Abstract
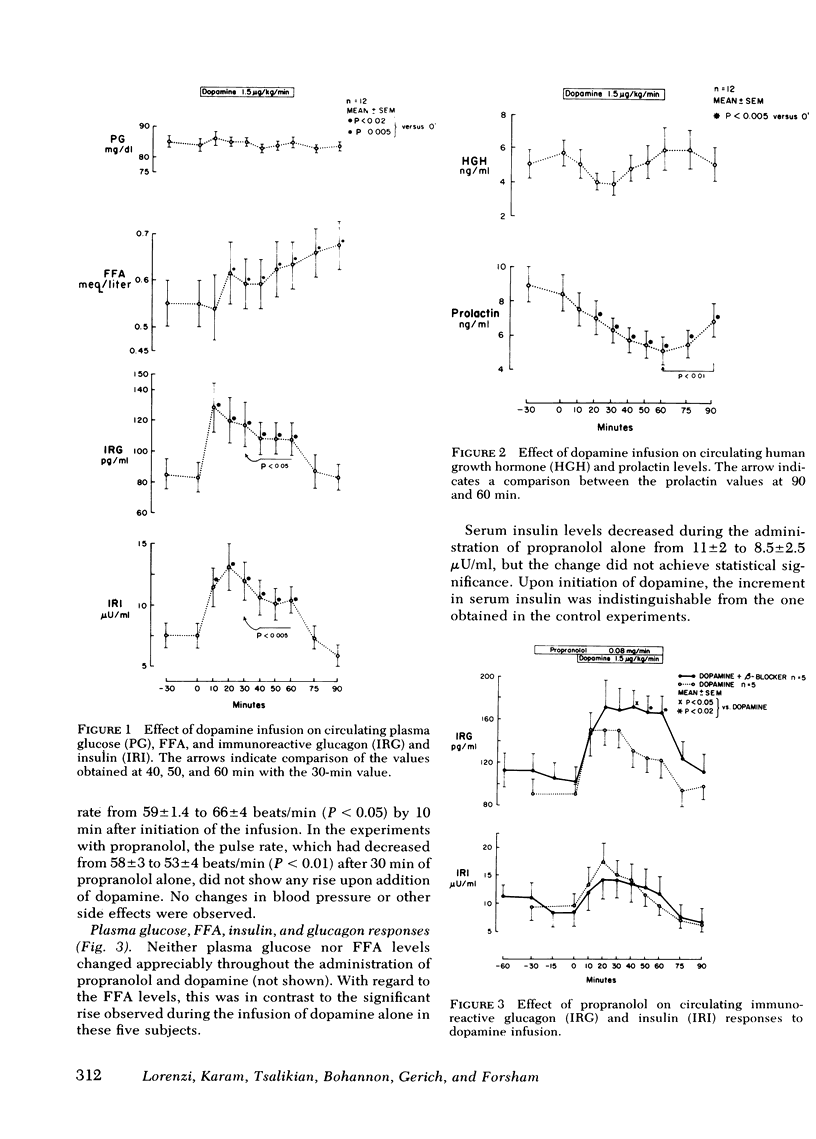
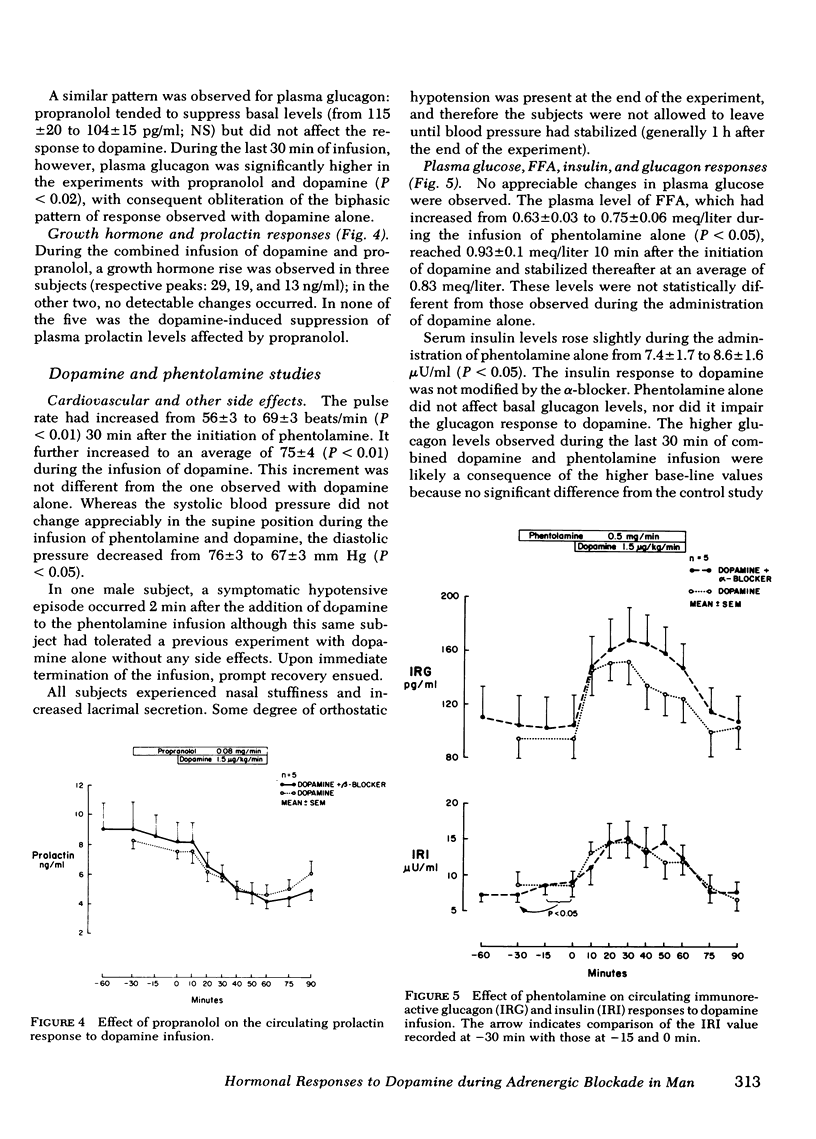
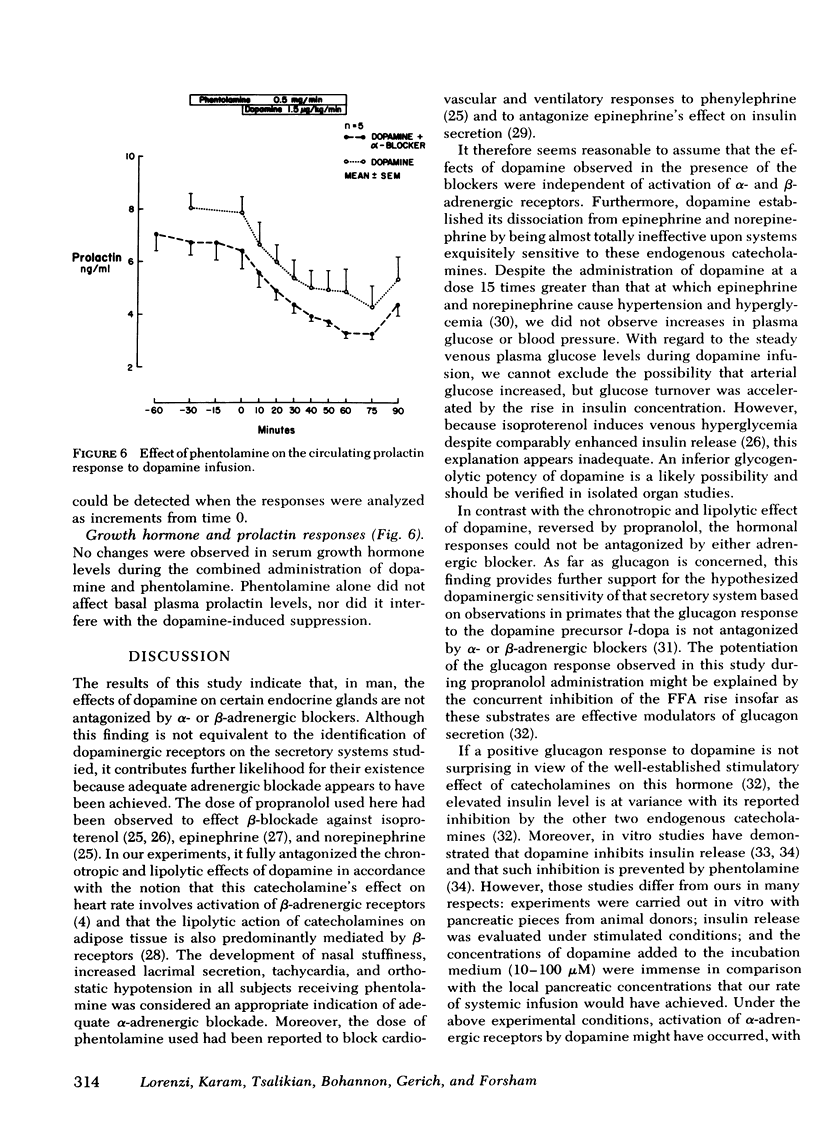
We studied the contribution of α- and β-adrenergic receptor activation to the cardiovascular, metabolic, and hormonal effects of dopamine. At a concentration of 1.5 μg/kg·min, the infusion of dopamine in 12 normal volunteers was associated with a transient but significant rise in pulse rate, which was prevented by propranolol. Venous plasma glucose did not change throughout the experiments, and a mild increase in plasma free fatty acid levels observed during the administration of dopamine alone was antagonized by propranolol. In contrast, neither the β-adrenergic blocker, propranolol, nor the α-adrenergic blocker, phentolamine, was effective in inhibiting the dopamine-induced rise in plasma glucagon (from 82±9 to 128±14 pg/ml; P < 0.005) and serum insulin (from 7.5±1 to 13±1.5 μU/ml; P < 0.005) or its suppression of plasma prolactin (from 8.5±1 to 5.2±0.8 ng/ml; P < 0.001). Although serum growth hormone levels did not change during the infusion of dopamine alone, an obvious rise occurred in three subjects during the combined infusion of propranolol and dopamine.
Whereas some metabolic and cardiovascular effects of dopamine are mediated through adrenergic mechanisms, these observations indicate that this is not the case for the effects of this catecholamine on glucagon, insulin, and prolactin secretion, and thus provide further support for the theory of a specific dopaminergic sensitivity of these hormonal systems in man.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubert M. L., Grumbach M. M., Kaplan S. L. Heterologous radioimmunoassay for plasma human prolactin (hPRL); values in normal subjects, puberty, pregnancy and in pituitary disorders. Acta Endocrinol (Copenh) 1974 Nov;77(3):460–476. doi: 10.1530/acta.0.0770460. [DOI] [PubMed] [Google Scholar]
- Bernardis L. L., Frohman L. A. Plasma growth hormone responses to electrical stimulation of the hypothalamus in the rat. Neuroendocrinology. 1971;7(4):193–201. doi: 10.1159/000121967. [DOI] [PubMed] [Google Scholar]
- Bertler A., Falck B., Owman C., Rosengrenn E. The localization of monoaminergic blood-brain barrier mechanisms. Pharmacol Rev. 1966 Mar;18(1):369–385. [PubMed] [Google Scholar]
- Birge C. A., Jacobs L. S., Hammer C. T., Daughaday W. H. Catecholamine inhibition of prolactin secretion by isolated rat adenohypophyses. Endocrinology. 1970 Jan;86(1):120–130. doi: 10.1210/endo-86-1-120. [DOI] [PubMed] [Google Scholar]
- Blackard W. G., Hubbell G. J. Stimulatory effect of exogenous catecholamines on plasma HGH concentrations in presence of beta adrenergic blockade. Metabolism. 1970 Jul;19(7):547–552. doi: 10.1016/0026-0495(70)90010-7. [DOI] [PubMed] [Google Scholar]
- Blackard W. G., Hull E. W., Lopez A. Effect of lipids on growth hormone secretion in humans. J Clin Invest. 1971 Jul;50(7):1439–1443. doi: 10.1172/JCI106627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A. E., 3rd, Lebovitz H. E., Pfeiffer J. B. Stimulation of human-growth-hormone secretion by L-dopa. N Engl J Med. 1970 Dec 24;283(26):1425–1429. doi: 10.1056/NEJM197012242832602. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Carroll R. J., Aurbach G. D. Dopaminergic stimulation of cyclic AMP accumulation and parathyroid hormone release from dispersed bovine parathyroid cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4210–4213. doi: 10.1073/pnas.74.10.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. M., Garfinkel P. E., Warsh J. J., Stancer H. C. Effect of carbidopa on prolactin, growth hormone and cortisol secretion in man. J Clin Endocrinol Metab. 1976 Jul;43(1):236–239. doi: 10.1210/jcem-43-1-236. [DOI] [PubMed] [Google Scholar]
- Camanni F., Massara F., Belforte L., Rosatello A., Molinatti G. M. Effect of dopamine on plasma growth hormone and prolactin levels in normal and acromegalic subjects. J Clin Endocrinol Metab. 1977 Mar;44(3):465–473. doi: 10.1210/jcem-44-3-465. [DOI] [PubMed] [Google Scholar]
- Christensen N. J., Mathias C. J., Frankel H. L. Plasma and urinary dopamine: studies during fasting and exercise and in tetraplegic man. Eur J Clin Invest. 1976 Sep 10;6(5):403–409. doi: 10.1111/j.1365-2362.1976.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Donoso A. O., Bishop W., Fawcett C. P., Krulich L., McCann S. M. Effects of drugs that modify brain monoamine concentrations on plasma gonadotropin and prolactin levels in the rat. Endocrinology. 1971 Sep;89(3):774–784. doi: 10.1210/endo-89-3-774. [DOI] [PubMed] [Google Scholar]
- Earll J. M., Sparks L. L., Forsham P. H. Glucose suppression of serum growth hormone in the diagnosis of acromegaly. JAMA. 1967 Aug 21;201(8):628–630. [PubMed] [Google Scholar]
- Feldman J. M., Boyd A. E., 3rd, Lebovitz H. E. Structural determinants of catecholamine action on in vitro insulin release. J Pharmacol Exp Ther. 1971 Mar;176(3):611–621. [PubMed] [Google Scholar]
- GRODSKY G. M., FORSHAM P. H. An immunochemical assay of total extractable insulin in man. J Clin Invest. 1960 Jul;39:1070–1079. doi: 10.1172/JCI104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Charles M. A., Grodsky G. M. Regulation of pancreatic insulin and glucagon secretion. Annu Rev Physiol. 1976;38:353–388. doi: 10.1146/annurev.ph.38.030176.002033. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Karam J. H., Forsham P. H. Stimulation of glucagon secretion by epinephrine in man. J Clin Endocrinol Metab. 1973 Sep;37(3):479–481. doi: 10.1210/jcem-37-3-479. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Langlois M., Noacco C., Schneider V., Forsham P. H. Adrenergic modulation of pancreatic glucagon secretion in man. J Clin Invest. 1974 May;53(5):1441–1446. doi: 10.1172/JCI107692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Tsalikian E., Karam J. H. Studies on the mechanism of epinephrine-induced hyperglycemia in man. Evidence for participation of pancreatic glucagon secretion. Diabetes. 1976 Jan;25(1):65–71. doi: 10.2337/diab.25.1.65. [DOI] [PubMed] [Google Scholar]
- Goldberg L. I. Cardiovascular and renal actions of dopamine: potential clinical applications. Pharmacol Rev. 1972 Mar;24(1):1–29. [PubMed] [Google Scholar]
- Goldberg L. I. The dopamine vascular receptor. Biochem Pharmacol. 1975 Mar 15;24(6):651–653. doi: 10.1016/0006-2952(75)90239-7. [DOI] [PubMed] [Google Scholar]
- Heistad D. D., Wheeler R. C., Mark A. L., Schmid P. G., Abboud F. M. Effects of adrenergic stimulation on ventilation in man. J Clin Invest. 1972 Jun;51(6):1469–1475. doi: 10.1172/JCI106943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J. Adrenergic receptors for metabolic responses in adipose tissue. Fed Proc. 1970 Jul-Aug;29(4):1388–1401. [PubMed] [Google Scholar]
- Hollenberg N. K., Adams D. F., Mendell P., Abrams H. L., Merrill J. P. Renal vascular responses to dopamine: haemodynamic and angiographic observations in normal man. Clin Sci Mol Med. 1973 Dec;45(6):733–742. doi: 10.1042/cs0450733. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- Imbs J. L., Schmidt M., Schwartz J. Effect of dopamine on renin secretion in the anesthetized dog. Eur J Pharmacol. 1975 Aug;33(1):151–157. doi: 10.1016/0014-2999(75)90150-8. [DOI] [PubMed] [Google Scholar]
- Kuku S. F., Zeidler A., Emmanouel D. S., Katz A. I., Rubenstein A. H. Heterogeneity of plasma glucagon: patterns in patients with chronic renal failure and diabetes. J Clin Endocrinol Metab. 1976 Jan;42(1):173–176. doi: 10.1210/jcem-42-1-173. [DOI] [PubMed] [Google Scholar]
- Lal S., De la Vega C. E., Sourkes T. L., Friesen H. G. Effect of apomorphine on growth hormone, prolactin, luteinizing hormone and follicle-stimulating hormone levels in human serum. J Clin Endocrinol Metab. 1973 Nov;37(5):719–724. doi: 10.1210/jcem-37-5-719. [DOI] [PubMed] [Google Scholar]
- Laurell S., Tibbling G. Colorimetric micro-determination of free fatty acids in plasma. Clin Chim Acta. 1967 Apr;16(1):57–62. doi: 10.1016/0009-8981(67)90269-0. [DOI] [PubMed] [Google Scholar]
- Leblanc H., Lachelin G. C., Abu-Fadil S., Yen S. S. Effects of dopamine infusion on pituitary hormone secretion in humans. J Clin Endocrinol Metab. 1976 Sep;43(3):668–674. doi: 10.1210/jcem-43-3-668. [DOI] [PubMed] [Google Scholar]
- Leblanc H., Lachelin G. C., Abu-Fadil S., Yen S. S. The effect of dopamine infusion on insulin and glucagon secretion in man. J Clin Endocrinol Metab. 1977 Jan;44(1):196–198. doi: 10.1210/jcem-44-1-196. [DOI] [PubMed] [Google Scholar]
- Leonard B. E. The effect of some beta-adrenergic receptor blocking drugs on carbohydrate metabolism in mouse brain. Neuropharmacology. 1971 Mar;10(21):127–144. doi: 10.1016/0028-3908(71)90034-7. [DOI] [PubMed] [Google Scholar]
- Lorenzi M., Tsalikian E., Bohannon N. V., Gerich J. E., Karam J. H., Forsham P. H. Differential effects of L-dopa and apomorphine on glucagon secretion in man: evidence against central dopaminergic stimulation of glucagon. J Clin Endocrinol Metab. 1977 Dec;45(6):1154–1158. doi: 10.1210/jcem-45-6-1154. [DOI] [PubMed] [Google Scholar]
- MacLeod R. M. Influence of norepinephrine and catecholamine-depleting agents on the synthesis and release of prolactin and growth hormone. Endocrinology. 1969 Nov;85(5):916–923. doi: 10.1210/endo-85-5-916. [DOI] [PubMed] [Google Scholar]
- MacLeod R. M., Lehmeyer J. E. Studies on the mechanism of the dopamine-mediated inhibition of prolactin secretion. Endocrinology. 1974 Apr;94(4):1077–1085. doi: 10.1210/endo-94-4-1077. [DOI] [PubMed] [Google Scholar]
- Massara F., Camanni F. Effect of various adrenergic receptor stimulating and blocking agents on human growth hormone secretion. J Endocrinol. 1972 Aug;54(2):195–206. doi: 10.1677/joe.0.0540195. [DOI] [PubMed] [Google Scholar]
- Myers M. G., Lewis P. J., Reid J. L., Dollery C. T. Brain concentration of propranolol in relation to hypotensive effect in the rabbit with observations on brain propranolol levels in man. J Pharmacol Exp Ther. 1975 Feb;192(2):327–335. [PubMed] [Google Scholar]
- Quickel K. E., Jr, Feldman J. M., Lebovitz H. E. Inhibition of insulin secretion by serotonin and dopamine: species variation. Endocrinology. 1971 Nov;89(5):1295–1302. doi: 10.1210/endo-89-5-1295. [DOI] [PubMed] [Google Scholar]
- Robertson R. P., Porte D., Jr Adrenergic modulation of basal insulin secretion in man. Diabetes. 1973 Jan;22(1):1–8. doi: 10.2337/diab.22.1.1. [DOI] [PubMed] [Google Scholar]
- Sandler M., Ruthven C. R. The biosynthesis and metabolism of the catecholamines. Prog Med Chem. 1969;6:200–265. doi: 10.1016/s0079-6468(08)70199-1. [DOI] [PubMed] [Google Scholar]


