Abstract
A defining characteristic of biofilms is antibiotic tolerance that can be up to 1,000-fold greater than that of planktonic cells. In Pseudomonas aeruginosa, biofilm tolerance to antimicrobial agents requires the biofilm-specific MerR-type transcriptional regulator BrlR. However, the mechanism by which BrlR mediates biofilm tolerance has not been elucidated. Genome-wide transcriptional profiling indicated that brlR was required for maximal expression of genes associated with antibiotic resistance, in particular those encoding the multidrug efflux pumps MexAB-OprM and MexEF-OprN. Chromatin immunoprecipitation (ChIP) analysis revealed a direct regulation of these genes by BrlR, with DNA binding assays confirming BrlR binding to the promoter regions of the mexAB-oprM and mexEF-oprN operons. Quantitative reverse transcriptase PCR (qRT-PCR) analysis further indicated BrlR to be an activator of mexAB-oprM and mexEF-oprN gene expression. Moreover, immunoblot analysis confirmed increased MexA abundance in cells overexpressing brlR. Inactivation of both efflux pumps rendered biofilms significantly more susceptible to five different classes of antibiotics by affecting MIC but not the recalcitrance of biofilms to killing by bactericidal agents. Overexpression of either efflux pump in a ΔbrlR strain partly restored tolerance of ΔbrlR biofilms to antibiotics. Expression of brlR in mutant biofilms lacking both efflux pumps partly restored antimicrobial tolerance of biofilms to wild-type levels. Our results indicate that BrlR acts as an activator of multidrug efflux pumps to confer tolerance to P. aeruginosa biofilms and to resist the action of antimicrobial agents.
INTRODUCTION
Pseudomonas aeruginosa is one of the principal human pathogens associated with cystic fibrosis (CF) pulmonary infection and chronic and burn wounds. The capacity of P. aeruginosa to form biofilms is an important requirement for chronic colonization of human tissues. Once established, P. aeruginosa biofilms are difficult to eradicate by antimicrobial treatment. Biofilms are surface-adhered bacterial communities encased in an extracellular matrix composed of DNA, bacterial polysaccharides, and proteins, and they are up to 1,000-fold more tolerant to antimicrobial agents than are their planktonic counterparts. Bacterial biofilms show enormous levels of antibiotic tolerance. Despite biofilms having been recognized as the predominant mode of bacterial growth in nature and for being responsible for the majority of refractory bacterial infections (1), little is known regarding the mechanisms of biofilm-specific antibiotic tolerance. It is likely that multiple mechanisms operate simultaneously in biofilms to contribute to antibiotic tolerance. Cells in a biofilm may be protected from antibiotic exposure due to the restricted penetration of antibiotics through the biofilm matrix (2–9). However, while β-lactams and aminoglycosides have been shown to be limited in their diffusion into biofilms, the penetration of fluoroquinolones occurs immediately and without delay (2–9). Moreover, once the matrix becomes saturated, diffusion and antimicrobial activity of the drug resume; thus, there is only a short-term protective effect. Other contributing mechanisms include the of subpopulations of multidrug-tolerant persister cells that neither grow nor die in the presence of bactericidal agents (10–14), reduced metabolic and divisional rates (15–18), and drug indifference of slow-growing, nutrient-limited cells (19), Recent reports further suggest that biofilm bacteria express specific protective factors such as multidrug efflux pumps and stress response regulons to counter the action of antimicrobial agents (7, 16, 20–27). In addition, we recently identified the transcriptional regulator BrlR to be required for P. aeruginosa biofilm tolerance to five classes of antimicrobial agents. BrlR conferred resistance by (indirectly) affecting the MIC required to inhibit P. aeruginosa growth and by contributing to the recalcitrance of P. aeruginosa biofilms to killing by bactericidal agents (28). However, the mechanism by which BrlR confers resistance to biofilms is unknown.
BrlR shares sequence similarities with members of the MerR family of multidrug efflux pump activators, including MerR, BmrR, BltR, and MtaN from Bacillus subtilis and TipA from Streptomyces lividans (28, 29). The MerR family regulators have homologous N-terminal DNA binding domains but differ in their variable C-terminal modulation or “coactivator” binding domains. These regulators are involved in modulating transcriptional activation of their own expression, as well as that of their target genes in response to an inducer(s) (30–32). They are also functionally similar, as they are all involved in controlling the expression of bacterial genes providing resistance to toxins via the induction of multidrug transporters. The MerR protein has been shown to activate the expression of mercury resistance genes upon binding of mercury ions (33), while TipA is induced upon thiostrepton binding (34, 35). Similarly, BmrR is induced upon exposure to rhodamine and tetraphenylphosphonium, while BltR is induced by rhodamine binding to the C-terminal domain, resulting in activation of transcription of multidrug transporters that export these toxic substances (30, 34, 36–40). Thus, MerR inducers are substrates of multidrug transporters, which are activated upon binding of the transporter substrate by the MerR regulatory proteins (38, 39), with multidrug resistance pumps being responsible for the extrusion of chemically unrelated antimicrobials from the bacterial cell.
While BrlR shares significant sequence similarity to the N-terminal DNA binding domain of MerR proteins and contributes to P. aeruginosa biofilm tolerance (28), BrlR differs from known MerR proteins in that brlR expression is specific to the biofilm mode of growth. This raised the question of whether BrlR confers resistance in a manner similar to that of known MerR proteins, via the activation of multidrug efflux pumps. To answer this question, we made use of genome-wide transcriptional profiling and chromatin immunoprecipitation (ChIP) to identify BrlR-regulated target genes and to initiate elucidation of the mechanism by which BrlR confers tolerance on P. aeruginosa biofilms. Here, we report that the transcriptional regulator BrlR plays a role in the high-level tolerance of biofilms formed by P. aeruginosa by activating the expression of genes encoding the multidrug efflux pumps MexAB-OprM and MexEF-OprN, thus establishing BrlR as a novel member of the MerR family of multidrug transport activators, and the first MerR-like protein in a Gram-negative bacterium.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and culture conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. P. aeruginosa strain PAO1 was used as the parental strain. All planktonic strains were grown in Lennox broth (LB; BD Biosciences) in Erlenmeyer flasks at 220 rpm. Escherichia coli cultures were grown in LB in the absence or presence of 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). For plasmid maintenance, antibiotics were used at the following concentrations: 50 to 75 μg/ml of gentamicin and 200 to 250 μg/ml of carbenicillin for P. aeruginosa and 20 μg/ml of gentamicin and 50 μg/ml of ampicillin for E. coli.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| DH5α | F− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) phoA supE44 thi-1 gyrA96 relA1 tonA | Invitrogen Corp. |
| BL21 | F− ompT hsdSB (rB− mB−) gal dcm rne131 (DE3) | Invitrogen Corp. |
| P. aeruginosa | ||
| PAO1 | Wild type | B. H. Holloway |
| ΔbrlR mutant | PAO1, ΔbrlR (PA4878) | 28 |
| PAO255 | PAO1, ΔmexAB-oprM-ΔmexEF-oprN | 67 |
| Plasmids | ||
| pCR2.1-TOPO | TA cloning vector; Kmr Apr | Invitrogen Corp. |
| pRK2013 | Helper plasmid for triparental mating; mob tra; Kmr | 75 |
| pJN105 | Arabinose-inducible gene expression vector; pBRR-1 MCS; araC-PBAD; Gmr | 41 |
| pMJT1 | Arabinose-inducible gene expression vector; pUCP18 MCS; araC-PBAD; Ampr/Carbr | 76 |
| pJN-brlR | brlR cloned into pJN105 | 28 |
| pMJT-brlR-V5/His6 | brlR-V5/His6 cloned into pMJT1 | 28 |
| pmexAB-oprM | mexAB-oprM operon, cloned into pJN105 at NheI-SacI; Gmr | This study |
| pmexEF-oprN | mexEF-oprN operon, cloned into pMJT1 at NheI-SacI; Ampr/Carbr | This study |
Strain construction.
Complementation and overexpression of brlR, mexAB-oprM, and mexEF-oprN were accomplished by placing the respective genes under the control of an arabinose-inducible promoter in the pMJT1 or pJN105 vector (41). The identity of vector inserts was confirmed by sequencing. Plasmids were introduced into P. aeruginosa via conjugation or electroporation. Primers used for strain construction are listed in Table 2.
Table 2.
Primers used in this study
| Oligonucleotide | Sequencea |
|---|---|
| RT-PCR, PCR, or cloning | |
| mreB-for | CTGTCGATCGACCTGGG |
| mreB-rev | CAGCCATCGGCTCTTCG |
| 16S rDNA_f | GACTCCTACGGGAGGCAGCAGT |
| 16S rDNA_r | GTATTACCGCGGCTGCTGGCAC |
| mexA-for | CAGCAGCTCTACCAGATCGAC |
| mexA-rev | GTATTGGCTACCGTCCTCCAG |
| mexE-for | GTCATCGAACAACCGCTG |
| mexE-rev | GTCGAAGTAGGCGTAGACC |
| mexAB-oprM-for NheI | GCGCGCGCTAGCGTAAGTATTTTGCCTGCCTTCTTC |
| mexAB-oprM-rev SacI | GCGCGCGAGCTCGATCAAGCCTGGGGATCTTCC |
| mexEF-oprN-for | GCGCGCGCTAGCGAGTCAAGCATGGAACAGTCATC |
| mexEF-oprN-rev | GCGCGCGAGCTCCTGGAGTGGCCGATTTCCATC |
| EMSA and streptavidin bead binding assays | |
| PmexAFb | GTAGTTCATTGGTTTGGCC |
| PmexAR | CATAGCGTTGTCCTCATG |
| PmexEFb | GGATCAGCATGTTCATCG |
| PmexER | CTGTTCCATGCTTGACTC |
| mexA_F4 | TTTTGCCTGCCTTCTTCG |
| mexA_R4 | TCGCTTTTTCCGCACCCG |
| pscE_GS_F | AAGGCGGTCTCGGCATTCTTTC |
| pscF_GS_R | CCACGGTATCGAGGGTATTC |
| ChIP or ChIP enrichment | |
| PmexAF | GTAGTTCATTGGTTTGGCC |
| PmexAR | CATAGCGTTGTCCTCATG |
| PmexEF | GGATCAGCATGTTCATCG |
| PmexER | CTGTTCCATGCTTGACTC |
| pscE_GS_F | AAGGCGGTCTCGGCATTCTTTC |
| pscF_GS_R | CCACGGTATCGAGGGTATTC |
Restriction sites are underlined.
Primer was biotinylated. Unbiotinylated primers were used for competition.
Biofilm growth.
Biofilms were grown using a once-through continuous-flow tube reactor system for 1 day for biofilm antibiotic tolerance testing and for up to 6 days for biofilm sample collection as previously described (21, 28, 42, 43). Biofilms were grown at 22°C in 20-fold-diluted LB medium.
RNA isolation and preparation for Affymetrix GeneChip analysis.
Samples were prepared identically as previously described (44, 45). For biofilm growth experiments, three independent replicates of the P. aeruginosa PAO1 parent strain and ΔbrlR mutant were grown as biofilms in a flowthrough system for 6 days as described above. Cells were treated with RNAprotect (Qiagen), and total RNA was extracted using an RNeasy minikit (Qiagen) per the manufacturer's instructions. RNA quality and the presence of residual DNA were checked on an Agilent Bioanalyzer 2100 electrophoretic system pre- and post-DNase treatment. Ten micrograms of total RNA was used for cDNA synthesis, fragmentation, and labeling according to the Affymetrix GeneChip P. aeruginosa genome array expression analysis protocol. Briefly, random hexamers (Invitrogen) were added (final concentration, 25 ng μl−1) to the 10 μg of total RNA along with in vitro-transcribed Bacillus subtilis control spikes (as described in the Affymetrix GeneChip P. aeruginosa genome array expression analysis protocol).
cDNA was synthesized using Superscript II (Invitrogen) according to the manufacturer's instructions under the following conditions: 25°C for 10 min, 37°C for 60 min, 42°C for 60 min, and 70°C for 10 min. RNA was removed by alkaline treatment and subsequent neutralization. The cDNA was purified with use of the QIAquick PCR purification kit (Qiagen) and eluted in 40 μl of buffer EB (10 mM Tris-HCl, pH 8.5). The cDNA was fragmented by DNase I (0.6 U μg−1 of cDNA; Amersham) at 37°C for 10 min and then end labeled with biotin-ddUTP with use of the Enzo BioArray terminal labeling kit (Affymetrix) at 37°C for 60 min. Proper cDNA fragmentation and biotin labeling were determined by gel mobility shift assay with NeutrAvadin (Pierce) followed by electrophoresis through a 5% polyacrylamide gel and subsequent DNA staining with SYBR green I (Roche).
Microarray data analysis.
Microarray data were generated using Affymetrix protocols as previously described (44, 46, 47). Absolute expression transcript levels were normalized for each chip by globally scaling all probe sets to a target signal intensity of 500. Three statistical algorithms (detection, change call, and signal log ratio) were then used to identify differential gene expression in experimental and control samples. The detection metric (presence, absence, or marginal status) for a particular gene was determined using default parameters in MAS software (version 5.0; Affymetrix). Batch analysis was performed in MAS to make pairwise comparisons between individual experimental and control GeneChips in order to generate change calls and a signal log ratio for each transcript. These data were imported into Data Mining Tools (version 3.0; Affymetrix). Transcripts that were absent under both control and experimental conditions were eliminated from further consideration. Statistical significance of signals between the control and experimental conditions (P < 0.05) for individual transcripts was determined using the t test. We defined a positive change call as one in which greater than 50% of the transcripts had a call of increased (I) or marginally increased (MI) for upregulated genes and decreased (D) or marginally decreased (MD) for downregulated genes. Finally, the mean value of the signal log ratios from each comparison file was calculated. Only those genes that met the above criteria and had a mean signal log ratio of greater than or equal to 1 for upregulated transcripts and less than or equal to 1 for downregulated transcripts were kept in the final list of genes. Signal log ratio values were converted from log 2 and expressed as fold changes.
qRT-PCR.
Quantitative reverse transcriptase PCR (qRT-PCR) was used to determine the expression levels of mexA and mexE using 1 μg of total RNA isolated from the P. aeruginosa PAO1 wild type and strains inactivated in or overexpressing brlR (ΔbrlR and PAO1/pJN-brlR strains) grown as biofilms and planktonically to exponential phase. Isolation of mRNA and cDNA synthesis were carried out as previously described (43, 48–50). qRT-PCR was performed using the Eppendorf Mastercycler ep realplex (Eppendorf AG, Hamburg, Germany) and the KAPA SYBR FAST qPCR kit (Kapa Biosystems, Woburn, MA), with oligonucleotides listed in Table 2. qRT-PCR and relative transcript quantitation were performed as previously described (51). mreB and 16S rRNA were used as controls.
Enrichment and detection of MexA.
Periplasmic proteins were obtained from P. aeruginosa PAO1, ΔbrlR mutant, and PAO1/pJN-brlR biofilms using the cold osmotic shock method described by Hiniker and Bardwell (52). Briefly, 3-day-old biofilm cells were harvested by centrifugation at 5,000 rpm for 10 min at 4°C and the pellets gently resuspended in 1 ml of TSE buffer (0.2 M Tris [pH 8.0], 0.5 M sucrose, 1 mM EDTA). The resuspended cells were incubated on ice for 30 min and centrifuged at 16,000 rpm for 30 min at 4°C, and supernatants were subsequently removed to a new microcentrifuge tube; this supernatant constituted the periplasmic extract. Trichloroacetic acid (100%) was added to these periplasmic extracts to a final concentration of 10%, followed by incubation at 4°C under static conditions for 15 to 16 h. Samples were centrifuged at 16,000 × g for 30 min at 4°C, and the resulting precipitate was washed twice with ice-cold acetone. Following acetone removal, the samples were dried using a SpeedVac and then resuspended in TE buffer (10 mM Tris [pH 8.0], 1 mM EDTA). Protein determination was carried out as previously described (50) using a modified Lowry assay. SDS loading buffer was mixed with the periplasmic protein fraction, followed by heat denaturation at 100°C for 10 min. The samples were resolved on an 11% polyacrylamide gel and subsequently transferred onto a polyvinylidene difluoride (PVDF) membrane using a TurboTransblot apparatus (Bio-Rad). Western blots were probed with anti-MexA antibodies (53) and developed with LumiGlo detection reagents (Cell Signaling). Quantitation of MexA abundance was done by determining the band intensity using ImageJ analysis software.
ChIP analysis.
In order to determine whether BrlR binds to the promoter region of mexAB-oprM (PmexA) and mexEF-oprN (PmexE) in vivo, 24-h-old biofilms of P. aeruginosa PAO1/pMJT-brlR-V5/His6, bearing His6/V5-tagged BrlR, were subjected to chromatin immunoprecipitation (ChIP) analysis as previously described (45). P. aeruginosa PAO1 expressing untagged brlR was used as a control. Briefly, in vivo DNA-protein cross-linking using 1% formaldehyde for 10 min at 37°C and immunoprecipitation using anti-V5 antibodies (Invitrogen Corp.) were done essentially as previously described (54–56). Following immunoprecipitation, DNA was liberated by reversing the cross-linking via incubation with 0.5 M NaCl in TE at 65°C for 4 h. Purified DNA from PAO1/pJN-brlR and PAO1/pMJT-brlR-V5/His6 samples was subjected to qPCR using primers listed in Table 2. The promoter region of pscEF was used as a control using primers pscE_GS_F/pscF_GS_R (Table 2). Relative transcript quantitation was accomplished using the ep realplex software (Eppendorf AG) by first normalizing transcript abundance (based on threshold cycle [CT] value) to mreB followed by determining transcript abundance ratios. Melting-curve analyses were employed to verify specific single product amplification.
Purification of His-tagged BrlR proteins.
V5/His6-tagged BrlR proteins were purified from E. coli supernatants following sonication of LB-grown planktonic cells and centrifugation at 21,200 × g. The supernatant was loaded onto nickel-nitrilotriacetic acid (Ni-NTA) affinity resin (Qiagen), washed with buffer, and eluted with an imidazole gradient according to the manufacturer's instructions for native protein purification. Protein preparations were examined for purity by SDS-PAGE, and fractions containing pure protein were pooled and desalted using VivaSpin centrifugal concentrator columns (10-kDa cutoff; Sartorius) and 10 mM Tris-HCl, pH 8. Protein determination was carried out as previously described (50) using a modified Lowry assay.
DNA binding assays.
BrlR binding to the putative brlR promoter was confirmed using the streptavidin magnetic bead DNA binding assay as previously described (45). Briefly, biotinylated target DNA fragments PmexA (−283 to +3 relative to translational start site) and PmexE (−269 to +9 relative to translational start site) were amplified using the primer pairs PmexAF/PmexAR and PmexEF/PmexER (Table 2). A total of 2.5 pmol of target DNA was incubated for 30 min at room temperature with 5 pmol of purified V5/His6-tagged BrlR in 25 mM Tris-HCl (pH 8), 5 mM MgCl2, 0.5 mM dithiothreitol, 1 mM EDTA, and 50 ng/μl of poly(dI-dC) as nonspecific competitor DNA. For specific competition, nonbiotinylated target DNA (0 to 50 pmol) was used. Streptavidin magnetic beads (Thermo Scientific; 100 μg) were used to capture biotinylated DNA. Following three washes, the proteins copurified with the biotinylated DNA were separated by 11% SDS-PAGE and assessed by immunoblot analysis for the presence of BrlR using anti-V5 antibodies (Invitrogen). An aliquot prior to addition of streptavidin magnetic beads was used to determine total BrlR present in each DNA binding assay.
DNA binding of BrlR to the region upstream of the mexA start codon was furthermore confirmed using electrophoretic mobility shift assays (EMSA) as described previously (57, 58) using purified V5/His6-tagged BrlR. Radiolabeled DNA promoter probes were generated by PCR using mexA_F4/mexA_R4 primers (Table 2) and end labeled using 10 μCi of [γ-32P]ATP (GE Healthcare) and 10 U of T4 polynucleotide kinase (New England BioLabs). The labeled DNA was subsequently separated from unincorporated nucleotides using Illustra microspin G-25 columns (GE Healthcare). Probes using pscE_GS_F/pscF_GS_R primers were generated accordingly and used as a control. Binding assays were performed for 30 min at 25°C in 25 mM Tris (pH 8.0), 25 mM KCl, 5% glycerol, 0.5 mM EDTA, 0.5 mM dithiothreitol, 2.5 ng/μl of poly(dI-dC), and 0.1 μg/μl of bovine serum albumin (BSA), using the protein and DNA concentrations indicated below. Samples were subjected to electrophoresis on a 6% polyacrylamide glycine gel (10 mM Tris [pH 7.5], 380 mM glycine, 1 mM EDTA) at 4°C. Imaging and data analyses were performed using a Molecular Imager FX phosphorimager (Bio-Rad) and Quantity One software (Bio-Rad).
MICs.
MICs of tobramycin, chloramphenicol, and trimethoprim were determined by 2-fold serial broth dilution in LB medium using 96-well microtiter plates. LB medium was used 10-fold diluted. The antibiotic concentrations used ranged from 0.02 to 200 μg/ml. The inoculum was ∼104 cells per well, and the results were read after overnight incubation at 37°C with shaking at 220 rpm. The MIC was defined as the lowest antibiotic concentration that yielded no visible growth. To ensure overexpression of brlR, PAO1/pJN-brlR and PAO255/pJN-brlR were grown in the presence of 0.5% arabinose. PAO1/pJN105 was used as a control.
Biofilm antibiotic tolerance assays.
Biofilms grown for 1 day under flowing conditions were treated for 1 h under flowing conditions with the following antimicrobial agents: tobramycin (50 to 150 μg/ml), norfloxacin (450 μg/ml), chloramphenicol (50 μg/ml), kanamycin (150 μg/ml), tetracycline (100 μg/ml), and trimethoprim (150 μg/ml). Following exposure of biofilms to the respective antimicrobial agents, biofilms were harvested, homogenized, serially diluted, and spread plated onto LB agar. Viability was determined via CFU counts. Susceptibility is expressed as log reduction. Biofilm MBC has been defined as the concentration of antibiotic at which no further increase in log reduction, i.e., decrease in CFU, is measured after addition of higher concentrations of antibiotic (59–61). To determine whether the two MDR pumps MexAB-OprM and MexEF-OprN contribute to resistance of biofilms to killing by bactericidal agents, PAO1, ΔbrlR mutant, and PAO255 biofilms were grown for 3 days, after which time the medium was switched to the same medium containing increasing concentrations of tobramycin or norfloxacin, ranging from 0.5 to 400 μg/ml. After 24 h of exposure to the antibiotic under continuous flow at 0.1 ml/min, biofilms were harvested and the surviving bacteria enumerated.
Statistical analysis.
Student's t test was performed for pairwise comparisons of groups, and multivariant analyses were performed using a 1-way analysis of variance (ANOVA) followed by a posteriori test using Sigma Stat software.
RESULTS
To initiate the characterization of the mechanism by which BrlR confers antimicrobial tolerance on P. aeruginosa biofilms, DNA microarray analysis was conducted (see Tables S1 and S2 in the supplemental material). Compared to wild-type biofilms, the transcriptomic study revealed decreased expression of genes involved in carbon catabolism and metabolism, secreted factors, and type I to III secretion (see Fig. S1 and Table S1 in the supplemental material). Even though ΔbrlR mutant biofilms were previously demonstrated to be more susceptible to hydrogen peroxide (28), none of the genes encoding catalases (katA, katB, katE, and katN) were differentially expressed, with the exception of katE, which decreased 2.3-fold in a ΔbrlR mutant compared to the wild type. Moreover, the organic hydroperoxide resistance protein (PA2850) was significantly less expressed in ΔbrlR mutant biofilms. No difference in the expression of psl genes required for Psl polysaccharide production and ndvB, required for glucan synthesis (26), was noted. The findings confirmed our previous results (28) and further indicated that BrlR-regulated biofilm resistance is independent of Psl and glucan synthesis. Moreover, ΔbrlR mutant biofilms were characterized by reduced expression of genes involved in adaptation, transport of small molecules, cell wall and lipopolysaccharide (LPS) biosynthesis, lipoproteins, and outer membrane proteins as well as genes involved in antimicrobial resistance (see Fig. S1). The latter included genes encoding probable resistance-nodulation-division (RND) efflux pumps (PA1435, PA3522-PA3523), MexAB-OprM, MexGHI-OpmD, and MexEF-OprN efflux pumps (Table S1). The tripartite multidrug resistance (MDR) pump MexAB-OprM has broad-range specificity. Substrates include aminoglycosides, tetracycline, β-lactams, SDS, and other compounds. The MDR pump MexEF-OprN has a different substrate specificity, including fluoroquinolones, trimethoprim, and chloramphenicol. While the expression of the mexAB-oprM and mexEF-oprN operons was reduced, expression of the oprH-phoPQ operon was increased in brlR mutant biofilms (see Tables S1 and S2). OprH-PhoPQ has been shown to be essential for resistance to cationic peptides, aminoglycosides, and polymyxin B (62–65). In addition, PA1874 was found to be less expressed in ΔbrlR biofilms. The gene is part of an operon (PA1874-PA1877) encoding a novel efflux pump which has recently been demonstrated to be involved in biofilm-specific resistance to a subset of antibiotics (66). However, PA1874 was found not to contribute to the tolerance of P. aeruginosa biofilms to antimicrobial agents.
Expression of mex efflux pumps is BrlR dependent.
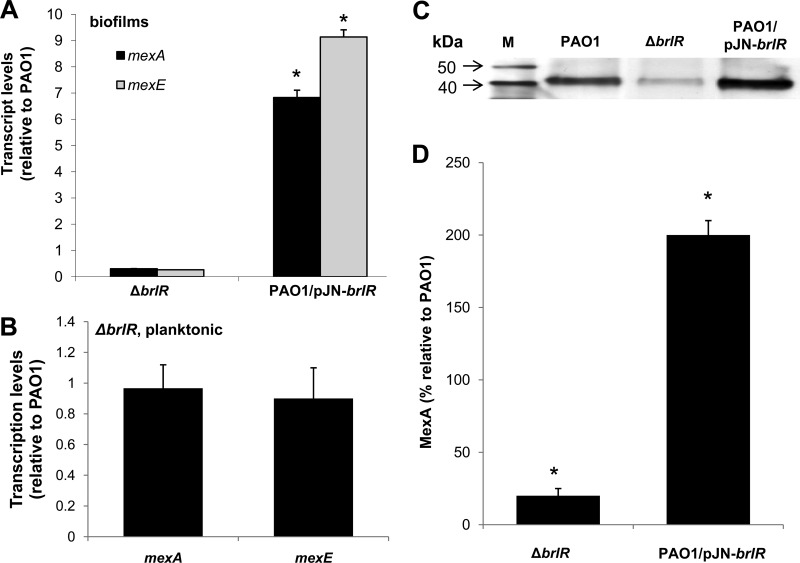
Based on sequence homology, BrlR has been previously characterized as a member of the MerR family of regulatory proteins that activate gene expression of multidrug efflux pumps. To determine whether BrlR is involved in the activation of multidrug efflux pumps in a manner similar to that of other MerR proteins, genes encoding efflux pumps were analyzed for BrlR-dependent expression. Of the efflux pumps that were detected by DNA microarray analysis to have decreased expression in ΔbrlR mutant biofilms, we chose to focus on the MexAB-OprM and MexEF-OprN MDR pumps, as inactivation of brlR had the greatest effect on the expression of the respective genes (see Table S1). The reduced expression of mexA and mexE in brlR mutant biofilms was confirmed by qRT-PCR (−3.3 ± 0.2 and −3.8 ± 0.3, respectively) (Fig. 1A). In contrast, significantly increased transcript levels of mexA and mexE were observed in biofilms overexpressing brlR (Fig. 1A). No difference in mexA and mexE transcript levels in the ΔbrlR mutant was noted under planktonic growth conditions (Fig. 1B).
Fig 1.
BrlR-dependent expression of the multidrug efflux pumps MexAB-oprM and MexEF-OprN. (A) Expression levels of efflux genes mexA and mexE are BrlR dependent. mexA and mexE transcripts were quantified by real-time qRT-PCR in the ΔbrlR mutant and PAO1/pJN-brlR. Relative transcript levels were based on comparison to PAO1. (B) No difference in mexA and mexE transcript levels are noted under planktonic growth conditions upon inactivation of brlR compared to the wild type. (C) Abundance of MexA in wild-type biofilms (PAO1) and strains inactivated in or overexpressing brlR (ΔbrlR mutant and PAO1/pJN-brlR) as determined by immunoblot analysis using 5 μg of the periplasmic protein fraction and anti-MexA antibody. Lane M, protein marker. (D) Quantitative analysis of MexA levels in ΔbrlR mutant and PAO1/pJN-brlR biofilms relative to P. aeruginosa PAO1 biofilms. *, significantly different from the values for P. aeruginosa PAO1 (P ≤ 0.01). Experiments were carried out at least in triplicate. Error bars denote standard deviations.
The differences in transcript levels observed for mexA correlated with MexA abundance. Immunoblot analysis using periplasmic fractions indicated reduced MexA levels in ΔbrlR biofilms but significantly increased MexA levels in PAO1/pJN-brlR biofilms compared to wild-type biofilms (Fig. 1C). Compared to wild-type biofilms, 4-fold-reduced MexA levels were detected in ΔbrlR mutant biofilms, while overexpression of brlR coincided with a 2-fold increase in MexA abundance (Fig. 1D). The findings indicated that both mexAB-oprM and mexEF-oprN are expressed in a BrlR-dependent manner.
BrlR is an activator of multidrug efflux pumps.
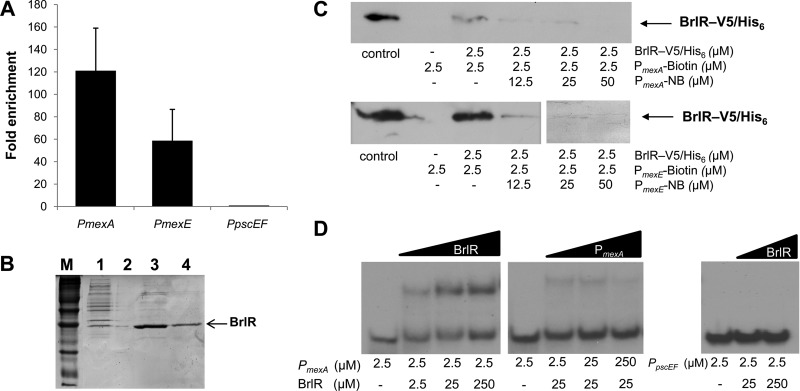
To determine whether the mex operons are direct targets of BrlR, chromatin immunoprecipitation was performed. DNA was enriched by using a strain expressing a V5/His6 C-terminally tagged BrlR construct (BrlR-V5/His6). A P. aeruginosa strain overexpressing the untagged protein was used as a control. DNA isolated via anti-V5 antibody immunoprecipitation from PAO1/pJN-brlR and PAO1/pMJT-brlR-V5/His6 biofilm samples was subjected to qPCR to determine whether the promoter regions of mexAB-oprM and mexEF-oprN were enriched compared to the control. On average, the promoter region of mexAB-oprM was enriched 120-fold, while the promoter region of mexEF-oprN was enriched 60-fold, compared to the control (Fig. 2A). In contrast, no enrichment was noted for pscEF operon encoding components of the type III secretion system (Fig. 2A).
Fig 2.
BrlR binds to the promoters of mexAB-oprM and mexEF-oprN. (A) Fold enrichment of the promoter sequences of mexA and mexE by ChIP compared to control (ChIP carried out in the absence of BrlR-V5/His6) as determined by qPCR. (B) Purification of BrlR-V5/His6. Lane M, protein marker; lane 1, lysate obtained from E. coli BL21/pET-brlR-V5/His6; lane 2, blank; lanes 3 and 4, eluates (fractions 1 and 2) obtained from Ni-NTA resin. Purified protein shown in lane 4 was used for EMSA and streptavidin binding assays. The molecular mass of tagged BrlR is 33 kDa. (C) Streptavidin magnetic bead binding assay demonstrating binding of V5/His6-tagged BrlR protein to 2.5 pmol of biotinylated PmexA and PmexE. Nonbiotinylated PmexA and PmexE (PmexA/mexE-NB) were used as specific competitor DNAs in 5-, 10-, and 20-fold excesses. BrlR binding to PmexA and PmexE was detected by immunoblot analysis using anti-V5 antibodies. +, presence of PmexA/mexE-biotin or PmexA/mexE-NB; −, absence of PmexA/mexE-biotin or PmexA/mexE-NB. Control, purified BrlR-V5/His6. (D) First gel, EMSA demonstrating BrlR binding to the 159-bp-long PmexA promoter region. BrlR concentrations were increased 10-fold over three concentrations. Second gel, BrlR binding to PmexA was outcompeted by increasing the concentration of unlabeled PmexA competitor DNA. Third gel, BrlR binding to the 92-bp-long PpscEF promoter region used as a control was not detected regardless of the BrlR concentration used. All experiments were carried out in triplicate.
The respective promoter regions were subsequently used in streptavidin bead pulldown assays to further confirm DNA binding of the BrlR protein to the promoter regions of the MexAB-OprM and MexEF-OprN efflux pumps. To do so, biotinylated promoter DNA and purified V5/His6-tagged BrlR (BrlR-V5/His6) (Fig. 2B) were used. BrlR binding to both promoter regions was observed and outcompeted by nonbiotinylated competitor DNA (Fig. 2C). In addition, electrophoretic mobility shift assays were performed using PmexA and V5/His6 C-terminally tagged BrlR. BrlR binding to PmexA was evident at 50 pmol of BrlR and outcompeted by nonradioactive competitor DNA (Fig. 2D). No specific binding to the PpscEF promoter region, which was used as negative control, was observed. These results indicate that BrlR binds specifically to the mexA and mexE promoter regions. Taken together with the expression results, our findings indicate that BrlR is involved in transcriptional regulation of the mexAB-oprM and mexEF-oprN operons.
MexAB-OprM and MexEF-OprN contribute to tolerance of biofilms to antimicrobial agents.
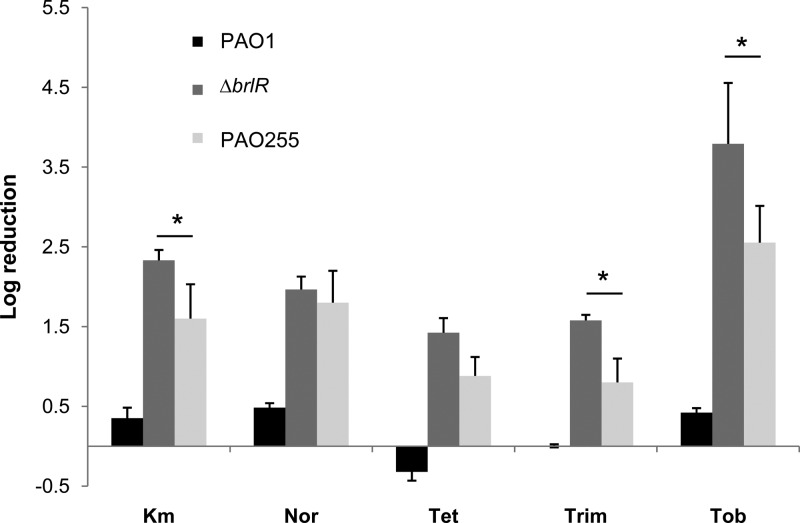
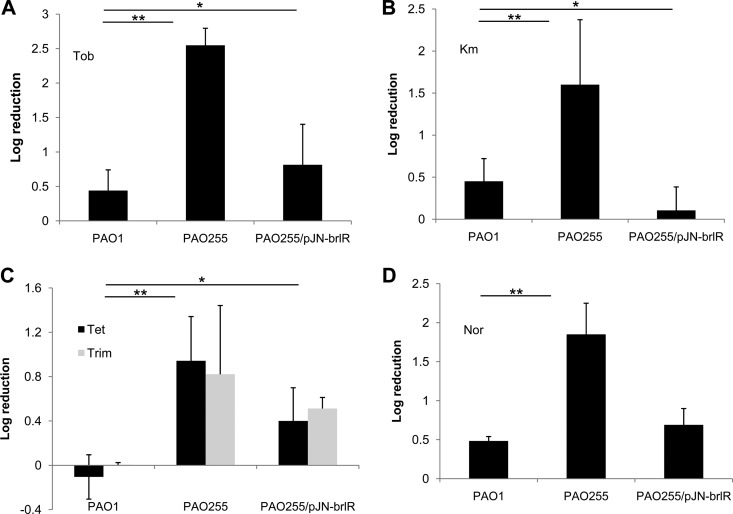
Considering the BrlR-dependent regulation of mexAB-oprM and mexEF-oprN gene expression, we next wished to determine whether the MexAB-OprM and MexEF-OprN efflux pumps contribute to P. aeruginosa biofilm tolerance to antimicrobial agents. To do so, we made use of the mutant strain PAO255 lacking both mex operons. Compared to wild-type biofilms, PAO255 biofilms were more susceptible to tobramycin, as treatment of 1-day-old biofilms with tobramycin (Tob; 150 μg/ml) for 1 h resulted in a 0.8-log reduction for the wild type but a 2.55-fold log reduction for PAO255 (Fig. 3). We reasoned that if BrlR-dependent biofilm resistance required only the presence of both the MexAB-OprM and MexEF-OprN efflux pumps, biofilms lacking both mex operons should be comparable to ΔbrlR mutant biofilms with respect to their susceptibility to antimicrobial agents. However, PAO255 biofilms were less susceptible than ΔbrlR biofilms to tobramycin. Similar results were obtained upon treatment with trimethoprim, tetracycline, and kanamycin but not with norfloxacin (Fig. 3).
Fig 3.
The MexAB-OprM or MexEF-OprN efflux pumps contribute to the tolerance of P. aeruginosa biofilms to antimicrobial agents. P. aeruginosa PAO1, the ΔbrlR mutant, and PAO255 (inactivated in mexAB-oprM and mexEF-oprN) were grown for 1 day as biofilms and subsequently treated for 1 h with tobramycin (150 μg/ml; Tob), kanamycin (150 μg/ml; Km), trimethoprim (150 μg/ml; Trim), tetracycline (100 μg/ml; Tet), and norfloxacin (450 μg/ml; Nor). *, significantly different from the values for the P. aeruginosa ΔbrlR mutant (P ≤ 0.01). Experiments were carried out at least three times. Error bars denote standard deviations.
Multicopy expression of mexAB-oprM and mexEF-oprN partially restores biofilm antibiotic resistance to the ΔblrR mutant.
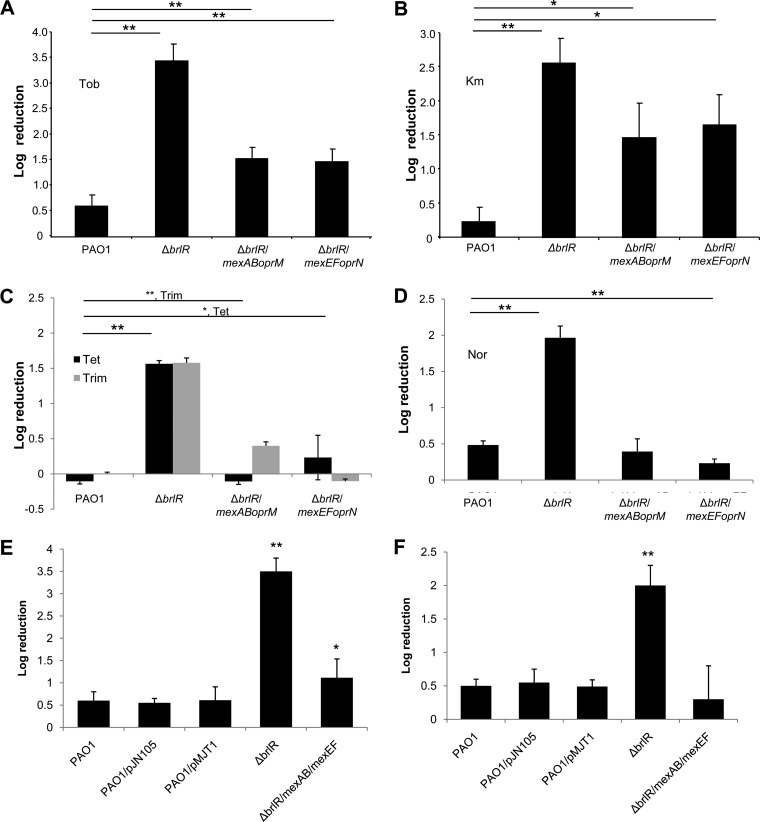
To further elucidate the role of the MexAB-OprM and MexEF-OprN efflux pumps in BrlR-dependent antibiotic tolerance of P. aeruginosa biofilms, the mexAB-oprM and the mexEF-oprN operons were cloned into pJN105 and pMJT1, respectively, under the control of the PBAD promoter and mated into a ΔbrlR mutant. The resulting strains were subsequently tested for their susceptibility to tobramycin. Treatment of ΔbrlR biofilms with tobramycin for 1 h resulted in a 3.5-log reduction, in contrast to wild-type biofilms, which were reduced by only 0.6 log after the same treatment. Plasmid-borne expression of mexAB-oprM or mexEF-oprN in ΔbrlR only partly restored the resistance phenotype (Fig. 4A). Similarly, overexpression of efflux pumps only partially restored ΔbrlR susceptibility to kanamycin (Fig. 4B). Overexpression of mexAB-oprM or mexEF-oprN rendered ΔbrlR mutant biofilms as resistant to tetracycline and trimethoprim as wild-type biofilms (Fig. 4). Likewise, resistance to norfloxacin was partly restored by expression of mexEF-oprN or mexAB-oprM (Fig. 4D). It is of interest to note that expression of the mexAB-oprM operon in a ΔbrlR mutant restored MexA to levels comparable to those detected in wild-type biofilms (Fig. S2), indicating that plasmid-borne expression of mexAB-oprM (induced with low levels of arabinose present in LB medium) results in wild-type-like levels of MexAB-OprM.
Fig 4.
Expression of mexAB-oprM or mexEF-oprN partially restores ΔbrlR susceptibility to antimicrobial agents. Biofilms of P. aeruginosa PAO1, the ΔbrlR mutant, and the ΔbrlR mutant overexpressing mexAB-oprM or mexEF-oprN were grown for 1 day and subsequently treated for 1 h with tobramycin (150 μg/ml) (A), kanamycin (150 μg/ml) (B), trimethoprim (150 μg/ml) and tetracycline (100 μg/ml) (C), and norfloxacin (400 μg/ml) (D). Biofilms of P. aeruginosa PAO1, the ΔbrlR mutant, and the ΔbrlR mutant coexpressing both mexAB-oprM and mexEF-oprN were grown for 1 day and subsequently treated for 1 h with tobramycin (150 μg/ml) (E) and norfloxacin (400 μg/ml) (F). P. aeruginosa PAO1 isolates harboring empty vectors (pMJT1 and pJN105) were used as controls. Experiments were carried out at least five times. Error bars denote standard deviations. * and **, significantly different from the values for P. aeruginosa PAO1 (P ≤ 0.05 and P ≤ 0.01, respectively).
To determine whether both efflux pumps are required to restore ΔbrlR mutant biofilm tolerance to wild-type levels, mexEF-oprN and mexAB-oprM were coexpressed in ΔbrlR and the respective strains tested for susceptibility to tobramycin and norfloxacin. While no difference in susceptibility to tobramycin or norfloxacin was noted for the vector controls compared to the wild type (Fig. 4E and F), coexpression of both mex pump operons rendered the ΔbrlR mutant less susceptible to tobramycin than ΔbrlR biofilms expressing mexEF-oprN or mexAB-oprM alone. However, the resulting strain was still more susceptible to tobramycin than wild-type biofilms (Fig. 4E). In contrast, coexpression of both mex pumps rendered ΔbrlR mutant biofilms tolerant to norfloxacin, with norfloxacin treatment not affecting viability. The tolerance upon coexpression of both mex operons in the ΔbrlR strain was comparable to that observed for ΔbrlR mutant biofilms expressing mexEF-oprN or mexAB-oprM alone (Fig. 4D and E).
MexAB-OprM and MexEF-OprN contribute to antimicrobial tolerance of P. aeruginosa by altering the MIC but not recalcitrance to killing by bactericidal agents.
To further determine the contribution of mexAB-oprM and mexEF-oprN to antimicrobial tolerance, MIC studies were carried out. Antimicrobial tolerance is the ability of a microorganism to grow in the presence of an elevated level of an antimicrobial agent, as indicated by an increased MIC. In particular, three different classes of antibiotics, including chloramphenicol, tobramycin, and trimethoprim, were tested. MICs were determined by 2-fold serial broth dilution in LB medium using 96-well microtiter plates and an inoculum of ∼104 cells per well. MICs of planktonic P. aeruginosa PAO1 and strain PAO255, a mutant harboring deletions in the respective efflux pumps (ΔmexAB-oprM and ΔmexEF-oprN ([67]), were compared. Lower MICs were consistently detected for PAO255 than for the wild type (Table 3).
Table 3.
MexAB-OprM and MexEF-OprN partly contribute to the BrlR-dependent resistance phenotype of P. aeruginosaa
| Strain | MIC (μg/ml) |
||
|---|---|---|---|
| Tobramycin | Trimethoprim | Chloramphenicol | |
| PAO1 | 1.25 | 12.5 | 6.25 |
| PAO1/brlR | 10 | 50 | 50 |
| Fold change | 6× | 4× | 6× |
| PAO255b | 0.3 | 3.1 | 2 |
| PAO255/brlR | 0.6 | 6.25 | 4 |
| Fold change | 2× | 2× | 2× |
Experiments were carried out in in triplicate.
PAO255, ΔmexAB-oprM ΔmexEF-oprN.
Previous findings indicated that BrlR contributed to P. aeruginosa antimicrobial tolerance by altering the MIC. To further determine whether the MexAB-OprM and MexEF-OprN efflux pumps are the only contributors to BrlR-dependent resistance of P. aeruginosa biofilms, PAO255 was complemented with brlR and the resulting strain tested using MIC assays. We reasoned that if BrlR-dependent biofilm resistance required only the presence of both MexAB-OprM and MexEF-OprN efflux pumps, expression of brlR in PAO255 should have no effect on the MIC. However, despite the lower MIC detected for PAO255, overexpression of brlR still resulted in an increase in MICs. For instance, overexpression of brlR in P. aeruginosa PAO255 resulted in a 2-fold-higher MIC (Table 3). In contrast, brlR expression in PAO1 correlated with a 6-fold increase in MIC to tobramycin and 4-fold increases in MIC to chloramphenicol and trimethoprim (Table 3).
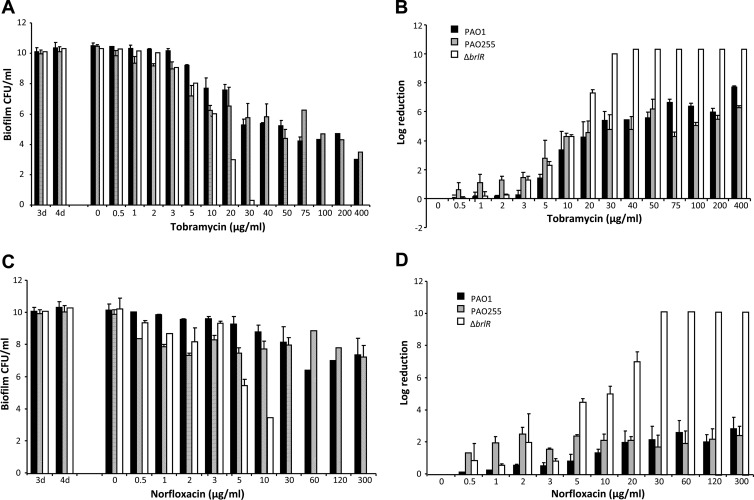
Biofilm MBC has been defined by Monzon et al. (59), Villain-Guillot et al. (60), and Moriarty et al. (61) as the concentration at which no further increase in log reduction following antimicrobial treatment is observed. We previously demonstrated that for P. aeruginosa wild-type biofilms, no further increase in log reduction was observed at concentrations higher than ∼75 μg/ml of tobramycin following 24 h of treatment. Higher concentrations resulted neither in increased log reduction nor in complete killing of P. aeruginosa wild-type biofilms (Fig. 5) (28). In contrast, inactivation of brlR rendered biofilms susceptible to tobramycin, as concentrations of tobramycin exceeding 30 μg/ml resulted in complete killing of the mutant biofilms. The finding indicated that BrlR contributes to the recalcitrance of P. aeruginosa biofilms to killing by bactericidal agents (Fig. 5) (28). To determine whether MexAB-OprM and MexEF-OprN efflux pumps contribute to this recalcitrant phenotype, biofilm MBC assays using tube reactor-grown biofilms were performed. Biofilms were grown for 3 days, after which time the medium was switched to the same medium containing increasing concentrations of tobramycin, ranging from 0.5 to 400 μg/ml. After 24 h of exposure to the antibiotic under continuous flow at 0.1 ml/min, biofilms were harvested and the surviving bacteria enumerated. Resistance of P. aeruginosa biofilms to tobramycin was dependent on the expression of MexAB-OprM and MexEF-OprN but only in the low concentration range (Fig. 5A and B). No difference in susceptibility compared to that of wild-type biofilms was noted at higher concentrations. In contrast, complete killing of ΔbrlR mutant biofilms was accomplished following treatment with tobramycin concentrations exceeding 30 μg/ml (Fig. 5A). Similar results were obtained when biofilms were treated with norfloxacin (Fig. 5C and D). The findings indicated that while MexAB-OprM and MexEF-OprN contribute to the resistance of biofilms at lower antibiotic concentrations, they do not contribute to the observed BrlR-dependent recalcitrance to killing by bactericidal agents.
Fig 5.
MexAB-OprM and MexEF-OprN do not contribute to resistance to killing of P. aeruginosa biofilms. P. aeruginosa PAO1, PAO255, and ΔbrlR mutant biofilms were grown as 3-day biofilms and subsequently treated for 24 h under continuous-flow conditions before surviving cells were recovered and enumerated. (A) Biofilm susceptibility to tobramycin as determined by viable counts (CFU). Viable ΔbrlR mutant cells were below the detection limit at the highest concentrations of tobramycin tested. (B) Biofilm susceptibility to tobramycin as determined by log reduction. Total killing of ΔbrlR biofilm cells was achieved at 40 μg/ml of tobramycin. In contrast, P. aeruginosa PAO1 and PAO255 biofilms maintained a steady level of persisting survivors at concentrations higher than 40 μg/ml of tobramycin. (C and D) Biofilm susceptibility to norfloxacin was determined similarly. Total killing of ΔbrlR biofilm cells was achieved at 30 μg/ml of norfloxacin, while PAO1 and PAO255 biofilms maintained a steady level of survivors through the highest concentrations tested. Error bars denote standard deviations.
Expression of brlR partly restores resistance in biofilms lacking MexAB-OprM and MexEF-OprN.
Our findings suggested BrlR confers biofilm tolerance by activating the expression of the two multidrug efflux pumps, MexAB-OprM and MexEF-OprN. We hypothesized that if BrlR confers resistance only through the action of MexAB-OprM and MexEF-OprN, expression of brlR in PAO255 lacking both mexAB-oprM and mexEF-oprN operons would not render this mutant more resistant. However, while PAO255 mutant biofilms expressing brlR were more susceptible to tobramycin than wild-type biofilms, PAO255/pJN-brlR biofilms were more resistant than PAO255 biofilms (Fig. 6A). Similar results were obtained upon treatment with tetracycline, trimethoprim, and norfloxacin (Fig. 6C and D). In contrast, expression of brlR rendered PAO255 biofilms as resistant to kanamycin as wild-type biofilms (Fig. 6B). The findings suggested that while BrlR contributes to biofilm tolerance through the activation of genes encoding the MexAB-OprM and MexEF-OprN multidrug efflux pumps, the regulon controlled by BrlR is not limited to these two multidrug efflux pumps.
Fig 6.
Expression of brlR partially restores resistance in the absence of MexAB-OprM and MexEF-OprN. P. aeruginosa PAO1, PAO255, and PAO255 biofilms overexpressing brlR (PAO255/pJN-brlR) were grown for 1 day and subsequently treated for 1 h with tobramycin (50 μg/ml) (A), kanamycin (150 μg/ml) (B), trimethoprim (150 μg/ml) and tetracycline (100 μg/ml) (C), and norfloxacin (400 μg/ml) (D). Experiments were carried out at least five times. Error bars denote standard deviations. * and **, significantly different from the values for P. aeruginosa PAO1 (P ≤ 0.05 and P ≤ 0.01, respectively).
DISCUSSION
A common feature of MerR-like regulatory proteins playing a role in tolerance to antibiotics, including BmrR, BltR, and MtaN from Bacillus subtilis and TipA from Streptomyces lividans, is activating expression of multidrug transporter genes upon binding of the transporter substrate (40, 68, 69). While the pattern of brlR expression is uncommon among members of the MerR family in that brlR transcription is biofilm specific (28), we nevertheless demonstrated that BrlR, similarly to known MerR proteins, activates the expression of operons encoding two multidrug efflux pumps, MexAB-OprM and MexEF-OprN. Moreover, our findings strongly suggested a contribution of both MexAB-OprM and MexEF-OprN to the BrlR-dependent tolerance of P. aeruginosa biofilms to antimicrobial agents.
While previous observations suggested that in P. aeruginosa, MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY had no impact on biofilm-specific resistance when mature biofilms were tested (70), we were able to demonstrate that MexAB-OprM and MexEF-OprN do contribute to biofilm resistance. However, the contribution of MexAB-OprM and MexEF-OprN was limited to biofilm tolerance upon short-term exposure, limited to 1 h. In addition, MexAB-OprM and MexEF-OprN only appeared to contribute to biofilm tolerance at lower concentrations of antibiotics, as inactivation of both efflux pumps did not eliminate the recalcitrance of P. aeruginosa biofilms to killing by bactericidal agents. Our findings, however, are in agreement with results obtained by Brooun et al. (12) indicating that resistance of P. aeruginosa biofilms to ofloxacin was dependent on the expression of mexAB-oprM but only in the low concentration range.
Previous findings indicated mexAB-oprM gene expression to be induced by quorum sensing. Maseda et al. (71) demonstrated that the quorum-sensing autoinducer N-butyryl-l-homoserine lactone (C4-HSL) enhanced the expression of mexAB-oprM, whereas N-(3-oxododecanoyl)-l-homoserine lactone had only a slight effect. Furthermore, this C4-HSL-mediated enhancement of mexAB-oprM expression was repressed by MexT, a positive regulator of the mexEF-oprN operon. Expression of mexAB-oprM is further regulated by the negative regulator of this efflux system, MexR (72). MexR binding to two sites in the mexR-mexA intergenic region (region of overlapping promoters for mexR and mexAB-oprM) was shown to repress expression of mexAB-oprM and mexR itself, which is located upstream of mexA and is transcribed in the opposite direction (72, 73). Recent evidence further suggested that MexR is a redox regulator that senses peroxide stress to mediate antibiotic resistance in P. aeruginosa, with MexR oxidation leading to its dissociation from promoter DNA, derepression of the mexAB-oprM operon, and increased antibiotic resistance of P. aeruginosa (74). The finding of BrlR functioning as an activator of mexAB-oprM and mexEF-oprN gene expression provides an additional level of control to the regulation of these two multidrug efflux pumps, enabling increased expression of both upon induction of brlR expression.
The expression of two highly similar multidrug transporters of Bacillus subtilis, Bmr and Blt, is regulated by specific MerR transcriptional activators, BmrR and BltR, respectively. Unlike BmrR and BltR, P. aeruginosa BrlR appears to be involved in the transcriptional regulation of more than one multidrug efflux pump, including MexAB-OprM and MexEF-OprN. Instead, the regulation of MDR pumps by BrlR is more reminiscent of the global transcriptional activator Mta, which interacts directly with the promoters of bmr and blt and induces transcription of these genes (39). However, the BrlR regulon does not appear to be limited to the promoters of mexAB-oprM and mexEF-oprN, as expression of brlR in P. aeruginosa PAO255 partly restored biofilm tolerance and increased MIC. Based on our transcriptomic and ChIP analyses, additional factors that may be regulated by BrlR to confer tolerance to biofilms may include the novel efflux pump PA1874-PA1877 (only PA1874 was detected). Previous studies indicated that expression of this efflux pump was 10-fold higher in biofilm cells than in planktonic cells (66). Complete deletion of the genes encoding this pump in a P. aeruginosa PA14 background resulted in a biofilm-specific increase in sensitivity to tobramycin, gentamicin, and ciprofloxacin (66). Analysis of additional efflux pumps that were found to be differentially expressed in ΔbrlR compared to wild-type biofilms may lead to a more complete understanding of BrlR-regulated antibiotic tolerance.
We have previously demonstrated that bacteria within microbial communities employ a specific regulatory mechanism to resist the action of antimicrobial agents in a BrlR-dependent manner, which affects MIC and recalcitrance to killing by bactericidal agents. The present work demonstrates that this is accomplished in part by BrlR activating the expression of two multidrug efflux pump systems, with indication of BrlR likely activating more than just mexAB-oprM and mexEF-oprN expression. Moreover, our findings establish BrlR as a true member of the MerR family of multidrug transporter activators. To our knowledge, this is the first description of a MerR-like multidrug transporter activator in a Gram-negative bacterium.
Supplementary Material
ACKNOWLEDGMENTS
We thank H. P. Schweizer at Colorado State University for providing the P. aeruginosa strain PAO255 and K. Poole at Queen's University for providing anti-MexA antibodies.
This work was supported by a grant from the National Institutes of Health (R01 AI080710).
Footnotes
Published ahead of print 17 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00318-13.
REFERENCES
- 1. Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 2. Campanac C, Pineau L, Payard A, Baziard-Mouysset G, Roques C. 2002. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob. Agents Chemother. 46:1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Picioreanu C, van Loosdrecht MCM, Heijnen JJ. 2001. Two-dimensional model of biofilm detachment caused by internal stress from liquid flow. Biotechnol. Bioeng. 72:205–218 [PubMed] [Google Scholar]
- 4. Thormann KM, Duttler S, Saville RM, Hyodo M, Shukla S, Hayakawa Y, Spormann AM. 2006. Control of formation and cellular detachment from Shewanella oneidensis MR-1 biofilms by cyclic di-GMP. J. Bacteriol. 188:2681–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderl JN, Franklin MJ, Stewart PS. 2000. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 44:1818–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewis K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138 [DOI] [PubMed] [Google Scholar]
- 8. Stewart PS. 1996. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 40:2517–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drenkard E. 2003. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. 5:1213–1219 [DOI] [PubMed] [Google Scholar]
- 10. Spoering AL, Lewis K. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183:6746–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. 2004. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 230:13–18 [DOI] [PubMed] [Google Scholar]
- 12. Brooun A, Liu S, Lewis K. 2000. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 44:640–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keren I, Shah D, Spoering A, Kaldalu N, Lewis K. 2004. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J. Bacteriol. 186:8172–8180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah D, Zhang Z, Khodursky A, Kaldalu N, Kurg K, Lewis K. 2006. Persisters: a distinct physiological state of E. coli. BMC Microbiol. 6:53. 10.1186/1471-2180-6-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sternberg C, Christensen BB, Johansen T, Toftgaard Nielsen A, Andersen JB, Givskov M, Molin S. 1999. Distribution of bacterial growth activity in flow-chamber biofilms. Appl. Environ. Microbiol. 65:4108–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anwar H, Strap JL, Chen K, Costerton JW. 1992. Dynamic interactions of biofilms of mucoid Pseudomonas aeruginosa with tobramycin and piperacillin. Antimicrob. Agents Chemother. 36:1208–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderl JN, Zahller J, Roe F, Stewart PS. 2003. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 47:1251–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fux CA, Wilson S, Stoodley P. 2004. Detachment characteristics and oxacillin resistance of Staphylococcus aureus biofilm emboli in an in vitro catheter infection model. J. Bacteriol. 186:4486–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y, Britigan BE, Singh PK. 2011. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334:982–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sauer K, Camper AK. 2001. Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J. Bacteriol. 183:6579–6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stoodley P, Sauer K, Davies DG, Costerton JW. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187–209 [DOI] [PubMed] [Google Scholar]
- 23. Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilbert P, Maira-Litran T, McBain AJ, Rickard AH, Whyte FW. 2002. The physiology and collective recalcitrance of microbial biofilm communities. Adv. Microb. Physiol. 46:202–256 [PubMed] [Google Scholar]
- 25. Mah TF, O'Toole GA. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34–39 [DOI] [PubMed] [Google Scholar]
- 26. Mah T-F, Pitts B, Pellock B, Walker GC, Stewart PS, O'Toole GA. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306–310 [DOI] [PubMed] [Google Scholar]
- 27. Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. 1998. The involvement of cell-to-ell signals in the development of a bacterial biofilm. Science 280:295–298 [DOI] [PubMed] [Google Scholar]
- 28. Liao J, Sauer K. 2012. The MerR-like transcriptional regulator BrlR contributes to Pseudomonas aeruginosa biofilm tolerance. J. Bacteriol. 194:4823–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Winsor GL, Van Rossum T, Lo R, Khaira B, Whiteside MD, Hancock REW, Brinkman FSL. 2009. Pseudomonas genome database: facilitating user-friendly, comprehensive comparisons of microbial genomes. Nucleic Acids Res. 37:D483–D488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Godsey MH, Zheleznova Heldwein EE, Brennan RG. 2002. Structural biology of bacterial multidrug resistance gene regulators. J. Biol. Chem. 277:40169–40172 [DOI] [PubMed] [Google Scholar]
- 31. Grkovic S, Brown MH, Skurray RA. 2002. Regulation of bacterial drug export systems. Microbiol. Mol. Biol. Rev. 66:671–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paulsen IT. 2003. Multidrug efflux pumps and resistance: regulation and evolution. Curr. Opin. Microbiol. 6:446–451 [DOI] [PubMed] [Google Scholar]
- 33. Summers AO. 1992. Untwist and shout: a heavy metal-responsive transcriptional regulator. J. Bacteriol. 174:3097–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahmed M, Borsch CM, Taylor SS, Vazquez-Laslop N, Neyfakh AA. 1994. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J. Biol. Chem. 269:28506–28513 [PubMed] [Google Scholar]
- 35. Holmes DJ, Caso JL, Thompson CJ. 1993. Autogenous transcriptional activation of a thiostrepton-induced gene in Streptomyces lividans. EMBO J. 12:3183–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neyfakh AA. 2001. The ostensible paradox of multidrug recognition. J. Mol. Microbiol. Biotechnol. 3:151–154 [PubMed] [Google Scholar]
- 37. Vazquez-Laslop N, Zheleznova EE, Markham PN, Brennan RG, Neyfakh AA. 2000. Recognition of multiple drugs by a single protein: a trivial solution of an old paradox. Biochem. Soc. Trans. 28:517–520 [PubMed] [Google Scholar]
- 38. Brown NL, Stoyanov JV, Kidd SP, Hobman JL. 2003. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 27:145–163 [DOI] [PubMed] [Google Scholar]
- 39. Baranova NN, Danchin A, Neyfakh AA. 1999. Mta, a global MerR-type regulator of the Bacillus subtilis multidrug-efflux transporters. Mol. Microbiol. 31:1549–1559 [DOI] [PubMed] [Google Scholar]
- 40. Zheleznova EE, Markham PN, Neyfakh AA, Brennan RG. 1999. Structural basis of multidrug recognition by BmrR, a transcription activator of a multidrug transporter. Cell 96:353–362 [DOI] [PubMed] [Google Scholar]
- 41. Newman JR, Fuqua C. 1999. Broad-host-range expression vectors that carry the arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 227:197–203 [DOI] [PubMed] [Google Scholar]
- 42. Sauer K, Cullen MC, Rickard AH, Zeef LAH, Davies DG, Gilbert P. 2004. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J. Bacteriol. 186:7312–7326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petrova OE, Sauer K. 2009. A novel signaling network essential for regulating Pseudomonas aeruginosa biofilm development. PLoS Pathog. 5:e1000668. 10.1371/journal.ppat.1000668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morici LA, Carterson AJ, Wagner VE, Frisk A, Schurr JR, zu Bentrup KH, Hassett DJ, Iglewski BH, Sauer K, Schurr MJ. 2007. Pseudomonas aeruginosa AlgR represses the Rhl quorum-sensing system in a biofilm-specific manner. J. Bacteriol. 189:7752–7764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Petrova OE, Schurr JR, Schurr MJ, Sauer K. 2011. The novel Pseudomonas aeruginosa two-component regulator BfmR controls bacteriophage-mediated lysis and DNA release during biofilm development through PhdA. Mol. Microbiol. 81:767–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frisk A, Schurr JR, Wang G, Bertucci DC, Marrero L, Hwang SH, Hassett DJ, Schurr MJ. 2004. Transcriptome analysis of Pseudomonas aeruginosa after interaction with human airway epithelial cells. Infect. Immun. 72:5433–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lizewski SE, Schurr JR, Jackson DW, Frisk A, Carterson AJ, Schurr MJ. 2004. Identification of AlgR-regulated genes in Pseudomonas aeruginosa by use of microarray analysis. J. Bacteriol. 186:5672–5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Allegrucci M, Sauer K. 2007. Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J. Bacteriol. 189:2030–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Allegrucci M, Sauer K. 2008. Formation of Streptococcus pneumoniae non-phase-variable colony variants is due to increased mutation frequency present under biofilm growth conditions. J. Bacteriol. 190:6330–6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Southey-Pillig CJ, Davies DG, Sauer K. 2005. Characterization of temporal protein production in Pseudomonas aeruginosa biofilms. J. Bacteriol. 187:8114–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Petrova OE, Sauer K. 2010. The novel two-component regulatory system BfiSR regulates biofilm development by controlling the small RNA rsmZ through CafA. J. Bacteriol. 192:5275–5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hiniker A, Bardwell JCA. 2004. In vivo substrate specificity of periplasmic disulfide oxidoreductases. J. Biol. Chem. 279:12967–12973 [DOI] [PubMed] [Google Scholar]
- 53. Nehme D, Li Elliot X-ZR, Poole K. 2004. Assembly of the MexAB-OprM multidrug efflux system of Pseudomonas aeruginosa: identification and characterization of mutations in mexA compromising MexA multimerization and interaction with MexB. J. Bacteriol. 186:2973–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chiu C-M, Thomas CM. 2004. Evidence for past integration of IncP-1 plasmids into bacterial chromosomes. FEMS Microbiol. Lett. 241:163–169 [DOI] [PubMed] [Google Scholar]
- 55. Solomon MJ, Varshavsky A. 1985. Formaldehyde-mediated DNA-protein crosslinking: a probe for in vivo chromatin structures. Proc. Natl. Acad. Sci. U. S. A. 82:6470–6474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leech AJ, Sprinkle A, Wood L, Wozniak DJ, Ohman DE. 2008. The NtrC family regulator AlgB, which controls alginate biosynthesis in mucoid Pseudomonas aeruginosa, binds directly to the algD promoter. J. Bacteriol. 190:581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fuchs EL, Brutinel ED, Jones AK, Fulcher NB, Urbanowski ML, Yahr TL, Wolfgang MC. 2010. The Pseudomonas aeruginosa Vfr regulator controls global virulence factor expression through cyclic AMP-dependent and -independent mechanisms. J. Bacteriol. 192:3553–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jones AK, Fulcher NB, Balzer GJ, Urbanowski ML, Pritchett CL, Schurr MJ, Yahr TL, Wolfgang MC. 2010. Activation of the Pseudomonas aeruginosa AlgU regulon through mucA mutation inhibits cyclic AMP/Vfr signaling. J. Bacteriol. 192:5709–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Monzón M, Oteiza C, Leiva J, Amorena B. 2001. Synergy of different antibiotic combinations in biofilms of Staphylococcus epidermidis. J. Antimicrob. Chemother. 48:793–801 [DOI] [PubMed] [Google Scholar]
- 60. Villain-Guillot P, Gualtieri M, Bastide L, Leonetti J-P. 2007. In vitro activities of different inhibitors of bacterial transcription against Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 51:3117–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moriarty TF, Elborn JS, Tunney MM. 2007. Effect of pH on the antimicrobial susceptibility of planktonic and biofilm-grown clinical Pseudomonas aeruginosa isolates. Br. J. Biomed. Sci. 64:101–104 [DOI] [PubMed] [Google Scholar]
- 62. Macfarlane ELA, Kwasnicka A, Hancock REW. 2000. Role of Pseudomonas aeruginosa PhoP-PhoQ in resistance to antimicrobial cationic peptides and aminoglycosides. Microbiology 146:2543–2554 [DOI] [PubMed] [Google Scholar]
- 63. Macfarlane ELA, Kwasnicka A, Ochs MM, Hancock REW. 1999. PhoP-PhoQ homologues in Pseudomonas aeruginosa regulate expression of the outer-membrane protein OprH and polymyxin B resistance. Mol. Microbiol. 34:305–316 [DOI] [PubMed] [Google Scholar]
- 64. McPhee JB, Bains M, Winsor G, Lewenza S, Kwasnicka A, Brazas MD, Brinkman FSL, Hancock REW. 2006. Contribution of the PhoP-PhoQ and PmrA-PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J. Bacteriol. 188:3995–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mulcahy H, Charron-Mazenod L, Lewenza S. 2008. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 4:e1000213. 10.1371/journal.ppat.1000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang L, Mah T-F. 2008. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J. Bacteriol. 190:4447–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chuanchuen R, Karkhoff-Schweizer RR, Schweizer HP. 2003. High-level triclosan resistance in Pseudomonas aeruginosa is solely a result of efflux. Am. J. Infect. Control 2:124–127 [DOI] [PubMed] [Google Scholar]
- 68. Heldwein EEZ, Brennan RG. 2001. Crystal structure of the transcription activator BmrR bound to DNA and a drug. Nature 409:378–382 [DOI] [PubMed] [Google Scholar]
- 69. Newberry KJ, Brennan RG. 2004. The structural mechanism for transcription activation by MerR family member multidrug transporter activation, N terminus. J. Biol. Chem. 279:20356–20362 [DOI] [PubMed] [Google Scholar]
- 70. De Kievit TR, Parkins MD, Gillis RJ, Srikumar R, Ceri H, Poole K, Iglewski BH, Storey DG. 2001. Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 45:1761–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maseda H, Sawada I, Saito K, Uchiyama H, Nakae T, Nomura N. 2004. Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 48:1320–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Poole K, Tetro K, Zhao Q, Neshat S, Heinrichs DE, Bianco N. 1996. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob. Agents Chemother. 40:2021–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Srikumar R, Paul CJ, Poole K. 2000. Influence of mutations in the mexR repressor gene on expression of the MexA-MexB-OprM multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 182:1410–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen H, Hu J, Chen PR, Lan L, Li Z, Hicks LM, Dinner AR, He C. 2008. The Pseudomonas aeruginosa multidrug efflux regulator MexR uses an oxidation-sensing mechanism. Proc. Natl. Acad. Sci. U. S. A. 105:13586–13591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U. S. A. 76:1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. 2007. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Invest. 117:877–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.