Abstract
Aminopeptidase-N (APN1) and alkaline phosphatase (ALP) proteins located in the midgut epithelium of Manduca sexta have been implicated as receptors for Cry1Aa, Cry1Ab, and Cry1Ac insecticidal proteins produced by Bacillus thuringiensis subsp. kurstaki. In this study, we analyzed the roles of ALP and APN1 in the toxicity of these three Cry1A proteins. Ligand blot analysis using brush border membrane vesicles of M. sexta showed that Cry1Aa and Cry1Ab bind preferentially to ALP during early instars while binding to APN was observed after the third instar of larval development. Cry1Ac binds to APN throughout all larval development, with no apparent binding to ALP. ALP was cloned from M. sexta midgut RNA and expressed in Escherichia coli. Surface plasmon resonance binding analysis showed that recombinant ALP binds to Cry1Ac with 16-fold lower affinity than to Cry1Aa or Cry1Ab. Downregulation of APN1 and ALP expression by RNA interference (RNAi) using specific double-stranded RNA correlated with a reduction of transcript and protein levels. Toxicity analysis of the three Cry1A proteins in ALP- or APN1-silenced larvae showed that Cry1Aa relies similarly on both receptor molecules for toxicity. In contrast, RNAi experiments showed that ALP is more important than APN for Cry1Ab toxicity, while Cry1Ac relied principally on APN1. These results indicated that ALP and APN1 have a differential role in the mode of action of Cry1A toxins, suggesting that B. thuringiensis subsp. kurstaki produces different Cry1A toxins that in conjunction target diverse midgut proteins to exert their insecticidal effect.
INTRODUCTION
Cry toxins produced by Bacillus thuringiensis are pore-forming toxins that have been used worldwide in the control of insect pests in agriculture, either in transgenic crops or as spray formulations (1–3). Their mechanism of action is complex and involves several steps. In the case of lepidopteran-active Cry1A proteins, it has been proposed that protease-activated toxins first bind to highly abundant glycosylphosphatidylinositol (GPI)-anchored alkaline phosphatase (ALP) or aminopeptidase-N (APN) proteins as a mechanism to bring the toxins close to the insect midgut epithelium. Then, the toxins could bind to the cadherin protein with high affinity. This interaction induces further proteolytic cleavage of the amino-terminal end of the toxin, including the helix α-1 region of domain I, leading to toxin oligomerization (4, 5). Finally, the Cry1A oligomers gain affinity to APN or ALP and bind again to these receptors, leading to the insertion of the oligomeric structure into the membrane forming ionic pores, causing osmotic lysis of midgut epithelial cells and insect death (6–8). Although this mechanism of action is generally accepted, the role of the two GPI-anchored proteins in the toxicity of different Cry1A toxins remains to be determined (9).
B. thuringiensis subsp. kurstaki produces several Cry1A toxins, such as Cry1Aa, Cry1Ab, and Cry1Ac, and also the less related Cry2Aa. Cadherin and APN1 Cry1A receptor molecules have been cloned, heterologously expressed, and shown to bind Cry1A proteins by ligand blot analysis (6). Although functional proof for cadherin as a Cry1A protein receptor has been demonstrated by cytolysis of insect cells expressing Manduca sexta cadherin protein and by gene silencing of cadherin expression by RNA interference (RNAi) (10–13), a similar characterization of APN-Cry1A interaction has not yet been done. However, compelling evidence that lepidopteran APN is a functional receptor for Cry1A comes from studies by Gill and Ellar, who expressed M. sexta APN1 in Drosophila melanogaster and demonstrated that the transgenic flies acquired sensitivity to Cry1Ac (14). Also, the functional relevance of APN of Spodoptera litura for Cry1C toxicity was demonstrated by an APN silencing strategy using RNAi (15). Nevertheless, some Cry1Ac mutants affected in binding to APN1 showed a marginal effect on toxicity against M. sexta larvae, suggesting that other Cry1Ac binding proteins may be involved in Cry1Ac toxicity (16). In the case of Bombyx mori, it was shown that anticadherin antibodies protected detached midgut cells from the toxic effects of Cry1Aa, in contrast to anti-APN antibodies, which had no effect on the toxicity of this toxin (17). These apparently contradictory data could be explained if an additional secondary receptor could play the same role as APN in midgut cells. In this regard, a GPI-anchored ALP was also identified as a receptor for Cry1Ac toxin in different lepidopteran species (18). Recently, we showed that a mutant of Cry1Ab toxin affected in binding to ALP was not toxic to M. sexta larvae, supporting the role of this receptor in toxicity (1, 7).
Cry1Aa, Cry1Ab, and Cry1Ac exhibit similar toxicities toward M. sexta larvae and show high degrees of sequence and presumed structural identities (19). The three toxins share almost identical domain I amino acid sequences, with 97 to 98% identity, but show differences in amino acid sequences in domains II and III. Cry1Aa and Cry1Ab share 97% identity in the amino acid sequence of domain III but only 71% domain II sequence identity. Specifically, the domain II loop regions, which are involved in receptor interaction, show low amino acid similarity. In contrast, Cry1Ac and Cry1Ab share 98% identity in the amino acid sequence of domain II but only 45% sequence identity in domain III. Domain I is a seven-alpha-helix bundle involved in membrane insertion, oligomerization, and pore formation, while domains II and III, principally composed of beta-sheets, are involved in receptor interaction. Interestingly, Cry1Ac domain III contains an N-acetylgalactosamine binding pocket that has been shown to be crucial for APN1 binding (16, 20). Thus, differences in amino acid sequence identities of domain II and domain III among the three Cry1A toxins could determine different capabilities of binding to the diverse receptor molecules. To determine the role of both APN1 and ALP proteins in the toxicity of the three Cry1A toxins described above, we analyzed the binding properties of recombinant expressed ALP and analyzed the effect of downregulation of ALP and APN1 gene expression on the toxicity of these Cry toxins. Our results show that ALP and APN1 have a differential role in the mode of action of Cry1A toxins from B. thuringiensis subsp. kurstaki.
MATERIALS AND METHODS
Toxin expression and proteolytic activation.
The acrystalliferous strain 407cry− (21) transformed with pHT409 (22) harboring the cry1Aa gene or pHT315-1Ab harboring the cry1Ab gene was used for Cry1Aa and Cry1Ab production. Cry1Ac was produced from the wild-type B. thuringiensis strain HD73. Crystals of proteins were produced in B. thuringiensis transformant strains grown for 3 days at 29°C in nutrient broth sporulation medium (23). The crystal inclusions were purified from sporulated B. thuringiensis cultures using discontinuous sucrose gradients (24). The crystals were solubilized in 50 mM Na2CO3-NaHCO3 buffer containing 0.02% mercaptoethanol, pH 10.5, at 37°C for 2 h. The solubilized protoxins were centrifuged for 10 min at 14,000 rpm to remove insoluble material and stored at 4°C. For proteolytic activation, the solubilized protoxins were digested with 1:50 trypsin (Sigma) at 37°C for 2 h. Protein concentration of protoxins and toxins was determined by the Bradford method using bovine serum albumin (BSA) as a standard (25).
Bioassays.
Different doses of pure crystals (from 0.1 to 50 ng/cm2) were applied onto the artificial diet surface of 24-well polystyrene plates (cell wells; Corning). One first-instar M. sexta larva was used per well, and 24 larvae were assayed per toxin concentration in three repetitions. Mortality was recorded after 7 days, and the 50% lethal concentration (LC50) was estimated by using Probit analysis (Polo-PC; LeOra Software).
BBMV preparation and Western blotting.
Brush border membrane vesicles (BBMVs) were prepared from dissected midgut tissue of each instar of M. sexta larvae by following the protocol described by Wolfersberger (26). We resolved 2.5 μg of protein of BBMV isolated from the different larval instars in 9% SDS-PAGE gels, and then proteins were electrotransferred to a polyvinylidene fluoride (PVDF) membrane from Millipore. Blots were blocked with 5% nonfat dry milk in phosphate-buffered saline (PBS)-0.1% Tween 20 (PBST) and incubated with 1:30,000 anticadherin, 1:25,000 anti-APN, or 1:5,000 anti-ALP antibodies in PBST. Subsequently, blots were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:30,000) in PBST from Santa Cruz and developed with luminol (Pierce) as indicated by the manufacturer.
Ligand blot analysis.
Cry1Aa, Cry1Ab, and Cry1Ac toxins were biotinylated using biotinamido caproate N-hydroxysuccinimide ester (Amersham Biosciences) according to the manufacturer's instructions. Membrane preparations containing 2.5 μg of protein of BBMV from each instar were resolved in 9% SDS-PAGE and transferred to PVDF membranes. After blocking of the membrane in PBS buffer supplemented with 2% bovine serum albumin (bovine serum albumin fraction V; Roche), 5 nM Cry1A biotinylated toxin was added and the membrane was incubated for 1 h at room temperature. The blots were then washed twice with PBST and overlaid with streptavidin-horseradish peroxidase (Amersham) for 1 h. After three washes with PBST, the blot was developed with luminol (Pierce) as indicated by the manufacturer.
Cloning of alkaline phosphatase and heterologous expression from M. sexta larval gut tissue.
Total RNA from second-instar larval midguts of M. sexta was extracted using an RNeasy minikit (Qiagen). The 5′ and 3′ flanking regions of the alp gene were obtained by 5′ and 3′ rapid amplification of cDNA ends (RACE) using a GeneRacer kit (Invitrogen) according to the manufacturer's instructions. Primers were designed based on a larval midgut ALP expressed sequence tag (EST) of 1,193 nucleotides (nt) generated using 454 pyrosequencing (GenBank accession no. GR922057.1) (27). The 1,600-bp PCR product was subcloned into the TOPO vector (Invitrogen) and transformed into E. coli TOP10 cells (Invitrogen), and plasmids from transformants were purified and sequenced in the DNA sequencing facility of Instituto de Biotecnología-UNAM. M. sexta alkaline phosphatase (MsALP) was subcloned into the pET22b expression vector (Novagen) using PCR primers designed with EcoRI and HindIII restriction sites at the 5′ and 3′ ends, respectively. pET22b plasmid was selected since it adds a His tag at the C-terminal end of the protein. To ensure proper protein expression, the coding frame in transformants was verified by DNA sequencing in the forward and reverse directions.
For protein expression and purification of MsALP, the pET22b plasmid with MsALP was transformed into E. coli strain BL21(DE3). Transformants were grown overnight with constant agitation at 37°C in 5 ml of LB broth containing 50 μg/ml of ampicillin. The following morning, 100 μl of this overnight culture was used to inoculate 100 ml of 2× TY broth (in 1 liter: 10 g Bacto yeast extract, 16 g Bacto tryptone, 5 g NaCl) supplemented with 100 μg/ml of ampicillin in a 250-ml flask. This culture was incubated at 37°C with constant agitation until an optical density at 600 nm (OD600) of 0.7 was reached, and expression of MsALP was induced by addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma) for 5 h at 30°C. After this period, 200 μl of the culture was removed and mixed with Laemmli sample buffer (28), 15-μl aliquots were separated on a 12% SDS-PAGE gel, and expression of the MsALP clone was confirmed using Coomassie blue staining. For MsALP protein purification, cells were pelleted by centrifugation (5,000 rpm for 15 min at 4°C) and the pellet was suspended in STE buffer (10 mM Tris-HCl, 1 mM EDTA, 8 M urea [pH 8]). After sonication for 5 min on ice, cell debris was eliminated by centrifugation (70,000 rpm for 30 min at 15°C), and the supernatant was subjected to affinity purification using Ni-agarose beads (Qiagen). After a washing with 35 mM imidazole in PBS buffer (pH 7.5), the recombinant protein was eluted with 250 mM imidazole and gradually dialyzed against PBS buffer. The purified proteins were separated by 12% SDS-PAGE, and concentrations were measured as described above. Purified protein was used as antigen to develop antiserum to MsALP. A New Zealand White rabbit was immunized subcutaneously three times with 1 mg of MsALP, which had been mixed with incomplete Freund's adjuvant, at 15-day intervals. The serum was recovered 40 days after the primary immunization. A similar procedure was used to obtain anticadherin and anti-APN antibodies.
Biosensor analysis of Cry1Aa, Cry1Ab, and Cry1Ac toxin affinities.
A SensiQ instrument (ICX Nomadics) was programmed to conduct surface plasmon resonance (SPR) experiments and kinetic analysis. The sensogram was recorded as a plot of binding response (resonance unit) versus time. All the sensograms were processed using the double reference method to eliminate the nonspecific binding from background contribution and buffer artifacts by subtracting signals from the reference flow cell and from buffer blank injections. Purified MsALP protein was immobilized onto a COOH sensor (ICX Nomadics) by conventional amine coupling. The running buffer for all experiments was HBS buffer, pH 7.4, containing 10 mM HEPES, 150 mM NaCl, and 0.005% (vol/vol) Tween 20. Running buffer was freshly prepared, filtered (pore size of 0.22 μm), and degassed. Serial doubling dilutions from 0 to 100 nM Cry1Aa, Cry1Ab, and Cry1Ac were injected in randomized orders, and the surface was then regenerated with a 1-min injection of 20 mM NaOH. The data for all binding proteins were analyzed and fitted to 1:1 Langmuir binding, and binding constants were calculated using SensiQ software, version B.02, supplied by ICX Nomadics.
Reverse transcription and dsRNA synthesis.
Total RNA was extracted using an RNeasy minikit (Qiagen). The quantity and quality of RNA were determined spectrophotometrically using a NanoDrop 2000 (Thermo Scientific). cDNA was synthesized from 1 μg of total RNA using a SuperScript III reverse transcriptase kit (Invitrogen Life Technologies), by following the manufacturer's instructions. The cDNA was stored at −80°C prior to further analysis. A 308-bp internal fragment of apn1 and 434 bp for alp were obtained by PCR using the primers indicated in Table 1. The truncated fragments of M. sexta apn1 and alp were subcloned into pLITMUS 28i vector (New England BioLabs) and used for the preparation of double-stranded RNA (dsRNA). dsRNA was prepared with a TranscriptAid T7 high-yield transcription kit (Fermentas, Life Sciences) according to the manufacturer's protocols. Purified dsRNA was stored at −20°C until use.
Table 1.
Primers used to amplify APN and ALP regions for dsRNA synthesis and for qPCRa
| Region | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| APN1 | CGACAACTCGAGGTCGCCGAACCCTACCAC | GTCAATGACTCTAGACTGTCCAGTCATAAC |
| ALP | GTTGTTCAAGCTTCTGGCCTGCGCGATCGCGACGGCAGAGGATGT | CCGTCCATCTAGATGACTCCGTACTTAGTCTTCACTCCAGTCAAG |
| qAPN | CGACAACTCGAGGTCGCCGAACCCTACCAC | CATCTTGCCTGAACGAGAGTCATACGGCCG |
| qALP | GTTGTTCAAGCTTCTGGCCTGCGCGATCGCGACGGCAGAGGATGT | TTTCTCCTGGAGCTTGGCGTTCAGCGCTGC |
| RPS3 | GTGTCACCCCTACCCGATCGGAGATCATCA | ATCTCAAAGACTCGGCCTGAGCGATGGCGC |
Restrictions sites used for cloning in pLITMUS 28i plasmid are underlined.
Knockdown of APN1 and ALP using RNA interference and their effect on toxicity with Cry1Aa, Cry1Ab, and Cry1Ac toxins.
Newly emerged M. sexta larvae were starved for 2 h and then fed with a single drop of 1 μl containing 4 μg dsRNA (4 μg of dsRNA/larva). Each larva was located in front of the drop, and we took care to observe that each of them ingested the complete drop. Larvae that did not consume the drop were not further analyzed. Additional larvae were fed with water without dsRNA and used as control group. After feeding, larvae were reared on a diet without toxin under standard rearing conditions for 12 h. We used a total of 72 larvae in each treatment, and three repetitions were performed.
Larvae from the control and dsRNA feed treatments were randomly divided into two groups. One group was placed on an untreated control diet, and the other group was placed on a diet containing 25 ng/cm2 of Cry1Aa, Cry1Ab, or Cry1Ac purified crystals. Individual larvae were placed into separate wells of a 24-well plate, and larval mortality was evaluated after 7 days.
Quantitative real-time PCR.
Seven days postfeeding, larvae that were not exposed to toxin were evaluated for expression of apn1 and alp. Ten larval guts from each of the control or dsRNA feed groups were dissected into RNAlater, and total RNA was isolated (RNeasy kit; Qiagen). Quantitative real-time PCR was performed on each template using primers listed in Table 1 on a LightCycler 480 instrument (Roche) using a Sybr green I detection system (Fermentas Life Sciences). Relative-fold calculations were made with duplicates for each treatment group, analyzing the rps3 (ribosomal protein S3) gene to normalize gene expression.
RESULTS
Binding of Cry1Aa, Cry1Ab, and Cry1Ac toxins to M. sexta BBMV proteins.
We determined the LC50s for Cry1Aa, Cry1Ab, and Cry1Ac toxins and found that the three toxins have similar toxicities to first-instar M. sexta larvae, although Cry1Aa showed a slightly lower toxicity, with an LC50 of 3 ng/cm2 (fiducial limits, 0.4 to 5), than Cry1Ab and Cry1Ac, which showed LC50s of 1 ng/cm2 (fiducial limits, 0.4 to 1.8) and 0.7 ng/cm2 (fiducial limits, 0.1 to 1.1), respectively.
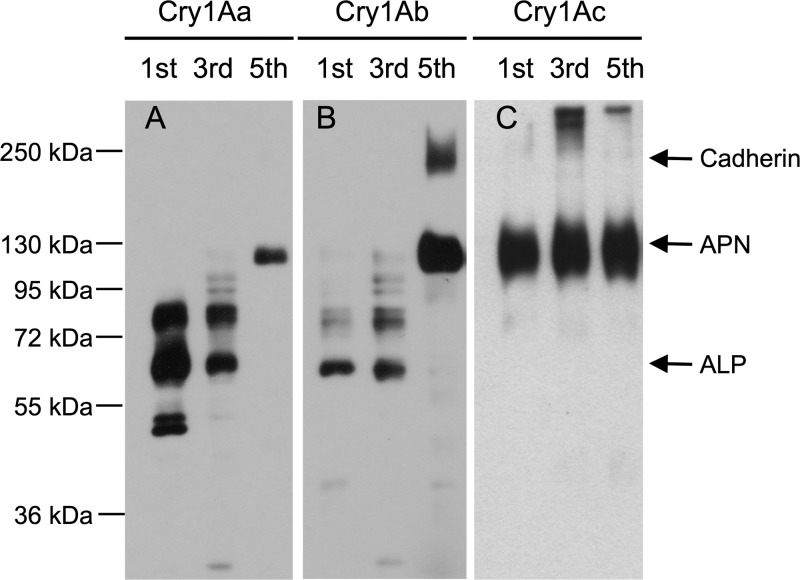
To determine the binding of the three Cry1A toxins to M. sexta BBMV proteins, ligand blot analysis of biotinylated Cry1A toxins was performed using BBMV samples obtained from first-, third-, and fifth-instar M. sexta larvae. When analyzing the binding to BBMV from the first and third instars, Cry1Aa and Cry1Ab were found to have similar binding properties (Fig. 1); both toxins bound to several proteins, where one of them could correspond to ALP protein of 65 kDa. The binding of these two toxins to BBMV prepared from fifth-instar larvae showed that both proteins bound to a 120-kDa protein that could correspond to APN1, and Cry1Ab also bound to a 210-kDa protein that could correspond to cadherin protein. In contrast, Cry1Ac toxin interacted only with APN1 in the three larval instars that were analyzed. The identity of the 65-, 120-, and 210-kDa proteins as ALP, APN1, and cadherin, respectively, was confirmed by Western blotting assays using specific polyclonal antibodies (see Fig. S1 in the supplemental material), suggesting that cadherin and APN1 expression is increased during larval development,while ALP is produced through larval development, with a higher expression during the third instar and a slightly lower expression in the last larval instar (see Fig. S1).
Fig 1.
Differential binding of Cry1A toxins to Manduca sexta BBMV proteins during larval development. Cry1A-binding proteins were revealed by ligand blot analysis of biotinylated Cry1Aa (A), Cry1Ab (B), and Cry1Ac (C) toxins using BBMV prepared from M. sexta larvae from the first, third, and fifth instars, respectively.
Binding of Cry1A toxins to recombinant expressed MsALP.
Previously, it was shown by surface plasmon resonance (SPR) binding analysis that Cry1Aa, Cry1Ab, and Cry1Ac bound to purified M. sexta APN1 protein with similar Kds (apparent affinities) ranging from 28 to 40 nM (29). To determine the rates of binding of the three Cry1A toxins to ALP receptor, we cloned a full-length ALP cDNA from RNA samples obtained from second-instar larvae where we observed binding of Cry1Ab to ALP (7). The full-length ALP clone (MsALP) revealed an open reading frame of 1,473 residues with a predicted molecular mass of 53.4 kDa and an isoelectric point of 5.08. The MsALP shared amino acid sequence identity with different insect ALPs, such as those of Bombyx mori (56%, GenBank accession no. AB013386.1), Heliothis virescens (54%, GenBank accession no. FJ416470.1); Helicoverpa armigera (53%, GenBank accession no. ACF40807.1), and Trichoplusia ni (53%, GenBank accession no. AEG79734.1) (Fig. 2). The full-length MsALP was cloned into the E. coli expression vector and purified from inclusion bodies.
Fig 2.
Protein sequence of MsALP from the midguts of M. sexta larvae. Multiple-sequence alignment of the deduced Ms-ALP amino acid sequence against ALPs from other lepidopteran insects was conducted using ClustalX. A dashed line indicates the amino-terminal signal peptide. The predicted phosphatase domain is included in a box, with the predicted active site in bold. Putative N-glycosylation sites are underlined. A predicted GPI anchor site is indicated with a triangle.
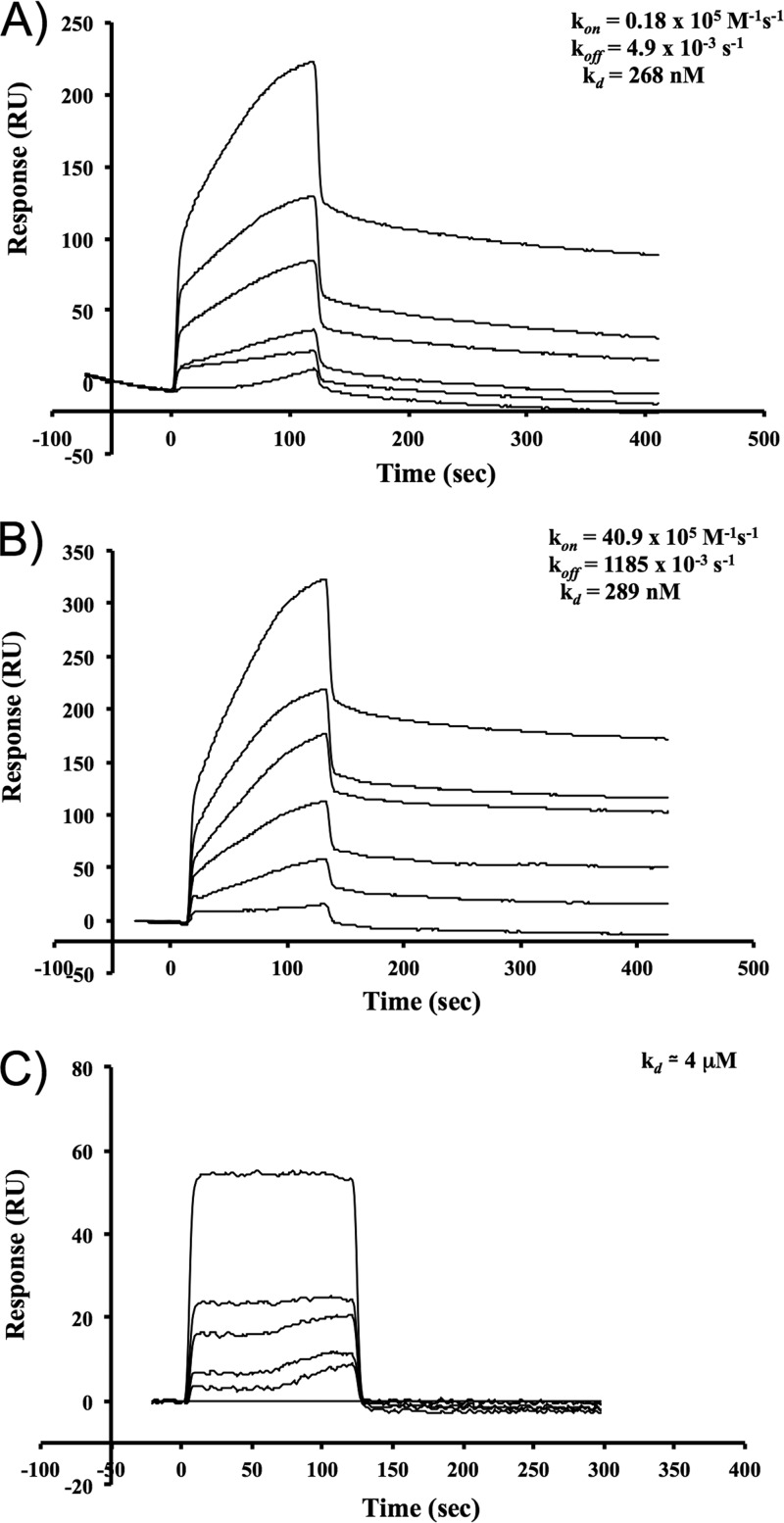
The affinities of binding of Cry1Aa, Cry1Ab, and Cry1Ac to MsALP were determined in real time by SPR analyses (Fig. 3). The overall affinities of Cry1Aa and Cry1Ab for binding to MsALP (Kd) were 268 nM and 289 nM, respectively, estimated from the association rate constant (kon) and dissociation rate constant (koff) values (Fig. 3A and B). In contrast, Cry1Ac bound immobilized MsALP with the low apparent binding affinity of 4 μM (Fig. 3C). These results show that Cry1Aa and Cry1Ab bound MsALP with a 16-fold-higher affinity than that of Cry1Ac.
Fig 3.
Cry1Ac binds to MsALP with lower affinity than Cry1Aa and Cry1Ab toxins. SPR analysis of binding of Cry1Aa (A), Cry1Ab (B), and Cry1Ac (C) to immobilized MsALP was conducted by conventional amine coupling. Sensograms of serial dilutions of Cry1A toxins from 1 to 100 nM are shown. For the kinetics of binding of Cry1Aa and Cry1Ab toxins to M. sexta alkaline phosphatase, kon is the association rate constant, koff is the dissociation rate constant, and Kd is the apparent affinity (koff/kon).
Toxicities of Cry1A toxins to M. sexta ALP- or APN1-silenced larvae.
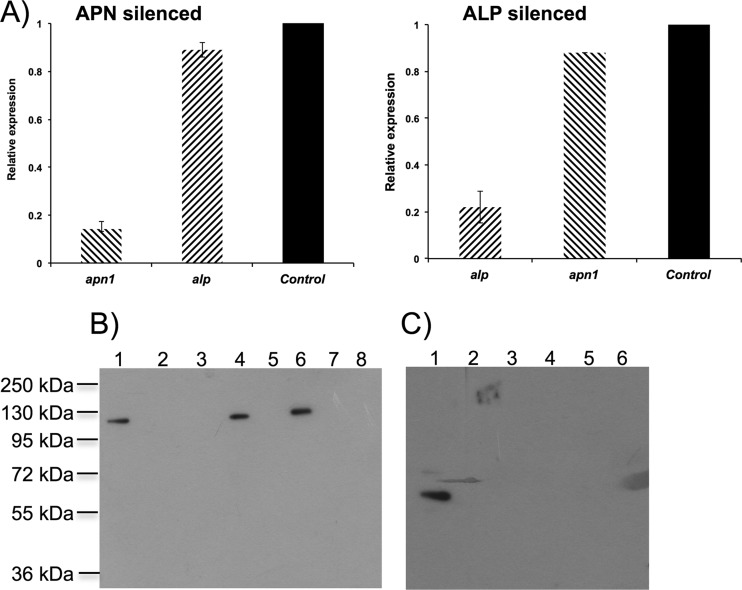
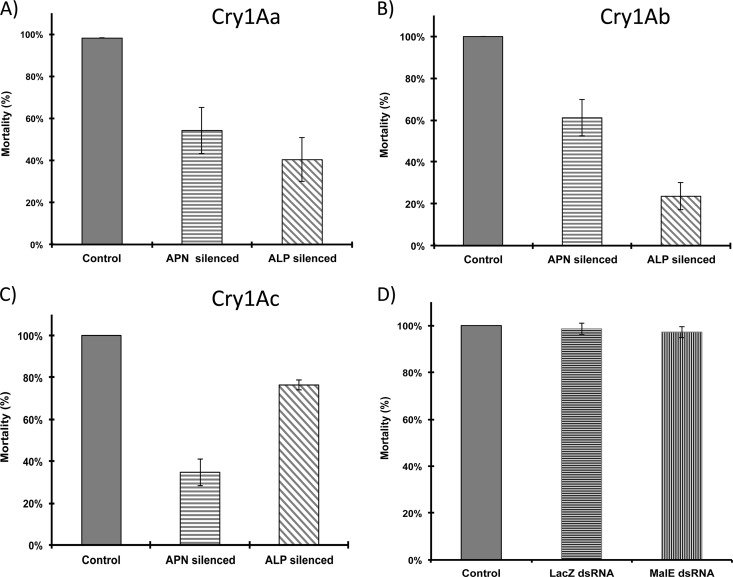
To determine the role of MsALP and APN1 in the toxicity of the three Cry1A toxins to M. sexta larvae, we determine the toxicity of the three toxins to MsALP- or APN1-silenced larvae. The dsRNAs of 308 bp from the MsAPN1 gene and 434 bp from the ALP gene were produced by in vitro transcription using T7 polymerase. M. sexta neonate larvae were starved for 2 h and then fed with 4 μg of dsRNA in a droplet, leaving the treated larvae on a normal diet until they reach the second instar. RNA and protein were extracted from the treated larvae to analyze MsALP and APN1 transcript and protein levels. qRT-PCR analyses showed reductions of MsALP and APN1 transcripts of 80% and 84%, respectively (Fig. 4A). Accordingly, a significant reduction of MsALP and APN1 proteins was observed by Western blotting of midgut tissues from individual silenced larvae (Fig. 4B and C). It is important to mention that silencing the expression of specific proteins by feeding the larvae with dsRNA was not 100% efficient, and some larvae showed expression of APN1 or ALP. In general, protein silencing by dsRNA was observed in 70 to 90% of the analyzed larvae. In addition, Fig. 4A shows that MsALP-silenced larvae were not affected in APN1 gene expression and that APN1-silenced larvae were not affected in MsALP expression. To determine the effect of MsALP and APN1 gene silencing on the toxicity of Cry1A toxins, larvae treated with alp dsRNA or with apn dsRNA as described above were fed a diet containing 25 ng/cm2 of each toxin, and mortality was recorded after 7 days of exposure to these toxins. As controls, we fed larvae with two unrelated dsRNAs from LacZ (30) and MalE (New England BioLabs). The larvae that survived in the bioassay with the different Cry1A toxins were analyzed by Western blotting, showing that in all cases the expression of toxin receptors of either APN or ALP was reduced (see Fig. S2 in the supplemental material). Figure 5 shows the mortality levels of the APN- or ALP-silenced M. sexta larvae compared to control larvae. It was observed that APN1- or MsALP-silenced larvae showed similarly reduced mortality due to Cry1Aa toxin, 50% or 60%, respectively, compared to control larvae, in which 100% mortality was observed. For Cry1Ab toxin, a higher reduction of mortality was observed in MsALP-silenced larvae, 80% reduction, compared to APN1-silenced larvae, in which a 40% reduction in mortality was observed compared to that of control larvae. Finally, for Cry1Ac toxin, the most significant reduction in mortality was observed with APN1 silencing (70%), in contrast to MsALP silencing, with which a reduction of only 20% in mortality was observed. These data show that Cry1Aa relies similarly on both receptor molecules, while Cry1Ab relies the most on MsALP and Cry1Ac on APN1 for toxicity.
Fig 4.
Efficient silencing of MsALP and APN1 by feeding specific dsRNA to neonate M. sexta larvae. (A) Transcript abundance was determined using qRT-PCR and Sybr green. Bars represent the means and standard errors for 5 individual midguts. (B) APN Western blot of midgut proteins (5 μg) prepared from individual control larvae (lane 1) and 5 apn dsRNA-fed larvae (lanes 2 to 8). (C) ALP Western blot of midgut proteins (5 μg) prepared from individual control larvae (lane 1) and 5 alp dsRNA-fed larvae (lanes 2 to 6).
Fig 5.
Effects of MsALP and APN1 gene silencing on the toxicity of Cry1A toxins. M. sexta MsALP-silenced, APN-silenced, or control larvae were exposed to a diet containing 25 ng/cm2 of Cry1Aa (A), Cry1Ab (B), or Cry1Ac (C), and mortality was recorded after 7 days. Seventy-two larvae were used in each treatment. (D) Mortality of larvae that were fed with nonrelated dsRNA sequences (LacZ and MalE) after treatment with Cry1Ab toxin. Bars represent the means and standard errors.
DISCUSSION
In the mode of action of Cry1A toxins, it has been suggested that ALP and APN could have redundant roles on Cry1A toxicity, in which these insect molecules have been proposed to fulfill two roles: first, as abundant low-affinity binding sites for monomeric Cry1A toxins that have a role in carrying the toxin to the insect epithelium, where the toxins bind to cadherin receptor, and second, as a high-affinity binding site for Cry1A oligomers, facilitating oligomer insertion into the membrane. However, previous work suggested that Cry1Ac preferentially binds APN1, whereas Cry1Ab preferentially interacts with ALP (7, 8, 31). As mentioned previously, Cry1Aa, Cry1Ab, and Cry1Ac differ in domain II and domain III, which are involved in receptor interaction. These findings could suggest that both GPI-anchored proteins may play a differential role in the toxicity of the three Cry1A toxins produced by B. thuringiensis subsp. kurstaki.
The analysis of the binding of Cry1A toxins to BBMV proteins, along with the analysis of the expression of APN1, ALP, and cadherin during larval development, suggested that Cry1Aa and Cry1Ab interact principally with ALP during the first larval instar, while Cry1Ac interacts principally with APN1 through all larval development, suggesting that Cry1Ac relies principally on APN interaction, in contrast to Cry1Aa and Cry1Ab. The observation that Cry1Aa and Cry1Ab proteins bound to ALP only in early instars despite the fact that ALP was observed in all instars by Western blotting suggests that a second ALP isoform that does not bind Cry1A toxins is produced during late-instar stages of development.
Here we show that Cry1Aa and Cry1Ab bound recombinant MsALP with similar binding affinities, in contrast to Cry1Ac, which bound MsALP with a 16-fold-lower binding affinity. Nevertheless, it has been shown that Cry1Ac relies on N-acetylgalactosamine interaction for binding to APN1 (16), and recombinant MsALP produced in E. coli is not glycosylated, explaining the low affinity of binding of Cry1Ac to this MsALP. In Heliothis virescens, Cry1Ac binding to ALP also relies on sugar interaction (32).
Finally, we evaluated the in vivo role of M. sexta APN1 and ALP in the toxicity of the three Cry1A toxins by gene silencing with specific dsRNA molecules. We observed a differential effect of APN1 or ALP downregulation in the toxicity of the three Cry1A toxins. The toxicity data shown in Fig. 5 indicate that Cry1Aa relies similarly on both APN1 and ALP, indicating a redundant role of these molecules in Cry1Aa toxicity. However, toxicity data for ALP-silenced larvae clearly indicate that Cry1Ab principally interacts with ALP to exert its toxic effect, while Cry1Ac relies principally on APN1. In the case of Cry1Ab, we showed previously that a single mutation in domain III residue L511 reduced binding to ALP, in contrast to APN, and affected the toxicity, indicating that domain III is important for ALP interaction and that Cry1Ab relies primarily on interaction with ALP rather than on APN binding (7). Cry1Aa and Cry1Ab have similar domain III sequences, explaining the important role of MsALP in the toxicity of both toxins. In the case of Cry1Ac, the domain III N-acetylgalactosamine binding pocket is an important binding epitope for APN1 interaction, explaining the differential binding of the three toxins to both receptor molecules (29). Overall, the results presented here indicate that Cry1A toxins differentially interact with the two GPI-anchored receptors to exert their toxicity and show that ALP and APN1 have a differential role in the mode of action of each one of the B. thuringiensis subsp. kurstaki Cry1A toxins.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported in part by a grant from DGAPA/UNAM IN209011 and CONACyT 83135. I.G. is especially thankful for a L'Oreal-UNESCO-AMC fellowship supporting this work.
We thank Jorge Sánchez for providing excellent technical assistance and Lizbeth Cabrera for insect maintenance.
Footnotes
Published ahead of print 17 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01062-13.
REFERENCES
- 1. Bravo A, Likitvivatanavong S, Gill SS, Soberon M. 2011. Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 41:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tabashnik BE, Van Rensburg JB, Carriere Y. 2009. Field-evolved insect resistance to Bt crops: definition, theory, and data. J. Econ. Entomol. 102:2011–2025 [DOI] [PubMed] [Google Scholar]
- 3. Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soberón M, Pardo L, Munoz-Garay C, Sanchez J, Gomez I, Porta H, Bravo A. 2010. Pore formation by Cry toxins. Adv. Exp. Med. Biol. 677:127–142 [DOI] [PubMed] [Google Scholar]
- 5. Gómez I, Sanchez J, Miranda R, Bravo A, Soberon M. 2002. Cadherin-like receptor binding facilitates proteolytic cleavage of helix alpha-1 in domain I and oligomer pre-pore formation of Bacillus thuringiensis Cry1Ab toxin. FEBS Lett. 513:242–246 [DOI] [PubMed] [Google Scholar]
- 6. Pigott CR, Ellar DJ. 2007. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 71:255–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arenas I, Bravo A, Soberon M, Gomez I. 2010. Role of alkaline phosphatase from Manduca sexta in the mechanism of action of Bacillus thuringiensis Cry1Ab toxin. J. Biol. Chem. 285:12497–12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pacheco S, Gomez I, Arenas I, Saab-Rincon G, Rodriguez-Almazan C, Gill SS, Bravo A, Soberon M. 2009. Domain II loop 3 of Bacillus thuringiensis Cry1Ab toxin is involved in a “ping pong” binding mechanism with Manduca sexta aminopeptidase-N and cadherin receptors. J. Biol. Chem. 284:32750–32757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soberón M, Gill SS, Bravo A. 2009. Signaling versus punching hole: how do Bacillus thuringiensis toxins kill insect midgut cells? Cell. Mol. Life Sci. 66:1337–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X, Candas M, Griko NB, Taussig R, Bulla LA., Jr 2006. A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc. Natl. Acad. Sci. U. S. A. 103:9897–9902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagamatsu Y, Koike T, Sasaki K, Yoshimoto A, Furukawa Y. 1999. The cadherin-like protein is essential to specificity determination and cytotoxic action of the Bacillus thuringiensis insecticidal CryIAa toxin. FEBS Lett. 460:385–390 [DOI] [PubMed] [Google Scholar]
- 12. Fabrick J, Oppert C, Lorenzen MD, Morris K, Oppert B, Jurat-Fuentes JL. 2009. A novel Tenebrio molitor cadherin is a functional receptor for Bacillus thuringiensis Cry3Aa toxin. J. Biol. Chem. 284:18401–18410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soberón M, Pardo-Lopez L, Lopez I, Gomez I, Tabashnik BE, Bravo A. 2007. Engineering modified Bt toxins to counter insect resistance. Science 318:1640–1642 [DOI] [PubMed] [Google Scholar]
- 14. Gill M, Ellar D. 2002. Transgenic Drosophila reveals a functional in vivo receptor for the Bacillus thuringiensis toxin Cry1Ac1. Insect Mol. Biol. 11:619–625 [DOI] [PubMed] [Google Scholar]
- 15. Rajagopal R, Sivakumar S, Agrawal N, Malhotra P, Bhatnagar RK. 2002. Silencing of midgut aminopeptidase N of Spodoptera litura by double-stranded RNA establishes its role as Bacillus thuringiensis toxin receptor. J. Biol. Chem. 277:46849–46851 [DOI] [PubMed] [Google Scholar]
- 16. Burton SL, Ellar DJ, Li J, Derbyshire DJ. 1999. N-Acetylgalactosamine on the putative insect receptor aminopeptidase N is recognised by a site on the domain III lectin-like fold of a Bacillus thuringiensis insecticidal toxin. J. Mol. Biol. 287:1011–1022 [DOI] [PubMed] [Google Scholar]
- 17. You TH, Lee MK, Jenkins JL, Alzate O, Dean DH. 2008. Blocking binding of Bacillus thuringiensis Cry1Aa to Bombyx mori cadherin receptor results in only a minor reduction of toxicity. BMC Biochem. 9:3. 10.1186/1471-2091-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jurat-Fuentes JL, Karumbaiah L, Jakka SR, Ning C, Liu C, Wu K, Jackson J, Gould F, Blanco C, Portilla M, Perera O, Adang M. 2011. Reduced levels of membrane-bound alkaline phosphatase are common to lepidopteran strains resistant to Cry toxins from Bacillus thuringiensis. PLoS One 6:e17606. 10.1371/journal.pone.0017606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hofte H, Whiteley HR. 1989. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 53:242–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Maagd RA, Bakker PL, Masson L, Adang MJ, Sangadala S, Stiekema W, Bosch D. 1999. Domain III of the Bacillus thuringiensis delta-endotoxin Cry1Ac is involved in binding to Manduca sexta brush border membranes and to its purified aminopeptidase N. Mol. Microbiol. 31:463–471 [DOI] [PubMed] [Google Scholar]
- 21. Lereclus D, Arantes O, Chaufaux J, Lecadet M. 1989. Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol. Lett. 51:211–217 [DOI] [PubMed] [Google Scholar]
- 22. Arantes O, Lereclus D. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108:115–119 [DOI] [PubMed] [Google Scholar]
- 23. Lereclus D, Agaisse H, Gominet M, Chaufaux J. 1995. Overproduction of encapsulated insecticidal crystal proteins in a Bacillus thuringiensis spo0A mutant. Biotechnology 13:67–71 [DOI] [PubMed] [Google Scholar]
- 24. Thomas WE, Ellar DJ. 1983. Bacillus thuringiensis var. israelensis crystal delta-endotoxin: effects on insect and mammalian cells in vitro and in vivo. J. Cell Sci. 60:181–197 [DOI] [PubMed] [Google Scholar]
- 25. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 26. Wolfersberger MG. 1993. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the gypsy moth (Lymantria dispar). Arch. Insect Biochem. Physiol. 24:139–147 [DOI] [PubMed] [Google Scholar]
- 27. Pauchet Y, Wilkinson P, Vogel H, Nelson DR, Reynolds SE, Heckel DG, Ffrench-Constant RH. 2010. Pyrosequencing the Manduca sexta larval midgut transcriptome: messages for digestion, detoxification and defence. Insect Mol. Biol. 19:61–75 [DOI] [PubMed] [Google Scholar]
- 28. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 29. Masson L, Lu YJ, Mazza A, Brousseau R, Adang MJ. 1995. The CryIA(c) receptor purified from Manduca sexta displays multiple specificities. J. Biol. Chem. 270:20309–20315 [DOI] [PubMed] [Google Scholar]
- 30. Jiménez AI, Reyes EZ, Cancino-Rodezno A, Bedoya-Perez LP, Caballero-Flores GG, Muriel-Millan LF, Likitvivatanavong S, Gill SS, Bravo A, Soberon M. 2012. Aedes aegypti alkaline phosphatase ALP1 is a functional receptor of Bacillus thuringiensis Cry4Ba and Cry11Aa toxins. Insect Biochem. Mol. Biol. 42:683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen J, Brown MR, Hua G, Adang MJ. 2005. Comparison of the localization of Bacillus thuringiensis Cry1A delta-endotoxins and their binding proteins in larval midgut of tobacco hornworm, Manduca sexta. Cell Tissue Res. 321:123–129 [DOI] [PubMed] [Google Scholar]
- 32. Perera OP, Willis JD, Adang MJ, Jurat-Fuentes JL. 2009. Cloning and characterization of the Cry1Ac-binding alkaline phosphatase (HvALP) from Heliothis virescens. Insect Biochem. Mol. Biol. 39:294–302 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.