtica dioica agglutinin. A superantigenic lectin from stinging nettle rhizome. J. Immunol. 151:1821–1831 [PubMed] [Google Scholar]
- 84. Schlievert PM, Blomster DA. 1983. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J. Infect. Dis. 147:236–242 [DOI] [PubMed] [Google Scholar]
- 85. Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick RP. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol. Gen. Genet. 202:58–61 [DOI] [PubMed] [Google Scholar]
- 86. Osterholm MT, Davis JP, Gibson RW, Mandel JS, Wintermeyer LA, Helms CM, Forfang JC, Rondeau J, Vergeront JM. 1982. Tri-state toxic-state syndrome study. I. Epidemiologic findings. J. Infect. Dis. 145:431–440 [DOI] [PubMed] [Google Scholar]
- 87. Schlievert PM. 1983. Alteration of immune function by staphylococcal pyrogenic exotoxin type C: possible role in toxic-shock syndrome. J. Infect. Dis. 147:391–398 [DOI] [PubMed] [Google Scholar]
- 88. Schlievert PM, Bettin KM, Watson DW. 1979. Production of pyrogenic exotoxin by groups of streptococci: association with group A. J. Infect. Dis. 140:676–681 [DOI] [PubMed] [Google Scholar]
- 89. Poindexter NJ, Schlievert PM. 1986. Suppression of immunoglobulin-secreting cells from human peripheral blood by toxic-shock-syndrome toxin-1. J. Infect. Dis. 153:772–779 [DOI] [PubMed] [Google Scholar]
- 90. Fast DJ, Schlievert PM, Nelson RD. 1988. Nonpurulent response to toxic shock syndrome toxin 1-producing Staphylococcus aureus. Relationship to toxin-stimulated production of tumor necrosis factor. J. Immunol. 140:949–953 [PubMed] [Google Scholar]
- 91. Cockerill FR, III, MacDonald KL, Thompson RL, Roberson F, Kohner PC, Besser-Wiek J, Manahan JM, Musser JM, Schlievert PM, Talbot J, Frankfort B, Steckelberg JM, Wilson WR, Osterholm MT. 1997. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA 277:38–43 [PubMed] [Google Scholar]
- 92. Cockerill FR, III, Thompson RL, Musser JM, Schlievert PM, Talbot J, Holley KE, Harmsen WS, Ilstrup DM, Kohner PC, Kim MH, Frankfort B, Manahan JM, Steckelberg JM, Roberson F, Wilson WR. 1998. Molecular, serological, and clinical features of 16 consecutive cases of invasive streptococcal disease. Southeastern Minnesota Streptococcal Working Group. Clin. Infect. Dis. 26:1448–1458 [DOI] [PubMed] [Google Scholar]
- 93. Davis JP, Chesney PJ, Wand PJ, LaVenture M. 1980. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N. Engl. J. Med. 303:1429–1435 [DOI] [PubMed] [Google Scholar]
- 94. Watson DW. 1960. Host-parasite factors in group A streptococcal infections. Pyrogenic and other effects of immunologic distinct exotoxins related to scarlet fever toxins. J. Exp. Med. 111:255–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bergdoll MS. 1988. Monkey feeding test for staphylococcal enterotoxin. Methods Enzymol. 165:324–333 [DOI] [PubMed] [Google Scholar]
- 96. McCormick JK, Bohach GA, Schlievert PM. 2003. Pyrogenic, lethal, and emetic properties of superantigens in rabbits and primates. Methods Mol. Biol. 214:245–253 [DOI] [PubMed] [Google Scholar]
- 97. Dick GF, Dick GH. 1983. Landmark article Jan 26, 1924: the etiology of scarlet fever. JAMA 250:3096. [DOI] [PubMed] [Google Scholar]
- 98. Rantz LA, Boisvert PJ, Spink WW. 1946. The Dick test in military personnel, with special reference to the pathogenesis of the skin reaction. N. Engl. J. Med. 235:39–43 [DOI] [PubMed] [Google Scholar]
- 99. Reingold AL, Hargrett NT, Dan BB, Shands KN, Strickland BY, Broome CV. 1982. Nonmenstrual toxic shock syndrome: a review of 130 cases. Ann. Intern. Med. 96:871–874 [DOI] [PubMed] [Google Scholar]
- 100. Kim YB, Watson DW. 1970. A purified group A streptococcal pyrogenic exotoxin. Physiochemical and biological properties including the enhancement of susceptibility to endotoxin lethal shock. J. Exp. Med. 131:611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schlievert PM, Watson DW. 1978. Group A streptococcal pyrogenic exotoxin: pyrogenicity, alteration of blood-brain barrier, and separation of sites for pyrogenicity and enhancement of lethal endotoxin shock. Infect. Immun. 21:753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schlievert PM, Bettin KM, Watson DW. 1977. Purification and characterization of group A streptococcal pyrogenic exotoxin type C. Infect. Immun. 16:673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schlievert PM, Schoettle DJ, Watson DW. 1980. Ganglioside and monosaccharide inhibition of nonspecific lymphocyte mitogenicity by group A streptococcal pyrogenic exotoxins. Infect. Immun. 27:276–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Marrack P, Kappler J. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705–711 [DOI] [PubMed] [Google Scholar]
- 105. Kotzin BL, Leung DY, Kappler J, Marrack P. 1993. Superantigens and their potential role in human disease. Adv. Immunol. 54:99–166 [DOI] [PubMed] [Google Scholar]
- 106. Li H, Llera A, Malchiodi EL, Mariuzza RA. 1999. The structural basis of T cell activation by superantigens. Annu. Rev. Immunol. 17:435–466 [DOI] [PubMed] [Google Scholar]
- 107. Leung DYM, Huber BT, Schlievert PM. 1997. Historical perspective of superantigens and their biological activities, p 1–14 In Leung DYM, Huber BT, Schlievert PM. (ed.), Superantigens: molecular biology, immunology, and relevance to human disease. Marcel Dekker, Inc., New York, NY [Google Scholar]
- 108. Mitchell DT, Levitt DG, Schlievert PM, Ohlendorf DH. 2000. Structural evidence for the evolution of pyrogenic toxin superantigens. J. Mol. Evol. 51:520–531 [DOI] [PubMed] [Google Scholar]
- 109. Brosnahan AJ, Schlievert PM. 2011. Gram positive bacterial superantigen outside-in signaling causes toxic shock syndrome. FEBS J. 278:4649–4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Acharya KR, Passalacqua EF, Jones EY, Harlos K, Stuart DI, Brehm RD, Tranter HS. 1994. Structural basis of superantigen action inferred from crystal structure of toxic-shock syndrome toxin-1. Nature 367:94–97 [DOI] [PubMed] [Google Scholar]
- 111. Prasad GS, Earhart CA, Murray DL, Novick RP, Schlievert PM, Ohlendorf DH. 1993. Structure of toxic shock syndrome toxin 1. Biochemistry 32:13761–13766 [DOI] [PubMed] [Google Scholar]
- 112. Kim J, Urban RG, Strominger JL, Wiley DC. 1994. Toxic shock syndrome toxin-1 complexed with a class II major histocompatibility molecule HLA-DR1. Science 266:1870–1874 [DOI] [PubMed] [Google Scholar]
- 113. McCormick JK, Tripp TJ, Llera AS, Sundberg EJ, Dinges MM, Mariuzza RA, Schlievert PM. 2003. Functional analysis of the TCR binding domain of toxic shock syndrome toxin-1 predicts further diversity in MHC class II/superantigen/TCR ternary complexes. J. Immunol. 171:1385–1392 [DOI] [PubMed] [Google Scholar]
- 114. Moza B, Varma AK, Buonpane RA, Zhu P, Herfst CA, Nicholson MJ, Wilbuer AK, Seth NP, Wucherpfennig KW, McCormick JK, Kranz DM, Sundberg EJ. 2007. Structural basis of T-cell specificity and activation by the bacterial superantigen TSST-1. EMBO J. 26:1187–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schlievert PM, Jablonski LM, Roggiani M, Sadler I, Callantine S, Mitchell DT, Ohlendorf DH, Bohach GA. 2000. Pyrogenic toxin superantigen site specificity in toxic shock syndrome and food poisoning in animals. Infect. Immun. 68:3630–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Earhart CA, Vath GM, Roggiani M, Schlievert PM, Ohlendorf DH. 2000. Structure of streptococcal pyrogenic exotoxin A reveals a novel metal cluster. Protein Sci. 9:1847–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jardetzky TS, Brown JH, Gorga JC, Stern LJ, Urban RG, Chi YI, Stauffacher C, Strominger JL, Wiley DC. 1994. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature 368:711–718 [DOI] [PubMed] [Google Scholar]
- 118. Papageorgiou AC, Collins CM, Gutman DM, Kline JB, O'Brien SM, Tranter HS, Acharya KR. 1999. Structural basis for the recognition of superantigen streptococcal pyrogenic exotoxin A (SpeA1) by MHC class II molecules and T-cell receptors. EMBO J. 18:9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fields BA, Malchiodi EL, Li H, Ysern X, Stauffacher CV, Schlievert PM, Karjalainen K, Mariuzza RA. 1996. Crystal structure of a T-cell receptor beta-chain complexed with a superantigen. Nature 384:188–192 [DOI] [PubMed] [Google Scholar]
- 120. Leder L, Llera A, Lavoie PM, Lebedeva MI, Li H, Sekaly RP, Bohach GA, Gahr PJ, Schlievert PM, Karjalainen K, Mariuzza RA. 1998. A mutational analysis of the binding of staphylococcal enterotoxins B and C3 to the T cell receptor beta chain and major histocompatibility complex class II. J. Exp. Med. 187:823–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Li H, Llera A, Tsuchiya D, Leder L, Ysern X, Schlievert PM, Karjalainen K, Mariuzza RA. 1998. Three-dimensional structure of the complex between a T cell receptor beta chain and the superantigen staphylococcal enterotoxin B. Immunity 9:807–816 [DOI] [PubMed] [Google Scholar]
- 122. Petersson K, Thunnissen M, Forsberg G, Walse B. 2002. Crystal structure of a SEA variant in complex with MHC class II reveals the ability of SEA to crosslink MHC molecules. Structure 10:1619–1626 [DOI] [PubMed] [Google Scholar]
- 123. Kozono H, Parker D, White J, Marrack P, Kappler J. 1995. Multiple binding sites for bacterial superantigens on soluble class II MHC molecules. Immunity 3:187–196 [DOI] [PubMed] [Google Scholar]
- 124. Antonsson P, Wingren AG, Hansson J, Kalland T, Varga M, Dohlsten M. 1997. Functional characterization of the interaction between the superantigen staphylococcal enterotoxin A and the TCR. J. Immunol. 158:4245–4251 [PubMed] [Google Scholar]
- 125. Li Y, Li H, Dimasi N, McCormick JK, Martin R, Schuck P, Schlievert PM, Mariuzza RA. 2001. Crystal structure of a superantigen bound to the high-affinity, zinc-dependent site on MHC class II. Immunity 14:93–104 [DOI] [PubMed] [Google Scholar]
- 126. Hudson KR, Tiedemann RE, Urban RG, Lowe SC, Strominger JL, Fraser JD. 1995. Staphylococcal enterotoxin A has two cooperative binding sites on major histocompatibility complex class II. J. Exp. Med. 182:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Unnikrishnan M, Altmann DM, Proft T, Wahid F, Cohen J, Fraser JD, Sriskandan S. 2002. The bacterial superantigen streptococcal mitogenic exotoxin Z is the major immunoactive agent of Streptococcus pyogenes. J. Immunol. 169:2561–2569 [DOI] [PubMed] [Google Scholar]
- 128. Gunther S, Varma AK, Moza B, Kasper KJ, Wyatt AW, Zhu P, Rahman AK, Li Y, Mariuzza RA, McCormick JK, Sundberg EJ. 2007. A novel loop domain in superantigens extends their T cell receptor recognition site. J. Mol. Biol. 371:210–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Brouillard JN, Gunther S, Varma AK, Gryski I, Herfst CA, Rahman AK, Leung DY, Schlievert PM, Madrenas J, Sundberg EJ, McCormick JK. 2007. Crystal structure of the streptococcal superantigen SpeI and functional role of a novel loop domain in T cell activation by group V superantigens. J. Mol. Biol. 367:925–934 [DOI] [PubMed] [Google Scholar]
- 130. Argudin MA, Mendoza MC, Rodicio MR. 2010. Food poisoning and Staphylococcus aureus enterotoxins. Toxins (Basel) 2:1751–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sospedra I, Soler C, Manes J, Soriano JM. 2011. Analysis of staphylococcal enterotoxin A in milk by matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Anal. Bioanal. Chem. 400:1525–1531 [DOI] [PubMed] [Google Scholar]
- 132. Arad G, Levy R, Hillman D, Kaempfer R. 2000. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat. Med. 6:414–421 [DOI] [PubMed] [Google Scholar]
- 133. Brosnahan AJ, Schaefers MM, Amundson WH, Mantz MJ, Squier CA, Peterson ML, Schlievert PM. 2008. Novel toxic shock syndrome toxin-1 amino acids required for biological activity. Biochemistry 47:12995–13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Spaulding AR, Lin YC, Merriman JA, Brosnahan AJ, Peterson ML, Schlievert PM. 2012. Immunity to Staphylococcus aureus secreted proteins protects rabbits from serious illnesses. Vaccine 30:5099–5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, Supper E, Shpilka T, Minis A, Kaempfer R. 2011. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 9:e1001149. 10.1371/journal.pbio.1001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kushnaryov VM, Bergdoll MS, MacDonald HS, Vellinga J, Reiser R. 1984. Study of staphylococcal toxic shock syndrome toxin in human epithelial cell culture. J. Infect. Dis. 150:535–545 [DOI] [PubMed] [Google Scholar]
- 137. Kushnaryov VM, MacDonald HS, Reiser R, Bergdoll MS. 1984. Staphylococcal toxic shock toxin specifically binds to cultured human epithelial cells and is rapidly internalized. Infect. Immun. 45:566–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kushnaryov VM, MacDonald HS, Reiser RF, Bergdoll MS. 1989. Reaction of toxic shock syndrome toxin 1 with endothelium of human umbilical cord vein. Rev. Infect. Dis. 11(Suppl 1):S282–S288 [DOI] [PubMed] [Google Scholar]
- 139. Lee PK, Vercellotti GM, Deringer JR, Schlievert PM. 1991. Effects of staphylococcal toxic shock syndrome toxin 1 on aortic endothelial cells. J. Infect. Dis. 164:711–719 [DOI] [PubMed] [Google Scholar]
- 140. Hamad AR, Marrack P, Kappler JW. 1997. Transcytosis of staphylococcal superantigen toxins. J. Exp. Med. 185:1447–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shands KN, Schmid GP, Dan BB, Blum D, Guidotti RJ, Hargrett NT, Anderson RL, Hill DL, Broome CV, Band JD, Fraser DW. 1980. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N. Engl. J. Med. 303:1436–1442 [DOI] [PubMed] [Google Scholar]
- 142. Gaventa S, Reingold AL, Hightower AW, Broome CV, Schwartz B, Hoppe C, Harwell J, Lefkowitz LK, Makintubee S, Cundiff DR, et al. 1989. Active surveillance for toxic shock syndrome in the United States, 1986. Rev. Infect. Dis. 11(Suppl 1):S28–S34 [DOI] [PubMed] [Google Scholar]
- 143. Schlievert PM, Case LC, Strandberg KL, Tripp TJ, Lin YC, Peterson ML. 2007. Vaginal Staphylococcus aureus superantigen profile shift from 1980 and 1981 to 2003, 2004, and 2005. J. Clin. Microbiol. 45:2704–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. DeVries AS, Lesher L, Schlievert PM, Rogers T, Villaume LG, Danila R, Lynfield R. 2011. Staphylococcal toxic shock syndrome—2006: epidemiology, clinical features, and molecular characteristics. PLoS One 6:e22997. 10.1371/journal.pone.0022997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Schlievert PM, Tripp TJ, Peterson ML. 2004. Reemergence of staphylococcal toxic shock syndrome in Minneapolis-St. Paul, Minnesota, during the 2000-2003 surveillance period. J. Clin. Microbiol. 42:2875–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Schlievert PM, Nemeth KA, Davis CC, Peterson ML, Jones BE. 2010. Staphylococcus aureus exotoxins are present in vivo in tampons. Clin. Vaccine Immunol. 17:722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Schlievert PM, Case LC, Nemeth KA, Davis CC, Sun Y, Qin W, Wang F, Brosnahan AJ, Mleziva JA, Peterson ML, Jones BE. 2007. Alpha and beta chains of hemoglobin inhibit production of Staphylococcus aureus exotoxins. Biochemistry 46:14349–14358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Schlievert PM, Bohach GA. 2007. Staphylococcal and streptococcal superantigens: an update, p 21–36 In Kotb M, Fraser JD. (ed), Superantigens: molecular basis for their role in human diseases. ASM Press, Washington, DC [Google Scholar]
- 149. Todd JK, Kapral FA, Fishaut M, Welch TR. 1978. Toxic shock syndrome associated with phage group 1 staphylococci. Lancet ii:1116–1118 [DOI] [PubMed] [Google Scholar]
- 150. John CC, Niermann M, Sharon B, Peterson ML, Kranz DM, Schlievert PM. 2009. Staphylococcal toxic shock syndrome erythroderma is associated with superantigenicity and hypersensitivity. Clin. Infect. Dis. 49:1893–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Parsonnet J. 1998. Case definition of staphylococcal TSS: a proposed revision incorporating laboratory findings. Int. Congr. Symp. Ser. 229:15 [Google Scholar]
- 152. MacDonald KL, Osterholm MT, Hedberg CW, Schrock CG, Peterson GF, Jentzen JM, Leonard SA, Schlievert PM. 1987. Toxic shock syndrome. A newly recognized complication of influenza and influenzalike illness. JAMA 257:1053–1058 [DOI] [PubMed] [Google Scholar]
- 153. Cone LA, Woodard DR, Byrd RG, Schulz K, Kopp SM, Schlievert PM. 1992. A recalcitrant, erythematous, desquamating disorder associated with toxin-producing staphylococci in patients with AIDS. J. Infect. Dis. 165:638–643 [DOI] [PubMed] [Google Scholar]
- 154. Kravitz G, Dries DJ, Peterson ML, Schlievert PM. 2005. Purpura fulminans due to Staphylococcus aureus. Clin. Infect. Dis. 40:941–947 [DOI] [PubMed] [Google Scholar]
- 155. Adem PV, Montgomery CP, Husain AN, Koogler TK, Arangelovich V, Humilier M, Boyle-Vavra S, Daum RS. 2005. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N. Engl. J. Med. 353:1245–1251 [DOI] [PubMed] [Google Scholar]
- 156. Assimacopoulos AP, Strandberg KL, Rotschafer JH, Schlievert PM. 2009. Extreme pyrexia and rapid death due to Staphylococcus aureus infection: analysis of 2 cases. Clin. Infect. Dis. 48:612–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kotler DP, Sandkovsky U, Schlievert PM, Sordillo EM. 2007. Toxic shock-like syndrome associated with staphylococcal enterocolitis in an HIV-infected man. Clin. Infect. Dis. 44:e121–e123 [DOI] [PubMed] [Google Scholar]
- 158. Lin Z, Kotler DP, Schlievert PM, Sordillo EM. 2010. Staphylococcal enterocolitis: forgotten but not gone? Dig. Dis. Sci. 55:1200–1207 [DOI] [PubMed] [Google Scholar]
- 159. Jarraud S, Cozon G, Vandenesch F, Bes M, Etienne J, Lina G. 1999. Involvement of enterotoxins G and I in staphylococcal toxic shock syndrome and staphylococcal scarlet fever. J. Clin. Microbiol. 37:2446–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, Killgore G, Tenover FC. 2006. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J. Infect. Dis. 193:172–179 [DOI] [PubMed] [Google Scholar]
- 161. Altemeier WA, Lewis S, Schlievert PM, Bjornson HS. 1981. Studies of the staphylococcal causation of toxic shock syndrome. Surg. Gynecol. Obstet. 153:481–485 [PubMed] [Google Scholar]
- 162. Altemeier WA, Lewis SA, Schlievert PM, Bergdoll MS, Bjornson HS, Staneck JL, Crass BA. 1982. Staphylococcus aureus associated with toxic shock syndrome: phage typing and toxin capability testing. Ann. Intern. Med. 96:978–982 [DOI] [PubMed] [Google Scholar]
- 163. Lin YC, Anderson MJ, Kohler PL, Strandberg KL, Olson ME, Horswill AR, Schlievert PM, Peterson ML. 2011. Proinflammatory exoprotein characterization of toxic shock syndrome Staphylococcus aureus. Biochemistry 50:7157–7167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. O'Reilly M, Kreiswirth B, Foster TJ. 1990. Cryptic alpha-toxin gene in toxic shock syndrome and septicaemia strains of Staphylococcus aureus. Mol. Microbiol. 4:1947–1955 [DOI] [PubMed] [Google Scholar]
- 165. Schlievert PM, Osterholm MT, Kelly JA, Nishimura RD. 1982. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic shock syndrome. Ann. Intern. Med. 96:937–940 [DOI] [PubMed] [Google Scholar]
- 166. Deleo FR, Kennedy AD, Chen L, Bubeck Wardenburg J, Kobayashi SD, Mathema B, Braughton KR, Whitney AR, Villaruz AE, Martens CA, Porcella SF, McGavin MJ, Otto M, Musser JM, Kreiswirth BN. 2011. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 108:18091–18096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Schlievert PM, Peterson ML. 2012. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS One 7:e40350. 10.1371/journal.pone.0040350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Yarwood JM, Schlievert PM. 2000. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J. Clin. Microbiol. 38:1797–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Hill DR, Brunner ME, Schmitz DC, Davis CC, Flood JA, Schlievert PM, Wang-Weigand SZ, Osborn TW. 2005. In vivo assessment of human vaginal oxygen and carbon dioxide levels during and post menses. J. Appl. Physiol. 99:1582–1591 [DOI] [PubMed] [Google Scholar]
- 170. Yarwood JM, McCormick JK, Schlievert PM. 2001. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J. Bacteriol. 183:1113–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Schlievert PM. 1996. Effect of Merocel vaginal sponge on growth of Staphylococcus aureus and production of toxic shock syndrome-associated toxins. J. Am. Coll Surg. 183:19–24 [PubMed] [Google Scholar]
- 172. Melish ME, Murata S, Fukunaga C, Frogner K, McKissick C. 1989. Vaginal tampon model for toxic shock syndrome. Rev. Infect. Dis. 11(Suppl 1):S238–S246 [DOI] [PubMed] [Google Scholar]
- 173. Tierno PM, Jr, Hanna BA. 1989. Ecology of toxic shock syndrome: amplification of toxic shock syndrome toxin 1 by materials of medical interest. Rev. Infect. Dis. 11(Suppl 1):S182–S186 [DOI] [PubMed] [Google Scholar]
- 174. Parsonnet J, Modern PA, Giacobbe KD. 1996. Effect of tampon composition on production of toxic shock syndrome toxin-1 by Staphylococcus aureus in vitro. J. Infect. Dis. 173:98–103 [DOI] [PubMed] [Google Scholar]
- 175. Schlievert PM. 1995. Comparison of cotton and cotton/rayon tampons for effect on production of toxic shock syndrome toxin. J. Infect. Dis. 172:1112–1114 [DOI] [PubMed] [Google Scholar]
- 176. Schlievert PM. 1996. Tampons and toxic shock syndrome. Med. J. Aust. 164:635–636 [DOI] [PubMed] [Google Scholar]
- 177. Fischetti VA, Chapman F, Kakani R, James J, Grun E, Zabriskie JB. 1989. Role of air in growth and production of toxic shock syndrome toxin 1 by Staphylococcus aureus in experimental cotton and rayon tampons. Rev. Infect. Dis. 11(Suppl. 1):S176–S181 [DOI] [PubMed] [Google Scholar]
- 178. Davis CC, Kremer MJ, Schlievert PM, Squier CA. 2003. Penetration of toxic shock syndrome toxin-1 across porcine vaginal mucosa ex vivo: permeability characteristics, toxin distribution, and tissue damage. Am. J. Obstet. Gynecol. 189:1785–1791 [DOI] [PubMed] [Google Scholar]
- 179. Squier CA, Mantz MJ, Schlievert PM, Davis CC. 2008. Porcine vagina ex vivo as a model for studying permeability and pathogenesis in mucosa. J. Pharm. Sci. 97:9–21 [DOI] [PubMed] [Google Scholar]
- 180. Peterson M, Ault K, Kremer MJ, Klingelhutz AJ, Davis CC, Squier CA, Schlievert PM. 2005. Innate immune system is activated by stimulation of vaginal epithelial cells with Staphylococcus aureus and toxic shock syndrome toxin-1. Infect. Immun. 73:2164–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Brosnahan AJ, Mantz MJ, Squier CA, Peterson ML, Schlievert PM. 2009. Cytolysins augment superantigen penetration of stratified mucosa. J. Immunol. 182:2364–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Breshears LM, Schlievert PM, Peterson ML. 2012. A disintegrin and metalloproteinase 17 (ADAM17) and epidermal growth factor receptor (EGFR) signaling drive the epithelial response to Staphylococcus aureus toxic shock syndrome toxin-1 (TSST-1). J. Biol. Chem. 287:32578–32587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Schlievert PM, Bettin KM, Watson DW. 1978. Effect of antipyretics on group A streptococcal pyrogenic exotoxin fever production and ability to enhance lethal endotoxin shock. Proc. Soc. Exp. Biol. Med. 157:472–475 [DOI] [PubMed] [Google Scholar]
- 185. Schlievert PM, Watson DW. 1979. Biogenic amine involvement in pyrogenicity and enhancement of lethal endotoxin shock by group A streptococcal pyrogenic exotoxin. Proc. Soc. Exp. Biol. Med. 162:269–274 [DOI] [PubMed] [Google Scholar]
- 186. Schlievert PM. 1982. Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect. Immun. 36:123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Stone RL, Schlievert PM. 1987. Evidence for the involvement of endotoxin in toxic shock syndrome. J. Infect. Dis. 155:682–689 [DOI] [PubMed] [Google Scholar]
- 188. Dinges MM, Schlievert PM. 2001. Comparative analysis of lipopolysaccharide-induced tumor necrosis factor alpha activity in serum and lethality in mice and rabbits pretreated with the staphylococcal superantigen toxic shock syndrome toxin 1. Infect. Immun. 69:7169–7172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lee PK, Deringer JR, Kreiswirth BN, Novick RP, Schlievert PM. 1991. Fluid replacement protection of rabbits challenged subcutaneous with toxic shock syndrome toxins. Infect. Immun. 59:879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Schlievert PM, Bettin KM, Watson DW. 1980. Inhibition of ribonucleic acid synthesis by group A streptococcal pyrogenic exotoxin. Infect. Immun. 27:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Schlievert PM. 2009. Cytolysins, superantigens, and pneumonia due to community-associated methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 200:676–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Chow AW, Percival-Smith R, Bartlett KH, Goldring AM, Morrison BJ. 1986. Vaginal colonization with Escherichia coli in healthy women. Determination of relative risks by quantitative culture and multivariate statistical analysis. Am. J. Obstet. Gynecol. 154:120–126 [DOI] [PubMed] [Google Scholar]
- 193. Musser JM, Schlievert PM, Chow AW, Ewan P, Kreiswirth BN, Rosdahl VT, Naidu AS, Witte W, Selander RK. 1990. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc. Natl. Acad. Sci. U. S. A. 87:225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Chu MC, Melish ME, James JF. 1989. Growth of toxic shock syndrome toxin 1-producing, tryptophan-requiring strains of Staphylococcus aureus associated with the presence of Escherichia coli. Rev. Infect. Dis. 11(Suppl 1):S101–S103 [DOI] [PubMed] [Google Scholar]
- 195. Chu MC, Melish ME, James JF. 1985. Tryptophan auxotypy associated with Staphylococcus aureus that produce toxic-shock-syndrome toxin. J. Infect. Dis. 151:1157–1158 [DOI] [PubMed] [Google Scholar]
- 196. Vergeront JM, Stolz SJ, Crass BA, Nelson DB, Davis JP, Bergdoll MS. 1983. Prevalence of serum antibody to staphylococcal enterotoxin F among Wisconsin residents: implications for toxic-shock syndrome. J. Infect. Dis. 148:692–698 [DOI] [PubMed] [Google Scholar]
- 197. Parsonnet J, Hansmann MA, Delaney ML, Modern PA, Dubois AM, Wieland-Alter W, Wissemann KW, Wild JE, Jones MB, Seymour JL, Onderdonk AB. 2005. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J. Clin. Microbiol. 43:4628–4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kansal R, Davis C, Hansmann M, Seymour J, Parsonnet J, Modern P, Gilbert S, Kotb M. 2007. Structural and functional properties of antibodies to the superantigen TSST-1 and their relationship to menstrual toxic shock syndrome. J. Clin. Immunol. 27:327–338 [DOI] [PubMed] [Google Scholar]
- 199. Kotb M, Norrby-Teglund A, McGeer A, El-Sherbini H, Dorak MT, Khurshid A, Green K, Peeples J, Wade J, Thomson G, Schwartz B, Low DE. 2002. An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat. Med. 8:1398–1404 [DOI] [PubMed] [Google Scholar]
- 200. Schlievert PM, Kim MH. 1991. Reporting of toxic shock syndrome Staphylococcus aureus in 1982 to 1990. J. Infect. Dis. 164:1245–1246 [DOI] [PubMed] [Google Scholar]
- 201. Giantonio BJ, Alpaugh RK, Schultz J, McAleer C, Newton DW, Shannon B, Guedez Y, Kotb M, Vitek L, Persson R, Gunnarsson PO, Kalland T, Dohlsten M, Persson B, Weiner LM. 1997. Superantigen-based immunotherapy: a phase I trial of PNU-214565, a monoclonal antibody-staphylococcal enterotoxin A recombinant fusion protein, in advanced pancreatic and colorectal cancer. J. Clin. Oncol. 15:1994–2007 [DOI] [PubMed] [Google Scholar]
- 202. Fey PD, Said-Salim B, Rupp ME, Hinrichs SH, Boxrud DJ, Davis CC, Kreiswirth BN, Schlievert PM. 2003. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Kim JS, Kim HS, Song W, Cho HC, Lee KM, Kim EC. 2007. Molecular epidemiology of methicillin-resistant Staphylococcus aureus isolates with toxic shock syndrome toxin and staphylococcal enterotoxin C genes. Korean J. Lab Med. 27:118–123 [DOI] [PubMed] [Google Scholar]
- 204. Nagao M, Okamoto A, Yamada K, Hasegawa T, Hasegawa Y, Ohta M. 2009. Variations in amount of TSST-1 produced by clinical methicillin resistant Staphylococcus aureus (MRSA) isolates and allelic variation in accessory gene regulator (agr) locus. BMC Microbiol. 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Robert J, Tristan A, Cavalie L, Decousser JW, Bes M, Etienne J, Laurent F. 2011. Panton-Valentine leukocidin-positive and toxic shock syndrome toxin 1-positive methicillin-resistant Staphylococcus aureus: a French multicenter prospective study in 2008. Antimicrob. Agents Chemother. 55:1734–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Centers for Disease Control 2003. Methicillin-resistant Staphylococcus aureus infections in correctional facilities—Georgia, California, and Texas, 2001-2003. MMWR Morb. Mortal. Wkly. Rep. 52:992–996 [PubMed] [Google Scholar]
- 207. Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl T, Ticehurst JR, Carroll K, Thomas DL, Nuermberger E, Bartlett JG. 2005. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin. Infect. Dis. 40:100–107 [DOI] [PubMed] [Google Scholar]
- 208. Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739 [DOI] [PubMed] [Google Scholar]
- 209. Vugia DJ, Peterson CL, Meyers HB, Kim KS, Arrieta A, Schlievert PM, Kaplan EL, Werner SB. 1996. Invasive group A streptococcal infections in children with varicella in Southern California. Pediatr. Infect. Dis. J. 15:146–150 [DOI] [PubMed] [Google Scholar]
- 210. Bradley JS, Schlievert PM, Sample TG., Jr 1991. Streptococcal toxic shock-like syndrome as a complication of varicella. Pediatr. Infect. Dis. J. 10:77–79 [DOI] [PubMed] [Google Scholar]
- 211. Davies HD, McGeer A, Schwartz B, Green K, Cann D, Simor AE, Low DE. 1996. Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N. Engl. J. Med. 335:547–554 [DOI] [PubMed] [Google Scholar]
- 212. Hoge CW, Schwartz B, Talkington DF, Breiman RF, MacNeill EM, Englender SJ. 1993. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. A retrospective population-based study. JAMA 269:384–389 [PubMed] [Google Scholar]
- 213. Demers B, Simor AE, Vellend H, Schlievert PM, Byrne S, Jamieson F, Walmsley S, Low DE. 1993. Severe invasive group A streptococcal infections in Ontario, Canada: 1987-1991. Clin. Infect. Dis. 16:792–800 [DOI] [PubMed] [Google Scholar]
- 214. Schlievert PM, Kotb MY, Stevens DL. 2000. Streptococcal superantigens: streptococcal toxic shock syndrome. In Cunningham MW, Fujinami RS. (ed), Effects of microbes on the immune system. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 215. Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, Tang AW, Phung TO, Spellberg B. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445–1453 [DOI] [PubMed] [Google Scholar]
- 216. Regev A, Weinberger M, Fishman M, Samra Z, Pitlik SD. 1998. Necrotizing fasciitis caused by Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 17:101–103 [DOI] [PubMed] [Google Scholar]
- 217. Birkhaug KE. 1925. Studies in scarlet fever. II. Studies on the use of convalescent scarlet fever serum and Dochez scarlatinal antistreptococcic serum for the treatment of scarlet fever. Bull. Johns Hopkins Hosp. 36:134–171 [Google Scholar]
- 218. Cleary PP, Kaplan EL, Handley JP, Wlazlo A, Kim MH, Hauser AR, Schlievert PM. 1992. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 339:518–521 [DOI] [PubMed] [Google Scholar]
- 219. Belani K, Schlievert PM, Kaplan EL, Ferrieri P. 1991. Association of exotoxin-producing group A streptococci and severe disease in children. Pediatr. Infect. Dis. J. 10:351–354 [DOI] [PubMed] [Google Scholar]
- 220. Cavalieri SJ, Allais JM, Schlievert PM, Dworzack DL, Clark RB. 1989. Group A streptococcal peritonitis in a patient undergoing continuous ambulatory peritoneal dialysis. Am. J. Med. 86:249–250 [DOI] [PubMed] [Google Scholar]
- 221. Goshorn SC, Schlievert PM. 1989. Bacteriophage association of streptococcal pyrogenic exotoxin type C. J. Bacteriol. 171:3068–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kaplan EL, Hill HR. 1987. Return of rheumatic fever: consequences, implications, and needs. J. Pediatr. 111:244–246 [DOI] [PubMed] [Google Scholar]
- 223. Veasy LG, Tani LY, Daly JA, Korgenski K, Miner L, Bale J, Kaplan EL, Musser JM, Hill HR. 2004. Temporal association of the appearance of mucoid strains of Streptococcus pyogenes with a continuing high incidence of rheumatic fever in Utah. Pediatrics 113:e168–172 [DOI] [PubMed] [Google Scholar]
- 224. Hauser AR, Stevens DL, Kaplan EL, Schlievert PM. 1991. Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J. Clin. Microbiol. 29:1562–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Kapur V, Nelson K, Schlievert PM, Selander RK, Musser JM. 1992. Molecular population genetic evidence of horizontal spread of two alleles of the pyrogenic exotoxin C gene (speC) among pathogenic clones of Streptococcus pyogenes. Infect. Immun. 60:3513–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Norrby-Teglund A, Thulin P, Gan BS, Kotb M, McGeer A, Andersson J, Low DE. 2001. Evidence for superantigen involvement in severe group a streptococcal tissue infections. J. Infect. Dis. 184:853–860 [DOI] [PubMed] [Google Scholar]
- 227. Carroll RK, Musser JM. 2011. From transcription to activation: how group A streptococcus, the flesh-eating pathogen, regulates SpeB cysteine protease production. Mol. Microbiol. 81:588–601 [DOI] [PubMed] [Google Scholar]
- 228. Lee PK, Schlievert PM. 1989. Quantification and toxicity of group A streptococcal pyrogenic exotoxins in an animal model of toxic shock syndrome-like illness. J. Clin. Microbiol. 27:1890–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Parsonnet J, Gillis ZA, Richter AG, Pier GB. 1987. A rabbit model of toxic shock syndrome that uses a constant, subcutaneous infusion of toxic shock syndrome toxin 1. Infect. Immun. 55:1070–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Silverstein R. 2004. d-Galactosamine lethality model: scope and limitations. J. Endotoxin Res. 10:147–162 [DOI] [PubMed] [Google Scholar]
- 231. Strandberg KL, Rotschafer JH, Vetter SM, Buonpane RA, Kranz DM, Schlievert PM. 2010. Staphylococcal superantigens cause lethal pulmonary disease in rabbits. J. Infect. Dis. 202:1690–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. de Azavedo JC, Foster TJ, Hartigan PJ, Arbuthnott JP, O'Reilly M, Kreiswirth BN, Novick RP. 1985. Expression of the cloned toxic shock syndrome toxin 1 gene (tst) in vivo with a rabbit uterine model. Infect. Immun. 50:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Nooh MM, El-Gengehi N, Kansal R, David CS, Kotb M. 2007. HLA transgenic mice provide evidence for a direct and dominant role of HLA class II variation in modulating the severity of streptococcal sepsis. J. Immunol. 178:3076–3083 [DOI] [PubMed] [Google Scholar]
- 234. Rajagopalan G, Sen MM, David CS. 2004. In vitro and in vivo evaluation of staphylococcal superantigen peptide antagonists. Infect. Immun. 72:6733–6737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Le Loir Y, Baron F, Gautier M. 2003. Staphylococcus aureus and food poisoning. Genet. Mol. Res. 2:63–76 [PubMed] [Google Scholar]
- 236. Hu DL, Omoe K, Shimoda Y, Nakane A, Shinagawa K. 2003. Induction of emetic response to staphylococcal enterotoxins in the house musk shrew (Suncus murinus). Infect. Immun. 71:567–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Hu DL, Omoe K, Shimura H, Ono K, Sugii S, Shinagawa K. 1999. Emesis in the shrew mouse (Suncus murinus) induced by peroral and intraperitoneal administration of staphylococcal enterotoxin A. J. Food Prot. 62:1350–1353 [DOI] [PubMed] [Google Scholar]
- 238. Hu DL, Suga S, Omoe K, Abe Y, Shinagawa K, Wakui M, Nakane A. 2005. Staphylococcal enterotoxin A modulates intracellular Ca2+ signal pathway in human intestinal epithelial cells. FEBS Lett. 579:4407–4412 [DOI] [PubMed] [Google Scholar]
- 239. Hu DL, Zhu G, Mori F, Omoe K, Okada M, Wakabayashi K, Kaneko S, Shinagawa K, Nakane A. 2007. Staphylococcal enterotoxin induces emesis through increasing serotonin release in intestine and it is downregulated by cannabinoid receptor 1. Cell. Microbiol. 9:2267–2277 [DOI] [PubMed] [Google Scholar]
- 240. Sugiyama H, Hayama T. 1965. Abdominal viscera as site of emetic action for staphylococcal enterotoxin in the monkey. J. Infect. Dis. 115:330–336 [DOI] [PubMed] [Google Scholar]
- 241. Hennekinne JA, De Buyser ML, Dragacci S. 2012. Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol. Rev. 36:815–836 [DOI] [PubMed] [Google Scholar]
- 242. Langmuir AD, Worthen TD, Solomon J, Ray CG, Petersen E. 1985. The Thucydides syndrome. A new hypothesis for the cause of the plague of Athens. N. Engl. J. Med. 313:1027–1030 [DOI] [PubMed] [Google Scholar]
- 243. Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 13:1405–1406 [DOI] [PubMed] [Google Scholar]
- 244. Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. 2008. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J. Infect. Dis. 198:1166–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Bubeck Wardenburg J, Schneewind O. 2008. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 205:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, Deleo FR. 2011. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J. Infect. Dis. 204:937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Aubert V, Schneeberger D, Sauty A, Winter J, Sperisen P, Aubert JD, Spertini F. 2000. Induction of tumor necrosis factor alpha and interleukin-8 gene expression in bronchial epithelial cells by toxic shock syndrome toxin 1. Infect. Immun. 68:120–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Spaulding AR, Satterwhite EA, Lin YC, Chuang-Smith ON, Frank KL, Merriman JA, Schaefers MM, Yarwood JM, Peterson ML, Schlievert PM. 2012. Differential ability of Staphylococcus aureus to cause infective endocarditis and lethal sepsis in rabbits. Front. Cell Infect. Microbiol. 2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Bashore TM, Cabell C, Fowler V., Jr 2006. Update on infective endocarditis. Curr. Probl. Cardiol. 31:274–352 [DOI] [PubMed] [Google Scholar]
- 250. Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH, Barriere S, Woods CW, Chu VH, Marin M, Bukovski S, Garcia P, Corey GR, Korman T, Doco-Lecompte T, Murdoch DR, Reller LB, Fowler VG., Jr 2011. Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J. Infect. Dis. 204:704–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Pragman AA, Yarwood JM, Tripp TJ, Schlievert PM. 2004. Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J. Bacteriol. 186:2430–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Mattis DM, Spaulding AR, Chuang-Smith ON, Sundberg EJ, Schlievert PM, Kranz DM. 2013. Engineering a soluble high-affinity receptor domain that neutralizes staphylococcal enterotoxin C in rabbit models of disease. Protein Eng. Des. Sel. 26:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Dauwalder O, Thomas D, Ferry T, Debard AL, Badiou C, Vandenesch F, Etienne J, Lina G, Monneret G. 2006. Comparative inflammatory properties of staphylococcal superantigenic enterotoxins SEA and SEG: implications for septic shock. J. Leukoc. Biol. 80:753–758 [DOI] [PubMed] [Google Scholar]
- 254. Ferry T, Thomas D, Genestier AL, Bes M, Lina G, Vandenesch F, Etienne J. 2005. Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clin. Infect. Dis. 41:771–777 [DOI] [PubMed] [Google Scholar]
- 255. Becker K, Friedrich AW, Lubritz G, Weilert M, Peters G, Von Eiff C. 2003. Prevalence of genes encoding pyrogenic toxin superantigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. J. Clin. Microbiol. 41:1434–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Boguniewicz M, Leung DY. 2011. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol. Rev. 242:233–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Guttman-Yassky E, Nograles KE, Krueger JG. 2011. Contrasting pathogenesis of atopic dermatitis and psoriasis. I. Clinical and pathologic concepts. J. Allergy Clin. Immunol. 127:1110–1118 [DOI] [PubMed] [Google Scholar]
- 258. Laouini D, Kawamoto S, Yalcindag A, Bryce P, Mizoguchi E, Oettgen H, Geha RS. 2003. Epicutaneous sensitization with superantigen induces allergic skin inflammation. J. Allergy Clin. Immunol. 112:981–987 [DOI] [PubMed] [Google Scholar]
- 259. Kawakami Y, Tomimori Y, Yumoto K, Hasegawa S, Ando T, Tagaya Y, Crotty S, Kawakami T. 2009. Inhibition of NK cell activity by IL-17 allows vaccinia virus to induce severe skin lesions in a mouse model of eczema vaccinatum. J. Exp. Med. 206:1219–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Skov L, Olsen JV, Giorno R, Schlievert PM, Baadsgaard O, Leung DY. 2000. Application of staphylococcal enterotoxin B on normal and atopic skin induces up-regulation of T cells by a superantigen-mediated mechanism. J. Allergy Clin. Immunol. 105:820–826 [DOI] [PubMed] [Google Scholar]
- 261. Brauweiler AM, Bin L, Kim BE, Oyoshi MK, Geha RS, Goleva E, Leung DYM. 2013. Filaggrin dependent secretion of sphingomyelinase protects against staphylococcal alpha toxin induced keratinocyte death. J. Allegy Clin. Immunol. 131:421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347:1151–1160 [DOI] [PubMed] [Google Scholar]
- 263. Broccardo CJ, Mahaffey S, Schwarz J, Wruck L, David G, Schlievert PM, Reisdorph NA, Leung DY. 2011. Comparative proteomic profiling of patients with atopic dermatitis based on history of eczema herpeticum infection and Staphylococcus aureus colonization. J. Allergy Clin. Immunol. 127:186–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Margolis DJ, Apter AJ, Gupta J, Hoffstad O, Papadopoulos M, Campbell LE, Sandilands A, McLean WH, Rebbeck TR, Mitra N. 2012. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J. Allergy Clin. Immunol. 130:912–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Irvine AD, McLean WH, Leung DY. 2011. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 365:1315–1327 [DOI] [PubMed] [Google Scholar]
- 266. Howell MD, Gao P, Kim BE, Lesley LJ, Streib JE, Taylor PA, Zaccaro DJ, Boguniewicz M, Beck LA, Hanifin JM, Schneider LC, Hata TR, Gallo RL, Kaplan MH, Barnes KC, Leung DY. 2011. The signal transducer and activator of transcription 6 gene (STAT6) increases the propensity of patients with atopic dermatitis toward disseminated viral skin infections. J. Allergy Clin. Immunol. 128:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, Mitsui H, Cardinale I, de Guzman Strong C, Krueger JG, Guttman-Yassky E. 2012. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 130:1344–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Ziegler SF. 2012. Thymic stromal lymphopoietin and allergic disease. J. Allergy Clin. Immunol. 130:845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Nakajima S, Igyarto BZ, Honda T, Egawa G, Otsuka A, Hara-Chikuma M, Watanabe N, Ziegler SF, Tomura M, Inaba K, Miyachi Y, Kaplan DH, Kabashima K. 2012. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J. Allergy Clin. Immunol. 129:1048–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Kawasaki H, Nagao K, Kubo A, Hata T, Shimizu A, Mizuno H, Yamada T, Amagai M. 2012. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J. Allergy Clin. Immunol. 129:1538–1546 [DOI] [PubMed] [Google Scholar]
- 271. Miajlovic H, Fallon PG, Irvine AD, Foster TJ. 2010. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J. Allergy Clin. Immunol. 126:1184–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Bin L, Kim BE, Brauweiler A, Goleva E, Streib J, Ji Y, Schlievert PM, Leung DY. 2012. Staphylococcus aureus alpha-toxin modulates skin host response to viral infection. J. Allergy Clin. Immunol. 130:683–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Beck LA, Boguniewicz M, Hata T, Schneider LC, Hanifin J, Gallo R, Paller AS, Lieff S, Reese J, Zaccaro D, Milgrom H, Barnes KC, Leung DY. 2009. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J. Allergy Clin. Immunol. 124:260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Kisich KO, Carspecken CW, Fieve S, Boguniewicz M, Leung DY. 2008. Defective killing of Staphylococcus aureus in atopic dermatitis is associated with reduced mobilization of human beta-defensin-3. J. Allergy Clin. Immunol. 122:62–68 [DOI] [PubMed] [Google Scholar]
- 275. Boguniewicz M, Leung DY. 2010. Recent insights into atopic dermatitis and implications for management of infectious complications. J. Allergy Clin. Immunol. 125:4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Herz U, Schnoy N, Borelli S, Weigl L, Kasbohrer U, Daser A, Wahn U, Kottgen E, Renz H. 1998. A human-SCID mouse model for allergic immune response bacterial superantigen enhances skin inflammation and suppresses IgE production. J. Invest. Dermatol. 110:224–231 [DOI] [PubMed] [Google Scholar]
- 277. Leung DY, Harbeck R, Bina P, Reiser RF, Yang E, Norris DA, Hanifin JM, Sampson HA. 1993. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J. Clin. Invest. 92:1374–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Bunikowski R, Mielke M, Skarabis H, Herz U, Bergmann RL, Wahn U, Renz H. 1999. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J. Allergy Clin. Immunol. 103:119–124 [DOI] [PubMed] [Google Scholar]
- 279. Leung DY, Gately M, Trumble A, Ferguson-Darnell B, Schlievert PM, Picker LJ. 1995. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J. Exp. Med. 181:747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Ou LS, Goleva E, Hall C, Leung DY. 2004. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J. Allergy Clin. Immunol. 113:756–763 [DOI] [PubMed] [Google Scholar]
- 281. Leung DY, Hanifin JM, Pariser DM, Barber KA, Langley RG, Schlievert PM, Abrams B, Hultsch T. 2009. Effects of pimecrolimus cream 1% in the treatment of patients with atopic dermatitis who demonstrate a clinical insensitivity to topical corticosteroids: a randomized, multicentre vehicle-controlled trial. Br. J. Dermatol. 161:435–443 [DOI] [PubMed] [Google Scholar]
- 282. Cornelissen C, Marquardt Y, Czaja K, Wenzel J, Frank J, Luscher-Firzlaff J, Luscher B, Baron JM. 2012. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J. Allergy Clin. Immunol. 129:426–433 [DOI] [PubMed] [Google Scholar]
- 283. Guttman-Yassky E, Nograles KE, Krueger JG. 2011. Contrasting pathogenesis of atopic dermatitis and psoriasis. II. Immune cell subsets and therapeutic concepts. J. Allergy Clin. Immunol. 127:1420–1432 [DOI] [PubMed] [Google Scholar]
- 284. Baker BS, Bokth S, Powles A, Garioch JJ, Lewis H, Valdimarsson H, Fry L. 1993. Group A streptococcal antigen-specific T lymphocytes in guttate psoriatic lesions. Br. J. Dermatol. 128:493–499 [DOI] [PubMed] [Google Scholar]
- 285. Schlievert PM, Gray ED. 1989. Group A streptococcal pyrogenic exotoxin (scarlet fever toxin) type A and blastogen A are the same protein. Infect. Immun. 57:1865–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Kim SW, Grant JE, Kim SI, Swanson TA, Bernstein GA, Jaszcz WB, Williams KA, Schlievert PM. 2004. A possible association of recurrent streptococcal infections and acute onset of obsessive-compulsive disorder. J. Neuropsychiatry Clin. Neurosci. 16:252–260 [DOI] [PubMed] [Google Scholar]
- 287. Schwab JH, Brown RR, Anderle SK, Schlievert PM. 1993. Superantigen can reactivate bacterial cell wall-induced arthritis. J. Immunol. 150:4151–4159 [PubMed] [Google Scholar]
- 288. Batsford SR, Mezzano S, Mihatsch M, Schiltz E, Rodriguez-Iturbe B. 2005. Is the nephritogenic antigen in post-streptococcal glomerulonephritis pyrogenic exotoxin B (SPE B) or GAPDH? Kidney Int. 68:1120–1129 [DOI] [PubMed] [Google Scholar]
- 289. Parra G, Rodriguez-Iturbe B, Batsford S, Vogt A, Mezzano S, Olavarria F, Exeni R, Laso M, Orta N. 1998. Antibody to streptococcal zymogen in the serum of patients with acute glomerulonephritis: a multicentric study. Kidney Int. 54:509–517 [DOI] [PubMed] [Google Scholar]
- 290. Cu GA, Mezzano S, Bannan JD, Zabriskie JB. 1998. Immunohistochemical and serological evidence for the role of streptococcal proteinase in acute post-streptococcal glomerulonephritis. Kidney Int. 54:819–826 [DOI] [PubMed] [Google Scholar]
- 291. Fast DJ, Schlievert PM, Nelson RD. 1989. Toxic shock syndrome-associated staphylococcal and streptococcal pyrogenic toxins are potent inducers of tumor necrosis factor production. Infect. Immun. 57:291–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Nyberg SL, Schlievert PM, Saul W, Johnson JR, Ferrieri P, Watkins VS. 1995. Successful management of a serious group A streptococcal infection during the third trimester of pregnancy. Clin. Infect. Dis. 21:1058–1059 [DOI] [PubMed] [Google Scholar]
- 293. Schlievert PM, Kelly JA. 1984. Clindamycin-induced suppression of toxic-shock syndrome-associated exotoxin production. J. Infect. Dis. 149:471. [DOI] [PubMed] [Google Scholar]
- 294. Stevens DL, Bryant AE, Hackett SP. 1995. Antibiotic effects on bacterial viability, toxin production, and host response. Clin. Infect. Dis. 20(Suppl. 2):S154–S157 [DOI] [PubMed] [Google Scholar]
- 295. Stevens DL, Gibbons AE, Bergstrom R, Winn V. 1988. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J. Infect. Dis. 158:23–28 [DOI] [PubMed] [Google Scholar]
- 296. Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. 2007. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 195:202–211 [DOI] [PubMed] [Google Scholar]
- 297. Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342–1347 [DOI] [PubMed] [Google Scholar]
- 298. Schlievert PM, Blomster DA, Kelly JA. 1984. Toxic shock syndrome Staphylococcus aureus: effect of tampons on toxic shock syndrome toxin 1 production. Obstet. Gynecol. 64:666–671 [PubMed] [Google Scholar]
- 299. Schlievert PM, Deringer JR, Kim MH, Projan SJ, Novick RP. 1992. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob. Agents Chemother. 36:626–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Projan SJ, Brown-Skrobot S, Schlievert PM, Vandenesch F, Novick RP. 1994. Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J. Bacteriol. 176:4204–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Strandberg KL, Peterson ML, Schaefers MM, Case LC, Pack MC, Chase DJ, Schlievert PM. 2009. Reduction in Staphylococcus aureus growth and exotoxin production and in vaginal interleukin 8 levels due to glycerol monolaurate in tampons. Clin. Infect. Dis. 49:1711–1717 [DOI] [PubMed] [Google Scholar]
- 302. Peterson ML, Schlievert PM. 2006. Glycerol monolaurate inhibits the effects of gram-positive select agents on eukaryotic cells. Biochemistry 45:2387–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Witcher KJ, Novick RP, Schlievert PM. 1996. Modulation of immune cell proliferation by glycerol monolaurate. Clin. Diagn. Lab. Immunol. 3:10–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Schlievert PM, Strandberg KL, Brosnahan AJ, Peterson ML, Pambuccian SE, Nephew KR, Brunner KG, Schultz-Darken NJ, Haase AT. 2008. Glycerol monolaurate does not alter rhesus macaque (Macaca mulatta) vaginal lactobacilli and is safe for chronic use. Antimicrob. Agents Chemother. 52:4448–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Strandberg KL, Peterson ML, Lin YC, Pack MC, Chase DJ, Schlievert PM. 2010. Glycerol monolaurate inhibits Candida and Gardnerella vaginalis in vitro and in vivo but not Lactobacillus. Antimicrob. Agents Chemother. 54:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Schaefers MM, Breshears LM, Anderson MJ, Lin YC, Grill AE, Panyam J, Southern PJ, Schlievert PM, Peterson ML. 2012. Epithelial proinflammatory response and curcumin-mediated protection from staphylococcal toxic shock syndrome toxin-1. PLoS One 7:e32813. 10.1371/journal.pone.0032813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Lin YC, Schlievert PM, Anderson MJ, Fair CL, Schaefers MM, Muthyala R, Peterson ML. 2009. Glycerol monolaurate and dodecylglycerol effects on Staphylococcus aureus and toxic shock syndrome toxin-1 in vitro and in vivo. PLoS One 4:e7499. 10.1371/journal.pone.0007499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. McNamara PJ, Syverson RE, Milligan-Myhre K, Frolova O, Schroeder S, Kidder J, Hoang T, Proctor RA. 2009. Surfactants, aromatic and isoprenoid compounds, and fatty acid biosynthesis inhibitors suppress Staphylococcus aureus production of toxic shock syndrome toxin 1. Antimicrob. Agents Chemother. 53:1898–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Schlievert PM. 2007. Chitosan malate inhibits growth and exotoxin production of toxic shock syndrome-inducing Staphylococcus aureus strains and group A streptococci. Antimicrob. Agents Chemother. 51:3056–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Meissner HC, Schlievert PM, Leung DY. 1994. Mechanisms of immunoglobulin action: observations on Kawasaki syndrome and RSV prophylaxis. Immunol. Rev. 139:109–123 [DOI] [PubMed] [Google Scholar]
- 311. Schlievert PM. 2001. Use of intravenous immunoglobulin in the treatment of staphylococcal and streptococcal toxic shock syndromes and related illnesses. J. Allergy Clin. Immunol. 108:107S–110S [DOI] [PubMed] [Google Scholar]
- 312. Norrby-Teglund A, Basma H, Andersson J, McGeer A, Low DE, Kotb M. 1998. Varying titers of neutralizing antibodies to streptococcal superantigens in different preparations of normal polyspecific immunoglobulin G: implications for therapeutic efficacy. Clin. Infect. Dis. 26:631–638 [DOI] [PubMed] [Google Scholar]
- 313. Norrby-Teglund A, Kaul R, Low DE, McGeer A, Andersson J, Andersson U, Kotb M. 1996. Evidence for the presence of streptococcal-superantigen-neutralizing antibodies in normal polyspecific immunoglobulin G. Infect. Immun. 64:5395–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Varshney AK, Wang X, Cook E, Dutta K, Scharff MD, Goger MJ, Fries BC. 2011. Generation, characterization, and epitope mapping of neutralizing and protective monoclonal antibodies against staphylococcal enterotoxin B-induced lethal shock. J. Biol. Chem. 286:9737–9747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Singh PK, Agrawal R, Kamboj DV, Gupta G, Boopathi M, Goel AK, Singh L. 2010. Construction of a single-chain variable-fragment antibody against the superantigen staphylococcal enterotoxin B. Appl. Environ. Microbiol. 76:8184–8191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Yang X, Buonpane RA, Moza B, Rahman AK, Wang N, Schlievert PM, McCormick JK, Sundberg EJ, Kranz DM. 2008. Neutralization of multiple staphylococcal superantigens by a single-chain protein consisting of affinity-matured, variable domain repeats. J. Infect. Dis. 198:344–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Wang N, Mattis DM, Sundberg EJ, Schlievert PM, Kranz DM. 2010. A single, engineered protein therapeutic agent neutralizes exotoxins from both Staphylococcus aureus and Streptococcus pyogenes. Clin. Vaccine Immunol. 17:1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. McCormick JK, Tripp TJ, Dunny GM, Schlievert PM. 2002. Formation of vegetations during infective endocarditis excludes binding of bacterial-specific host antibodies to Enterococcus faecalis. J. Infect. Dis. 185:994–997 [DOI] [PubMed] [Google Scholar]
- 319. Schlievert PM, Chuang-Smith ON, Peterson ML, Cook LC, Dunny GM. 2011. Enterococcus faecalis endocarditis severity in rabbits is reduced by IgG Fabs interfering with aggregation substance. PLoS One 5:e13194. 10.1371/journal.pone.0013194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Roggiani M, Stoehr JA, Leonard BA, Schlievert PM. 1997. Analysis of toxicity of streptococcal pyrogenic exotoxin A mutants. Infect. Immun. 65:2868–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Roggiani M, Stoehr JA, Olmsted SB, Matsuka YV, Pillai S, Ohlendorf DH, Schlievert PM. 2000. Toxoids of streptococcal pyrogenic exotoxin A are protective in rabbit models of streptococcal toxic shock syndrome. Infect. Immun. 68:5011–5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Bauer MJ, Georgousakis MM, Vu T, Henningham A, Hofmann A, Rettel M, Hafner LM, Sriprakash KS, McMillan DJ. 2012. Evaluation of novel Streptococcus pyogenes vaccine candidates incorporating multiple conserved sequences from the C-repeat region of the M-protein. Vaccine 30:2197–2205 [DOI] [PubMed] [Google Scholar]
- 323. Dale JB, Penfound TA, Chiang EY, Walton WJ. 2011. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine 29:8175–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Penfound TA, Chiang EY, Ahmed EA, Dale JB. 2010. Protective efficacy of group A streptococcal vaccines containing type-specific and conserved M protein epitopes. Vaccine 28:5017–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Cleary PP, Matsuka YV, Huynh T, Lam H, Olmsted SB. 2004. Immunization with C5a peptidase from either group A or B streptococci enhances clearance of group A streptococci from intranasally infected mice. Vaccine 22:4332–4341 [DOI] [PubMed] [Google Scholar]
- 326. Pereira N, Edlind TD, Schlievert PM, Nyirjesy P. 2013. Vaginal toxic shock reaction triggering desquamative inflammatory vaginitis. J. Low. Genit. Tract. Dis. 17:88–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Schlievert PM. 1986. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet i:1149–1150 [DOI] [PubMed] [Google Scholar]
- 328. Hall M, Hoyt L, Ferrieri P, Schlievert PM, Jenson HB. 1999. Kawasaki syndrome-like illness associated with infection caused by enterotoxin B-secreting Staphylococcus aureus. Clin. Infect. Dis. 29:586–589 [DOI] [PubMed] [Google Scholar]
- 329. Leung DY, Meissner HC, Fulton DR, Murray DL, Kotzin BL, Schlievert PM. 1993. Toxic shock syndrome toxin-secreting Staphylococcus aureus in Kawasaki syndrome. Lancet 342:1385–1388 [DOI] [PubMed] [Google Scholar]
- 330. Leung DY, Meissner HC, Fulton DR, Quimby F, Schlievert PM. 1995. Superantigens in Kawasaki syndrome. Clin. Immunol. Immunopathol. 77:119–126 [DOI] [PubMed] [Google Scholar]
- 331. Leung DY, Meissner HC, Shulman ST, Mason WH, Gerber MA, Glode MP, Myones BL, Wheeler JG, Ruthazer R, Schlievert PM. 2002. Prevalence of superantigen-secreting bacteria in patients with Kawasaki disease. J. Pediatr. 140:742–746 [DOI] [PubMed] [Google Scholar]
- 332. Leung DY, Sullivan KE, Brown-Whitehorn TF, Fehringer AP, Allen S, Finkel TH, Washington RL, Makida R, Schlievert PM. 1997. Association of toxic shock syndrome toxin-secreting and exfoliative toxin-secreting Staphylococcus aureus with Kawasaki syndrome complicated by coronary artery disease. Pediatr. Res. 42:268–272 [DOI] [PubMed] [Google Scholar]
- 333. Kikuchi K, Takahashi N, Piao C, Totsuka K, Nishida H, Uchiyama T. 2003. Molecular epidemiology of methicillin-resistant Staphylococcus aureus strains causing neonatal toxic shock syndrome-like exanthematous disease in neonatal and perinatal wards. J. Clin. Microbiol. 41:3001–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Takahashi N. 2003. Neonatal toxic shock syndrome-like exanthematous disease (NTED). Pediatr. Int. 45:233–237 [DOI] [PubMed] [Google Scholar]
- 335. Schlievert PM, Assimacopoulos AP, Cleary PP. 1996. Severe invasive group A streptococcal disease: clinical description and mechanisms of pathogenesis. J. Lab. Clin. Med. 127:13–22 [DOI] [PubMed] [Google Scholar]
- 336. Aziz RK, Pabst MJ, Jeng A, Kansal R, Low DE, Nizet V, Kotb M. 2004. Invasive M1T1 group A streptococcus undergoes a phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol. Microbiol. 51:123–134 [DOI] [PubMed] [Google Scholar]
- 337. Dhodapkar K, Corbacioglu S, Chang MW, Karpatkin M, DiMichele D. 2000. Purpura fulminans caused by group A beta-hemolytic streptococcus sepsis. J. Pediatr. 137:562–567 [DOI] [PubMed] [Google Scholar]
- 338. Matsubara K, Fukaya T. 2007. The role of superantigens of group A Streptococcus and Staphylococcus aureus in Kawasaki disease. Curr. Opin. Infect. Dis. 20:298–303 [DOI] [PubMed] [Google Scholar]
- 339. McCormick JK, Schlievert PM. 2003. Expression, purification, and detection of novel streptococcal superantigens. Methods Mol. Biol. 214:33–43 [DOI] [PubMed] [Google Scholar]
- 340. Bernstein JM, Ballow M, Schlievert PM, Rich G, Allen C, Dryja D. 2003. A superantigen hypothesis for the pathogenesis of chronic hyperplastic sinusitis with massive nasal polyposis. Am. J. Rhinol. 17:321–326 [PubMed] [Google Scholar]
- 341. Cu GA, Mezzano S, Zabriskie JB. 1997. Role of streptococcal proteinase in acute post-streptococcal glomerulonephritis. Adv. Exp. Med. Biol. 418:137–140 [DOI] [PubMed] [Google Scholar]
- 342. Manders SM, Heymann WR, Atillasoy E, Kleeman J, Schlievert PM. 1996. Recurrent toxin-mediated perineal erythema. Arch. Dermatol. 132:57–60 [PubMed] [Google Scholar]
- 343. Lindsay J. 1996. Infectious agents and sudden infant death syndrome (SIDS): an update. Mol. Med. Today 2:94–95 [DOI] [PubMed] [Google Scholar]
- 344. Newbould MJ, Malam J, McIllmurray JM, Morris JA, Telford DR, Barson AJ. 1989. Immunohistological localisation of staphylococcal toxic shock syndrome toxin (TSST-1) antigen in sudden infant death syndrome. J. Clin. Pathol. 42:935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]