Abstract
The rhizobacterium Pseudomonas aeruginosa M18 can produce a broad spectrum of secondary metabolites, including the antibiotics pyoluteorin (Plt) and phenazine-1-carboxylic acid (PCA), hydrogen cyanide, and the siderophores pyoverdine and pyochelin. The antibiotic biosynthesis of M18 is coordinately controlled by multiple distinct regulatory pathways, of which the GacS/GacA system activates Plt biosynthesis but strongly downregulates PCA biosynthesis. Here, we investigated the global influence of a gacA mutation on the M18 transcriptome and related metabolic and physiological processes. Transcriptome profiling revealed that the transcript levels of 839 genes, which account for approximately 15% of the annotated genes in the M18 genome, were significantly influenced by the gacA mutation during the early stationary growth phase of M18. Most secondary metabolic gene clusters, such as pvd, pch, plt, amb, and hcn, were activated by GacA. The GacA regulon also included genes encoding extracellular enzymes and cytochrome oxidases. Interestingly, the primary metabolism involved in the assimilation and metabolism of phosphorus, sulfur, and nitrogen sources was also notably regulated by GacA. Another important category of the GacA regulon was secretion systems, including H1, H2, and H3 (type VI secretion systems [T6SSs]), Hxc (T2SS), and Has and Apr (T1SSs), and CupE and Tad pili. More remarkably, GacA inhibited swimming, swarming, and twitching motilities. Taken together, the Gac-initiated global regulation, which was mostly mediated through multiple regulatory systems or factors, was mainly involved in secondary and primary metabolism, secretion systems, motility, etc., contributing to ecological or nutritional competence, ion homeostasis, and biocontrol in M18.
INTRODUCTION
Pseudomonas aeruginosa M18 is a unique and well-characterized biocontrol rhizobacterium showing a strong antifungal capability (1, 2). It can produce a diverse range of secondary metabolites, including antibiotics, siderophores, and extracellular enzymes, such as phenazine-1-carboxylic acid (PCA), pyoluteorin (Plt), hydrogen cyanide (HCN), l-2-amino-4-methoxy-trans-3-butenoic acid (AMB), pyoverdine, pyochelin, and alkaline protease (1, 2). In contrast to primary metabolism, which is essential for maintaining normal physiological processes, secondary metabolism is not absolutely required for bacterial survival but contributes to ecological and nutritional competence. AMB, a potent antibiotic and toxin, has received little attention (3). The antibiotics PCA and Plt mainly contribute to the strong antifungal capacity of the M18 strain. In the M18 genome, two copies of phenazine biosynthetic operons are highly homologous to each other and to those in P. aeruginosa PAO1 (2, 4). However, the Plt biosynthetic structural, regulatory, and transport gene cluster is conserved in gene organization and size between P. aeruginosa M18 and P. protegens Pf-5 (previously called P. fluorescens) but displays a certain level of difference in the nucleotide sequence, especially the noncoding sequence, between the two species (1, 5–7). The clinically isolated strain P. aeruginosa LESB58 shares an identical plt locus and nucleotide sequence with the rhizobacterium M18. However, strain LESB58 cannot produce Plt (8) because of a frameshift mutation in the pltB gene (9).
The biosynthesis of antibiotics, including PCA and Plt, in P. aeruginosa M18 is tightly controlled by a unique and complex regulatory network integrated by many global and pathway-specific regulatory systems or factors as well as environmental factors, especially temperature (1, 4, 10–16). Among the different P. aeruginosa strains, the phzM gene, which is necessary for the conversion of PCA to pyocyanin (PYO), shows a strain-specific and temperature-dependent expression pattern. The dominant phenazine product of the rhizobacterium M18 is PCA, whereas the clinically isolated P. aeruginosa strain PAO1 mainly accumulates PYO. A temperature of 37°C instead of 28°C is more favorable for phzM expression and PYO biosynthesis (14). Apart from the different and strong transcriptional regulation from all three P. aeruginosa quorum-sensing (QS) systems (Las, Rhl, and PQS) (11, 12, 15), antibiotic biosynthesis in the M18 strain is also subject to posttranscriptional regulation from the sRNA chaperone Hfq (16) and the Gac/Rsm signal transduction cascade (10, 17).
The well-known Gac/Rsm cascade has been characterized in detail in P. fluorescens CHA0 (18–20). The Gac/Rsm cascade is initiated by the Gac two-component regulatory system composed of the sensor kinase GacS and its corresponding response regulator, GacA. The GacS kinase responds to and senses an unidentified signal, causing the activation of the transcriptional regulator GacA through a phosphorelay mechanism. The active form of the GacA protein subsequently activates the transcription of the small RNAs (sRNAs) rsmXYZ. These sRNAs sequester the translational repressor proteins RsmAE, which block ribosome binding at the mRNA ribosome binding site of the target genes involved in secondary metabolism, including antibiotic biosynthesis (18–20). The modes of Gac system regulation of antibiotic biosynthesis in M18 are both similar to and different from those in CHA0. Plt biosynthesis and its gene expression need activation by both the GacS/GacA global regulatory system (10) and the plt pathway-specific transcriptional activator PltR (21, 22). Moreover, one section of the activating pathway driven by Gac may be mediated by PltR (13). Intriguingly, the Gac system exerts strong downregulation on PCA biosynthesis through an uncharacterized mechanism (10).
The global transcriptome profile of the gacA mutant has been measured both in the representative biocontrol rhizobacterium P. protegens strain Pf-5 grown in NBGly medium at 20°C (23) and in the clinically isolated P. aeruginosa strain PAK grown in Luria-Bertani (LB) medium at 37°C (24). In the Pf-5 genome, more than 10% of the genes are significantly influenced at the transcript level when the cell density reaches an optical density at 600 nm (OD600) of 2.4 (23). The transcript levels of a relatively smaller number of genes (n = 228) in the P. aeruginosa PAK genome show more than 2-fold induction or repression in the gacA mutant relative to the level of expression in its parental strain grown to an OD600 of approximately 6.0 (24). However, the RsmA regulon in P. aeruginosa strain PAK grown at the stationary phase includes over 500 genes (25). The Gac system also acts exclusively by controlling the transcription of sRNAs, RsmY, and RsmZ in P. aeruginosa PAK (24). The Gac/Rsm cascade covers a broad range of complex regulons regulating secondary metabolism and virulence factors.
During the long-term adaptation to rhizosphere environmental pressure, P. aeruginosa strain M18 may have evolved a unique set of secondary metabolic profiles involved in antibiotic biosynthesis and numerous corresponding global regulatory mechanisms, including Gac/Rsm (10, 17) and QS (11, 12, 15). The complex and diverse regulatory mechanisms of the Gac system in M18 urged us to analyze the global pattern of genomic expression of the gacA mutant and to further explore the importance of the Gac system to overall physiology and metabolism and the regulatory network of the Gac system. In this study, we used the global transcriptome approach and extensive phenotypic analysis to investigate the genome-wide effects of gacA inactivation on rhizosphere-isolated P. aeruginosa strain M18 grown in King's medium B (KMB) (26) at 28°C during the early stationary phase. The results highlight a broader and more distinct regulatory profile of the GacS/GacA system in M18 than in Pf-5 and PAK. Approximately 15% of all annotated genes (5,698 genes) in the M18 genome displayed more than 2-fold upregulation (367 genes) or downregulation (472 genes) at the transcript level of the gacA mutant compared with the level of expression in wild-type strain M18. The Gac-controlled genotypic and phenotypic profiles predominantly included the secondary metabolism involved in the biosynthesis of antibiotics and extracellular enzymes as well as the primary metabolism involved in utilization of sulfur, phosphorus, and nitrogen, a large number of secretion systems, three types of motility, multiple regulatory factors or systems, etc.
MATERIALS AND METHODS
Bacterial strains, plasmids, DNA primers, and culture conditions.
The bacterial strains, plasmids, and DNA primers used in this study are listed in Table S1 in the supplemental material. Escherichia coli strains were grown in Luria-Bertani medium for routine purposes. P. aeruginosa M18 and its derivative strains were grown in KMB (26) or pigment-producing medium (PPM) (27) at either 28°C or 37°C. For P. aeruginosa M18, antibiotics were added at the following final concentrations: 50 μg ml−1 kanamycin, 100 μg ml−1 spectinomycin, and 120 μg ml−1 tetracycline. Tetracycline (15 μg ml−1) or kanamycin (50 μg ml−1) was used for E. coli. For assaying β-galactosidase activity, 4 mg ml−1 ortho-nitrophenyl-β-d-galactopyranoside in 100 mM phosphate buffer (pH 7.0) was used. When required, 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) was added on solid medium at 0.02% (28).
Molecular biological methods.
All DNA manipulations not described in detail were carried out using standard methods (28). KOD plus (Toyobo), LA Taq, Taq DNA polymerase (TaKaRa), RNA reverse transcriptase, DNA restriction endonucleases, ligase, DNA markers (MBI Fermentas), and other related reagents were used according to the manufacturers' instructions. Genomic DNA was extracted from P. aeruginosa M18 with an EZ-10 spin column genomic DNA isolation kit provided by Bio Basic Inc. Plasmid DNA was extracted using BioDev plasmid minipreparation purification system B. DNA was purified from the gel with a Qiagen QIAquick gel extraction kit or an Axygen AxyPrep DNA gel extraction kit. DNA synthesis and sequencing were conducted by Invitrogen Biotechnology Corporation or the Beijing HuaDa Genomics Institute.
RNA isolation, microarray hybridization, and data analysis.
For microarray analysis, fresh overnight cultures of P. aeruginosa M18 and its gacA mutant were inoculated at a final concentration with an OD600 of 0.05 into 500-ml Erlenmeyer flasks containing 100 ml KMB. The cultures were grown at 28°C with shaking at 200 rpm. After 22 h of incubation, the cells were harvested in the early stationary phase with an OD600 of 5.08 for M18 and 5.29 for the gacA mutant. The cells were collected using centrifugation (3,000 rpm, 4°C, 3 min). The cell pellet was rapidly washed with prechilled phosphate-buffered saline and then recollected by centrifugation (3,000 rpm, 4°C, 3 min). The precipitated cells were immediately resuspended in 1 ml of TRIzol reagent (Invitrogen) at room temperature for 20 min and then added to 200 μl chloroform. The sample was mixed by vortexing for 15 s and then centrifuged at 4°C for 15 min at 4,600 × g. Subsequently, the liquid layer was transferred into a new tube and added to 480 μl isopropanol. Similarly, the mixed sample was centrifuged at 4,600 × g and 4°C for 15 min. The RNA pellet was washed with 70% ethanol, dissolved in RNase-free water, and purified using a Qiagen RNeasy minikit. The concentration and purity of RNA were determined by a BioAnalyzer apparatus (Agilent Technologies). RNA samples with an A260/A280 of between 1.8 and 2.0 and an RNA integrity number of ≥7.0 were kept for further reverse transcription-PCR (RT-PCR).
Approximately 2 μg of RNA sample was transformed into cDNA using one-step RT-PCR. The cRNA transcribed from cDNA by T7 RNA polymerase was purified using a Qiagen RNeasy minikit, modified with aminoallyl-UTP (aa-UTP), labeled with cyanine-3 (Cy3) fluorescence dye, and then quantified by the BioAnalyzer apparatus (Agilent Technologies). The Cy3-labeled cRNA was subsequently used for hybridization with a DNA microarray (8×15K array; Agilent). The in situ-synthesized oligonucleotide microarray (Gene Expression Omnibus [GEO] platform GPL11372) comprises 15,000 60-mer DNA oligonucleotide probes covering almost all open reading frames (ORFs) annotated in the P. aeruginosa M18 genome. Approximately 875 ng of Cy3-labeled cRNA was hybridized with the M18 genomic microarray at 65°C for 17 h. The hybridized microarray slides were washed in staining dishes with a gene expression wash buffer kit and stabilization and drying solution (Agilent Technologies). Thereafter, the slides were scanned twice using Agilent's high-performance microarray scanner at 100% and 10% photomultiplier tubes to reduce the signal-to-noise ratio of each probe on the DNA chip. Each microarray experiment consisted of three biological replicates and three technical replicates.
Background noise from the microarray raw data was corrected with the RMA algorithm and quantile normalization (29). Expression levels obtained from three replicates were compared using an SBC analysis system with an unpaired Student's t test. Given that the RMA algorithm diminishes the false-positive rate and compresses the fold change, only genes showing a P value of <0.05 and a fold change of ≥2 were considered to be significantly differentially expressed. The genes differentially regulated between the wild-type M18 strain and its gacA mutant were identified manually.
Construction of lacZ fusion plasmids.
The regulatory effect of GacA on three type VI secretion systems (T6SSs) was assessed by selecting hsiA1, hsiA2, and hsiB3, which correspond to the first genes of the hsiA1-hcp1, hsiA2-icmF2, and hsiB3-clpV3 operons located within the H1, H2, and H3 (T6SS) loci, respectively, in constructing the lacZ translational fusions in the pME6015 vector. In pME6015, the corresponding promoter/operator region and the first eight codons of the E. coli lacZ gene were deleted. Specifically, three fragments, including the promoter/operator region and the first several codons of hsiA1, hsiA2, and hsiB3, were PCR amplified and cloned into EcoRI/PstI-digested pME6015, generating the corresponding hsiA1′-′lacZ, hsiA2′-′lacZ, and hsiB3′-′lacZ translational fusion vectors.
Quantification of β-galactosidase activity.
P. aeruginosa M18 and its derivative strains harboring the lacZ reporter plasmid were inoculated from the overnight culture into 500-ml Erlenmeyer flasks containing 100 ml KMB to a final concentration of an OD600 of 0.05 and then cultured at 28°C with shaking at 200 rpm. At different time points, the cells were harvested for assaying β-galactosidase activities using the method of Miller (30).
In vitro assay for HCN and siderophore production.
The HCN production of M18 and its derivative strains was detected on an HCN induction medium plate (30 g tryptic soy broth, 4.4 g glycine, 15 g agar per liter) at 28°C using an indicator paper method (31). For each strain, 100 μl of culture (108 cells ml−1) was dropped at the center of the plate. Thereafter, a disk of filter paper impregnated with picric acid (0.5%) and sodium carbonate (2%) was fixed to the underside of the petri dish covers, which were then sealed with Parafilm. After 24 h of incubation at 28°C, HCN production was indicated by the orange-brown discoloration of the filter paper.
Siderophore production by the Pseudomonas strains in solid medium was determined by the universal chrome azurol S (CAS) method (32). CAS blue agar was prepared according to a detailed and step-by-step procedure provided by Schwyn and Neilands (32). The blue agar was poured along the glass wall of the CAS plate, and the plate was carefully agitated to avoid foaming. An inoculum (10 μl) of M18 or its derivative strains was dropped onto the center of the CAS plate. After incubation for 56 h to 60 h at 28°C, siderophore production was assessed by the formation of a distinct fluorescent orange zone on the CAS plate.
Assessment of phosphorus, sulfur, and nitrogen utilization.
The influence of the gacA mutation on the ability of P. aeruginosa M18 to utilize different phosphorus, sulfur, or nitrogen sources was assessed by growing M18 and its gacA mutant strains on minimal medium with or without the addition of corresponding phosphorus, sulfur, or nitrogen sources. Cell growth (OD600) was monitored spectrophotometrically at 600 nm on a rotary shaker with shaking at 200 rpm at 28°C. The influence of the P. aeruginosa M18 gacA gene on the utilization of KH2PO4 as a phosphorus source was measured in medium (0.5 g MgSO4, 2 g citric acid, 1 g l-asparagine, 0.3 g KCl, 1 g glycerol, 0.5 g Tween 80, 320 μl 0.5 M FeCl3, 100 μl 1 M NH4Cl per liter liquid) amended without or with 10 mM KH2PO4 as the sole phosphorus source (33). For the sulfur utilization assay, M18 and its gacA mutant were routinely grown in a minimal medium containing 50 mM Tris-HCl (pH 7.3), 25 mM glucose, 20 mM NH4Cl, 2 mM KH2PO4, and 0.5 mM MgCl2 (34). Five sulfur compounds, including MgSO4, Met, cysteine (Cys), sodium dodecyl sulfate (SDS), and taurine, were each added to the foregoing medium as an alternative source of sulfur to a final concentration of 500 μM. Assay for nitrogen assimilation was carried out in nitrogen-free medium amended without or with NH4Cl or NaNO3 as the nitrogen source at a final concentration of 0.03 mol l−1. The composition of the minimal medium per liter was as follows: 2.0 g glucose, 0.5 g K2HPO4, 0.1 g CaCl2·2H2O, 0.5 g NaH2PO4·H2O, and 0.2 g MgSO4·7H2O.
Bacterial motility assays.
Assays for swimming, swarming, and twitching motilities were performed as reported by Rashid and Kornberg (35). In the swimming and swarming motility assays, overnight cultures of M18 and its derivatives were inoculated with a sterile toothpick onto the center of swimming plates (10 g liter−1 tryptone, 5 g liter−1 NaCl, 3 g liter−1 agarose) and swarming plates (5 g liter−1 glucose, 10 g liter−1 tryptone, 5 g liter−1 yeast extract, 5 g liter−1 agar), respectively, and grown for 24 h at 28°C. Twitching motility was assayed by stabbing the bacteria with a sharp toothpick into the bottom of twitching motility plates (10 g liter−1 tryptone, 5 g liter−1 yeast extract, 5 g liter−1 NaCl, 10 g liter−1 agar). After incubation at 28°C for 24 h, the zone of motility at the petri dish interface was observed. In all motility assays, three independent experiments were carried out with at least three parallel replicates for each strain.
Microarray data accession number.
The microarray data sets have been deposited in the GEO database (http://www.ncbi.nlm.nih.gov/geo/) with accession number GSE43655.
RESULTS
Influences of gacA inactivation on transcriptome profile of P. aeruginosa M18.
DNA microarray experiments were conducted to investigate the influence of GacA on the genome-wide transcription of P. aeruginosa M18. The experiments compared the transcriptional profile of M18 with that of the gacA mutant grown to the early stationary phase (OD600 = 5.0 to 5.5) in King's medium B (KMB). The microarray data from three independent cultures were validated by quantitative real-time RT-PCR and lacZ fusion expression analyses (see Fig. S1 in the supplemental material). The transcriptome results were also confirmed by our previous report that the GacS/GacA two-component system (TCS) activated Plt biosynthesis and its operon expression and exerted strong downregulation on PCA biosynthesis and phz operon expression in M18 (10).
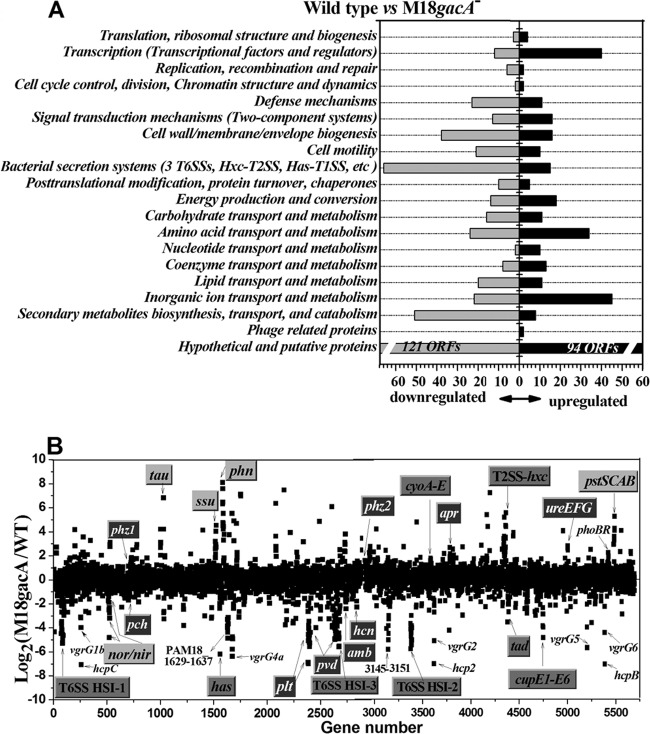
Compared with the M18 parental strain, the transcriptome profile of the gacA mutant revealed the significantly differential expression (increased or decreased by at least 2-fold; P < 0.05) of 839 genes, which represent approximately 15% of the 5,698 annotated genes in the M18 genome. Of these 839 genes, 472 were downregulated and 367 genes were upregulated by the gacA mutation (see Tables S2 and S3 in the supplemental material). The GacA regulon genes were grouped into 20 functional categories on the basis of PseudoCAP and were plotted with respect to downregulation and upregulation (Fig. 1A). The largest group consisted of hypothetical and putative genes, 121 of which were downregulated and 94 of which were upregulated in the gacA mutant compared with the expression in its parental strain, M18. The regulon under the positive control of GacA was mainly involved in secondary metabolism (51 genes), secretion systems (66 genes), cell membrane biogenesis (38 genes), etc. However, the negative regulon of GacA was predominantly involved in transcription factors and regulators (40 genes), amino acid transport and metabolism (34 genes), inorganic ion transport and metabolism (45 genes), etc. (Fig. 1A).
Fig 1.
Transcriptional profiles of P. aeruginosa M18 and the gacA mutants. (A) Functional classification of differential expression genes with a fold change of ≥2 and a P value of <0.05. Genes significantly upregulated or downregulated by gacA were classified into 20 different functional categories. (B) Differential gene transcription between M18 and its gacA mutant. Each dot represents 1 of 5,698 genes annotated in the M18 genome. The x axis shows the gene order; the y axis shows the log2 transcript abundance of each gene in the gacA mutant relative to that in wild-type (WT) strain M18. The highly modulated and well-characterized gene clusters are marked. The operons involved in secondary metabolism, primary metabolism, and secretion systems are shown with white text on a dark gray background, dark text on a light gray background, and dark text on a medium gray background, respectively. Other T6SS genes (hcp and vgrG), phoBR, and two uncharacterized operons are shown only as text with no box.
The well-characterized genes or gene clusters under GacA control are plotted in Fig. 1B. The secondary metabolic genes or gene clusters responsible for the biosynthesis of antibiotics (PCA, Plt, HCN, and AMB), siderophores (pyoverdine and pyochelin), and exoenzymes (alkaline protease, phospholipase C, chitinase, etc.) were strongly controlled by the Gac system. Surprisingly, the Gac system also exerted a significant influence on the primary metabolism involved in the assimilation and metabolism of phosphorus (phn, pst, and phoBR), sulfur (tau and ssu), and nitrogen (nor and nir). Many bacterial secretion systems also received strong and comprehensive regulation originating from GacA, including all three T6SSs (H1, H2, and H3), one T2SS (Hxc), two T1SSs (Has and Apr), one type I pilus system (CupE), and one type IVb pilus system (Tad). Among the genes in the GacA regulon, multiple operons encoding siderophores (pvd and pch) and hemophore (has) as well as phosphorus utilization operons (phn and pst) were established to play important roles in maintaining cellular ion homeostasis (36). The che2 chemotaxis operon (PAM18_0169 to PAM18_0178) was also shown to be subject to the positive control of GacA. Another important functional category occupying a large proportion of the GacA regulon was regulatory genes, such as two-component regulatory systems, transcriptional regulators, sigma factors, and regulators. This finding indicates that most members of the GacA regulon are likely under the indirect control of the Gac system.
GacA acts as a central regulator of secondary metabolism.
The biosynthesis of secondary metabolites, including antibiotics, siderophores, and extracellular enzymes, contributes to the biocontrol or virulence activity of Pseudomonas strains. The transcript levels of all genes within the Plt biosynthetic gene cluster were significantly downregulated by at least 4-fold in the gacA mutant relative to their expression in wild-type strain M18 (Fig. 2C). This further confirms our previous result (10) and is also consistent with results reported for P. protegens Pf-5 (23). Two homologous phz gene clusters revealed an approximately 2-fold increase at the transcript level in the gacA mutant compared with their expression in M18. Although the level of significance (P value) of the difference in transcript abundance of some phz genes between the two strains was more than 0.05 (Fig. 2D), the microarray result coincided with the results in our initial report (10). Similarly, the gacA mutation caused an obvious upregulation of the transcript level of the mexGHI-opmD efflux pump operon adjacent to the phz1 operon. The transcript level of qscR, a global QS regulator gene upstream of the phz2 operon, displayed a 3.3-fold decrease because of the gacA mutation. The qscR gene was also reported to mainly downregulate the expression of the phz2 operon (16).
Fig 2.
Influence of gacA on transcript levels of gene clusters involved in secondary metabolism and extracellular enzyme synthesis, including pyoverdine (A), pyochelin (B), pyoluteorin (C), phenazine (D), l-2-amino-4-methoxy-trans-3-butenoic acid (AMB) (E), hydrogen cyanide (F), potential secondary metabolites (G), and extracellular enzymes (H). Hollow arrows, transcript levels that differed significantly between wild-type strain M18 and the gacA mutant strain (P < 0.05); gray arrows, P > 0.05.
In addition to plt and phz, the amb (PAM18_2734 to PAM18_2739) and hcn (PAM18_2842 to PAM18_2840) operons responsible for AMB and HCN biosyntheses, respectively, were also present among the positive regulons of GacA (Fig. 2E and F). Similar to Plt biosynthesis, HCN biosynthesis was strongly repressed in the gacA mutant of M18 (Fig. 3A). The picrate paper on the plate inoculated with the gacA mutant remained yellow. This result indicates that minimal HCN was generated. In contrast, the picrate paper on the plates inoculated with M18 changed from yellow to a strong reddish brown after M18 was grown at 28°C for 24 h. The HCN production of the gacA mutant could also be restored to the wide-type level by complementation with plasmid pME6032 bearing the gacA gene (Fig. 3A). The Gac-initiated activation pathway of hcn expression was utilized as a classic model for clarifying the molecular regulatory mechanism of the Gac/Rsm cascade in CHA0 (20, 37). The expression of a functionally unknown gene cluster spanning from PAM18_1637 to PAM18_1629, which may be involved in secondary metabolism, was strongly positively regulated by GacA. The gacA mutation caused approximately 8-fold to 30-fold downregulation of these genes at the transcript levels (Fig. 2G).
Fig 3.

Assay for the biosynthesis of HCN and siderophore. (A) HCN production of wild-type strain M18, the gacA mutant (M18G), and the complemented gacA mutant strain (M18G/pME-gacA) was detected on an HCN induction medium plate with an indicator paper method. The strains were incubated at 28°C for 24 h. (B) Siderophore production of wild-type strain M18, the gacA mutant, and the complemented gacA mutant strain was detected on CAS blue agar plates. The strains were incubated for 56 to 60 h at 28°C. Siderophore production, indicated by the size of the orange zones, was significantly decreased due to the gacA mutation.
The siderophores pyoverdine and pyochelin, which can be produced by both the biocontrol rhizobacterium P. protegens Pf-5 (38) and the opportunistic pathogen P. aeruginosa PAK (39), play a critical role in iron uptake and metabolism, thus ensuring iron homeostasis (40). As shown in Fig. 2A, the genes associated with pyoverdine biosynthesis and transport underwent a notable downregulation at the transcript level in the gacA mutant relative to their expression in M18. The gacA mutation resulted in a relatively small degree of downregulation on the expression of pch genes encoding the other siderophore, pyochelin. Correspondingly, siderophore production was assayed for wild-type strain M18, the gacA mutant, and the complemented gacA mutant strain (Fig. 3B). The diameter of the fluorescent region around the inoculation point, which can reflect siderophore production, was significantly narrowed because of the gacA mutation. Consequently, the reduced capacity of siderophore biosynthesis in the gacA mutant was recovered to the wild-type level by introducing a gacA expression vector (Fig. 3B). These results indicate that the Gac system positively influences the biosynthesis of the siderophores pyoverdine and pyochelin in M18, which is the opposite of the influence in P. protegens Pf-5 (23).
In addition to the antibiotic and siderophore biosynthetic gene clusters under GacA control, the expression of genes encoding some extracellular enzymes was also subject to Gac regulation. As shown in Fig. 2H, the gacA mutation significantly upregulated the transcript levels of both the apr operon, encoding alkaline protease, and three phospholipase C genes (plcB, plcN, and plcH). The Gac system regulated apr expression in the opposite directions between P. aeruginosa M18 and P. protegens Pf-5. When the gacA mutant was compared with its parental strain, M18, a remarkable downregulation of the penicillin acylase gene PAM18_3149 and its flanking genes, the sodM gene (encoding a superoxide dismutase) and its flanking genes, and the chiC gene (encoding a chitinase) was also observed. These extracellular enzymes are usually secreted for biocontrol or virulence, depending on the Pseudomonas species (41, 42).
GacA targets involved in primary metabolism and energy metabolism.
The GacS/GacA system is considered a master regulator of Pseudomonas secondary metabolism. However, in the transcriptome profile of P. aeruginosa M18, many gene clusters involved in primary metabolism, including assimilation and metabolism of phosphorus, sulfur, and nitrogen sources, varied considerably at the transcript level because of the gacA mutation (Fig. 4). The gacA mutation resulted in the strong upregulation by up to several dozen times of the transcript levels of two phosphonate transport and metabolic operons, phnCDEFGHIJKLMNP and pstSCAB-phoU-PAM18_5484, and the phoR-phoB TCS (Fig. 4A and B). Similarly, the ssu and tau operons, which are required for the utilization of sulfonate and taurine as sulfur sources, respectively, displayed a relatively significant upregulation in transcript levels in the gacA mutant relative to their expression in the M18 strain (Fig. 4C and D). However, the expression of two gene clusters (nir and nor) involved in nitrogen metabolism was downregulated, although not at a significant level, because of gacA inactivation (Fig. 4E). In addition, the cyoABCDE operon (PAM18_3585 to PAM18_3581), encoding cytochrome O ubiquinol oxidase, and two cytochrome P450 genes (PAM18_1634 and PAM18_2564) involved in energy metabolism were, respectively, upregulated and downregulated in the gacA mutant compared with their expression in its parental strain (Fig. 4F).
Fig 4.
Influence of gacA on transcript levels of gene clusters involved in primary metabolism and cytochrome oxidase synthesis. (A, B) Two phosphonate transport and metabolic gene clusters, phn and pst, showed a significant enhancement in transcript levels due to the gacA mutation. (C, D) The transcript levels of two gene clusters involved in the utilization of sulfonate source and taurine, ssu and tauABCD, were significantly upregulated in the gacA mutant relative to their expression in wild-type strain M18. (E) Some of the nitric oxide reductase genes (nor) and nitrite reductase genes (nir) showed a larger but not significant downregulation in the gacA mutant relative to their expression in wild-type strain M18. (F) Influence of gacA on transcript abundance of the cyo operon and two cytochrome P450 genes. Hollow arrows, transcript levels that differed significantly between wild-type strain M18 and the gacA mutant strain (P < 0.05); gray arrows, P > 0.05.
Subsequently, we compared the ability of the M18 strain with its gacA mutant to grow on medium with various compounds as the sole source of phosphorus, sulfur, or nitrogen. As shown in Fig. 5A, when 10 mM KH2PO4 was added as the sole source of phosphorus in the minimal medium, the gacA mutant showed a significant increase in growth rate compared with that of wild-type strain M18. However, no obvious difference in growth between M18 and its gacA mutant was observed in the minimal medium without the addition of 10 mM KH2PO4. Five compounds, including MgSO4, methionine, cysteine, SDS, and taurine, were used as the sole source of sulfur in assessing the influence of GacA on the sulfur utilization capacity of P. aeruginosa M18. The results of assays for cell growth revealed that the increase in cell density caused by the gacA mutation in the minimal medium with the addition of MgSO4, methionine, or taurine as the sole source of sulfur was greater than that in the minimal medium without the corresponding addition of one of these three sulfur compounds (Fig. 5B, C, and F). However, the growth promotion caused by gacA inactivation did not show an obvious difference between the minimal medium amended with one of the other two sulfur compounds, cysteine and SDS, and the minimal medium not amended (Fig. 5D and E). The cell growth of the gacA mutant was markedly impaired compared with that of M18 in the minimal medium amended with 30 mM NH4Cl as the sole nitrogen source (Fig. 5G). However, the gacA mutation showed no significant influence on the cell growth of P. aeruginosa M18 in the minimal medium with NaNO3 as the sole nitrogen source (Fig. 5H). These results, together with the transcriptome data, indicate that the primary metabolism involved in the uptake or catabolism of phosphonate, sulfonate, and nitrate compounds is also under the global regulation of the M18 Gac system. The control of primary metabolism by the Gac system may ultimately contribute to nutritional competition and ion homeostasis in P. aeruginosa M18.
Fig 5.
Growth of P. aeruginosa M18 and the gacA mutant (M18G) in liquid minimal medium amended without or with different phosphorus (10 mM KH2PO4), sulfur (500 μM MgSO4, Met, Cys, SDS, or taurine), or nitrogen (NH4Cl or NaNO3) sources. Growth was monitored by measuring the OD600. Each value represents the mean and standard deviation of three replicate samples.
GacA strongly regulates many bacterial secretion systems. (i) GacA strongly upregulates the expression of all three type VI secretion systems.
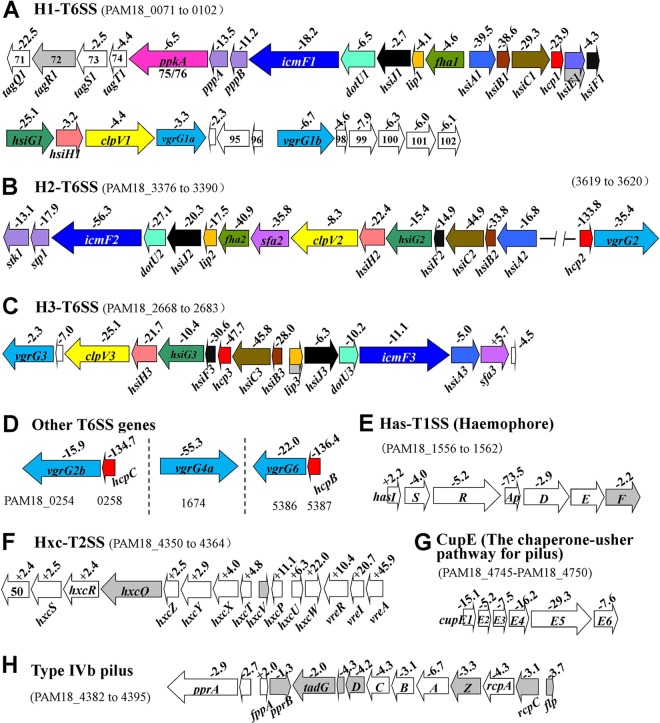
Numerous secretion systems, types I to VI, exist in Gram-negative bacteria. These systems are involved in the release of proteins, enzymes, or toxins, most of which belong to virulence or biocontrol factors (43, 44). The type VI secretion system (T6SS), a multipurpose delivery system with phage-like machinery, is one of the largest classes of bacterial secretion systems. In P. aeruginosa, three T6SSs (the H1, H2, and H3 T6SSs) secrete two types of homologous proteins, Hcp and VgrG (45). In the present study, microarray experiments determined that all three T6SSs, H1 (PAM18_0071 to PAM18_0102), H2 (PAM18_3390 to PAM18_3376), and H3 (PAM18_2668 to PAM18_2683), and other T6SS-related genes were strongly downregulated in the gacA mutant relative to their expression in its parental strain, M18. Among the T6SS regulons of GacA, the most significant downregulation at the transcript level was observed on the hcp genes encoding the secreted factor Hcp (hemolysin-coregulated protein), including hcp1, hcp2, hcp3, hcpB, and hcpC (Fig. 6A to D). To confirm further the positive control of gacA on the three T6SSs, we constructed three lacZ reporter plasmids carrying the hsiA1′-′lacZ, hsiA2′-′lacZ, and hsiB3′-′lacZ translational fusions. The β-galactosidase activities resulting from these three plasmids were assayed in the gacA mutant and wild-type strain M18. As shown in Fig. 7, the expression of the hsiA1, hsiA2, and hsiB3 operons, belonging to the H1, H2, and H3 T6SSs, respectively, was downregulated in the gacA mutant compared with their expression in M18. These results clearly suggest that the Gac system positively controls the expression of T6SSs in P. aeruginosa M18.
Fig 6.
Influence of gacA on transcript abundance of gene clusters encoding bacterial secretion systems in P. aeruginosa M18. (A to D) Positive control of gacA of all three P. aeruginosa T6SS gene clusters (H1, H2, and H3 T6SSs) and other related genes. In all three T6SSs, homologous genes are shown in the same color; (E) positive control of gacA on the has T1SS operon, involved in hemophore-dependent heme acquisition; (F) negative regulation of gacA on transcript levels of the hxc T2SS gene cluster; (G and H) the transcript levels of two pilus operons, cupE and tad, were under the positive control of gacA. Hollow arrows, transcript levels that differed significantly between wild-type strain M18 and the gacA mutant strain (P < 0.05); gray arrows, P value > 0.05.
Fig 7.

Analysis of β-galactosidase expression from the hsiA1′-′lacZ, hsiA2′-′lacZ, and hsiB3′-′lacZ translational fusion plasmids in P. aeruginosa M18 and the gacA mutant in KMB.
(ii) Positive control of the Has T1SS and negative control of the Apr T1SS and Hxc T2SS by GacA.
The Has type I secretion system (Has T1SS) HasDEF is responsible for the secretion of the hemophore HasAp (heme acquisition protein), which binds heme from hemoglobin and is then taken up via the cell surface receptor HasR in P. aeruginosa (44). Transcriptome data demonstrated that the hasISRApDEF gene cluster was significantly downregulated at the transcript level by the gacA mutation (Fig. 6E). The most significant downregulation of the gacA mutation was found at the transcript level of the gene encoding HasAp (Fig. 6E). These results suggest that the Gac system positively controls the expression of the Has T1SS. In contrast, another T1SS (Apr) involved in alkaline protease secretion (44) was under the negative control of GacA (Fig. 2H). Similarly, the hxc gene cluster (PAM18_4350 to PAM18_4361), encoding the Hxc T2SS, which is involved in the secretion of a low-molecular-weight alkaline phosphatase, LapA (44), displayed significant upregulation at the transcript level because of the gacA mutation. The downstream vreAIR operon encoding a cell surface signaling (CSS) system (46) was also strongly downregulated by GacA (Fig. 6F).
(iii) Strong and positive regulation of GacA on two pilus operons, the cupE type I pilus operon and the tad type IVb pilus operon.
Similar to P. aeruginosa PAO1, the M18 strain possesses four cup (chaperone-usher pathway) gene clusters, cupA, cupB, cupC, and cupE, which encode the fimbrial subunits for assembling type I pili. The Cup systems are important in surface attachment and biofilm formation (44). Among the four cup gene clusters, the cupE gene cluster showed the strongest downregulation at the transcript level in the gacA mutant compared with its expression in the M18 strain (Fig. 6G). Another type of pilus, the type IVb pilus, is mainly involved in adherence, motility, pathogenesis, etc. (47). The tad (tight adherence) locus encodes functions necessary for the biogenesis of the Flp subfamily of type IVb pili (47). The microarray result showed that the flp-rcp-tad locus was positively regulated by GacA (Fig. 6H).
Significant enhancement of bacterial motility by the gacA mutation.
Motility is important because it allows bacteria to colonize different environments, attach to surfaces, and form biofilms. Pseudomonas has three distinct modes of motility: swimming, swarming, and twitching. Pseudomonas motility, which is most closely related to flagella, pili, and chemotaxis, is also subject to the complex control of various regulatory systems and environmental factors (35). We measured the swimming, swarming, and twitching motilities of the gacA mutant and its parental strain, M18, in semisolid or soft agar to assess the influence of GacA on the motility of P. aeruginosa M18. As shown in Fig. 8, all three modes of motility were amazingly enhanced in the gacA mutant relative to wild-type strain M18. Compared with the wild-type strain, the gacA mutant strain showed a dramatic increase in flagellar swimming motility through the soft agar. In addition, it was hyperefficient in swarming motility, with an irregular hyperbranching pattern, and formed an increasing interstitial twitching zone through colony expansion. In turn, the motility of the gacA mutant could be recovered to the wild-type level through complementation with plasmid pME6032 carrying the gacA gene (Fig. 8). Unexpectedly, among the GacA regulons of M18, no entire or partial motility-related gene clusters were negatively regulated by GacA. In contrast, gene clusters potentially related to bacterial motility, such as che2, were positively controlled by GacA. These results imply that the Gac system negatively controls the motility of P. aeruginosa M18 through a series of indirect and complex pathways or mechanisms.
Fig 8.
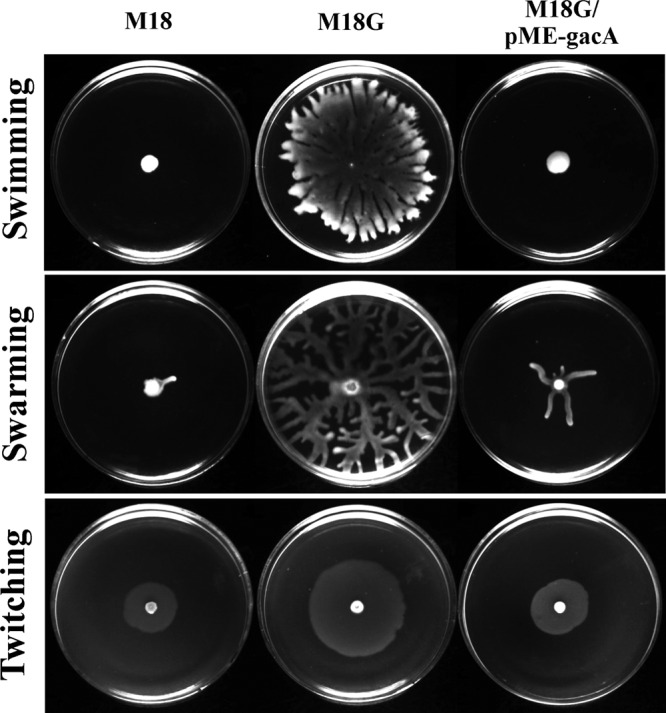
Swimming (0.3% agarose), swarming (0.5% agar), and twitching (1% agar) motility of M18, its gacA mutant, and the complemented gacA mutant strain after incubation 28°C for 24 h.
The Gac system regulates multiple transcriptional regulatory factors or systems.
The transcriptome profile revealed that 103 genes in the GacA regulon encode regulatory systems or factors, including 46 transcriptional regulators belonging to different families; 39 TCS elements; 15 sigma factors (RpoS, etc.), anti-sigma factors (VreR and FoxR), or sigma activators (Sfa2 and Sfa3); and 3 QS regulators (LasI, RsaL, and QscR). Among the TCSs regulated by GacA, PhoB/PhoR, NtrC/NtrB, PirS/PirR, and PAM18_2081/PAM18_2082 were significantly upregulated at the transcript level in the gacA mutant compared with their expression in wild-type strain M18. The PhoR/PhoB TCS globally regulates the expression of the phosphate transport and metabolism operons pst and phn and phoBR in other strains (36, 48). The pprA-pprB TCS, as part of the flp-tad-rcp gene cluster, activates the expression of the type IVb pilus Tad and type I pilus CupE in P. aeruginosa PAO1 (49, 50). In M18, the transcript level of the pprA sensor gene was significantly downregulated by the gacA mutation. The sensor kinase-response regulator hybrid RetS, which is involved in the regulation of secretion system expression, exopolysaccharide production, and biofilm formation (51), was also positively regulated by GacA in M18.
In addition to TCSs, multiple CSS systems constituted a larger group of the GacA regulon, such as VreI-VreR-VreA, which upregulates the expression of the Hxc T2SS (46), FoxI-FoxR-FoxA (52), and two CSS-related systems, TonB2-ExbB1-ExbD1 and TonB4-ExbD2-ExbB2. The CSS system typically consists of three components: an alternative σ70 factor with an extracytoplasmic function, a sigma factor regulator (anti-sigma factor) in the cytoplasmic membrane, and a TonB-dependent outer membrane receptor. The TonB-ExbB-ExbD protein complex provides energy for the transport of the iron-siderophore complex across the outer membrane (46). In the transcriptome profile of M18, the largest family of transcriptional regulators under the control of GacA is the LysR family, which includes 11 genes. Other families include GntR (5 genes), AraC (5 genes), Cro/CI (4 genes), MarR (2 genes), MerR (3 genes), TetR (3 genes), sigma factors (11 genes), and others (17 genes). For example, the redox-sensitive transcriptional activator SoxR, a member of the MerR family, was negatively controlled by GacA.
The presence of multiple regulators within the GacA regulon implies that, in most cases, the GacS-GacA system may indirectly regulate various cellular processes and functions (e.g., bacterial metabolism, secretion systems, and motility) in M18 through the mediation of these regulatory systems or factors. Most of the above-mentioned regulators controlled by GacA in M18 are involved in the global or pathway-specific regulation of the Gac-controlled phenotypes in other Pseudomonas strains. Most GacA-regulated gene clusters include or link with one or more regulatory genes, such as pch (pchR) (Fig. 2B), phz2 (qscR) (Fig. 2D), plt (pltR) (Fig. 2C), pst (pstU and PAM18_5484) (Fig. 4B), phn (phnF) (Fig. 4A), the H1 T6SS (ppkA-pppA) (Fig. 6A), the H2 T6SS (stk1-stp1 and sfa2) (Fig. 6B), and the H3 T6SS (sfa3) (Fig. 6C).
DISCUSSION
The conserved Gac-Rsm signal transduction pathway, which is constituted by the two-component regulatory system GacS/GacA (BarA/UvrY in E. coli), the small RNAs RsmYZ (CsrB), and the translational repressor RsmA (CsrA), is typically involved in the regulation of virulence or biocontrol factors and carbon storage in many gammaproteobacteria (53). Multiple distinct regulatory characteristics of secondary metabolism, including the antibiotic biosynthesis shown by P. aeruginosa strain M18 isolated from the rhizosphere (4, 10–16, 22), urged us to ascertain the genome-scale profiling of bacterial physiology and metabolism in the gacA mutant of the M18 strain. A general model of GacA-mediated global regulation in P. aeruginosa M18 is outlined in Fig. 9. The GacS/GacA system plays a pivotal role in the transcriptional regulation of diverse metabolic and physiological functions in the rhizobacterium P. aeruginosa M18, including secondary metabolism (involved in the biosynthesis of antibiotics, exoproducts, and siderophores), primary metabolism (involved in the assimilation and metabolism of phosphorus, sulfur, and nitrogen sources), ion homeostasis, ecological or nutritional competition, secretion systems, and cell motility (Fig. 9). The global transcriptional regulation driven by GacA is mostly mediated by many global or pathway-specific transcriptional regulatory systems or factors accounting for over 12% of all GacA regulons, such as transcriptional regulatory proteins of diverse families, TCSs, sigma factors or regulators, CSS systems, and QS systems. The Gac cascade, together with these regulatory systems and factors, is integrated into a cascaded and interwoven network to regulate globally a broad range of cell functions in M18.
Fig 9.
General model of GacA-mediated global regulation in P. aeruginosa M18. The GacS/GacA two-component system, directly or indirectly through a large number of transcriptional or posttranscriptional regulatory systems or factors, controls multiple sets of diverse biological processes, mainly including secondary metabolism, primary metabolism, bacterial secretion, and motility. Pvd, pyoverdine; Pch, pyochelin; T1P, type I pilus system; T4bP, type IVb pilus system.
The transcriptome profile of the gacA mutant was analyzed in both P. protegens Pf-5 (23) and P. aeruginosa PAK (24). The gacA mutation has a more extensive and significant impact on genomic expression in M18 than it does in either Pf-5 or PAK. This observation may mainly be attributed to the differences in strain specificity, culture conditions (including medium and temperature), sampling time, etc. The transcriptome profiles of the gacA mutant were assayed for P. protegens Pf-5 grown in NBGly medium at 20°C to an OD600 of 2.4 (23), P. aeruginosa PAO1 grown in LB medium at 37°C to an OD600 of approximately 6.0 (24), and P. aeruginosa M18 grown in KMB at 28°C for 22 h to an OD600 of 5.0 to 5.5. The culture conditions including the KMB and the 28°C temperature used in this study are generally used for Pseudomonas conventional cultivation and antibiotic fermentation (26). Also, P. aeruginosa M18 incubated in KMB at 28°C for 22 h enters into the early stationary phase, at which time bacteria start to biosynthesize secondary metabolites. Such culture conditions and such a sampling time were initially chosen for transcriptome profiling on the basis of the established viewpoint that the Gac system is a master regulatory system for Pseudomonas secondary metabolism (19). The transcriptome profile of P. protegens Pf-5 revealed that GacA significantly influenced the transcript levels of 635 genes. Of these genes, 124 were downregulated and 86 were upregulated (fold change, >2; P < 0.05) by the gacA mutation (23). Comparison of the gacA transcriptomes of M18 and Pf-5 showed that 34 genes, 10 genes, and 11 genes were, respectively, similarly downregulated, similarly upregulated, and reversely regulated (fold change, >2; P < 0.05) by a gacA mutation (see Table S4 in the supplemental material). Three gene clusters, those for Plt, HCN, and H1 T6SS, are under the positive control of GacA in both strains (23). However, two siderophore gene clusters, pch and pvd, are positively regulated in M18 and negatively regulated in Pf-5 by GacA. Similarly, the regulatory role of gacA on two type I secretion systems, the Apr T1SS and the Has T1SS, in M18 is opposite of that in Pf-5 (23). In addition, a number of cytochrome c (cco) and o (cyo) oxidase genes involved in energy metabolism were under the similar control of GacA in both Pf-5 and M18. Apart from these common GacA regulons, in M18 a more extensive list of GacA regulons is involved in bacterial secretion (the H2 T6SS, H3 T6SS, Hxc T2SS, type I pilus CupE, and type IVb pilus Tad), secondary metabolism (amb and phz), and primary metabolism (phn, pst, ssu, tau, nir, and nor).
The transcriptome profile of P. aeruginosa PAK revealed that 116 genes are positively controlled and 55 genes are negatively regulated (fold change, >2; P < 0.05) by GacA (24). Comparison of the transcriptome profiles of P. aeruginosa M18 and PAK revealed that 54 genes of the GacA regulon are under the common positive regulation of GacA in both strains (see Table S5 in the supplemental material). These GacA regulons with positive regulation include some genes of the H1 T6SS and H3 T6SS; the cat gene (PAM18_4330), encoding chloramphenicol acetyltransferase; the sodM gene (PAM18_4559), encoding superoxide dismutase; etc. (24). However, only two genes and four genes were, respectively, commonly downregulated and reversely regulated by GacA in both strains (see Table S5 in the supplemental material). In the PAK strain, the Gac system negatively controls the type III secretion system, which is encoded by the genes psc, pcr, exs, and pop, and the pyrroloquinoline quinone genes pqqABCDE (24). However, the expression of these genes is not significantly influenced by the gacA mutation in M18.
Typically, the GacS/GacA TCS plays an important role in the transition from primary to secondary metabolism and the control of the production of secondary metabolites and extracellular enzymes involved in biocontrol or pathogenicity and ecological fitness or competition (19, 53). This study shows that the Gac system regulates not only the secondary metabolism involved in the biosynthesis of antibiotics, siderophores, and extracellular enzymes but also the primary metabolism involved in the transport and metabolism of phosphate, sulfonate, and nitrogen compounds in P. aeruginosa M18. This observation implies another important function of the Gac system in ion homeostasis and the nutritional starvation response. For example, the PhoR/PhoB TCS, which is negatively controlled by the Gac system in M18, globally regulates the expression of phn, pst, phoBR, and other pho regulons in bacteria. These Pho regulons are important parts of a complex network involved in the stress response, phosphate homeostasis, and pathogenesis (36).
The cell surface structures responsible for bacterial motility vary among prokaryotes. In P. aeruginosa, the polar flagellum mediates swimming motility in aqueous environments or soft-agar plates, in which the movement direction is biased by chemotactic responses to chemical stimuli, and swarming motility, which is a type of social motility. However, twitching motility, a flagellum-independent mode of surface translocation, is mediated by the type IV pilus (35). All three modes of bacterial motility are repressed by the Gac system in M18. However, no obvious clustered genes directly related to bacterial motility, such as flagellar and pilus biosynthetic and assembly genes fli, flg, che, and pil, were systematically found to be upregulated by the gacA mutation. Instead, the motility-related che2 chemotaxis operon (54) was shown to be positively regulated by GacA in M18. Only a few individual motility-related genes, such as the two-component sensor gene fleS, the flagellar gene fliS, and the monorhamnolipid biosynthetic operon rhlAB, were shown to be under the negative regulation of GacA. Whether the upregulation of these genes is sufficient to significantly enhance the bacterial motility of the gacA mutant of M18 still needs to be assessed. In agreement with the negative control of M18 swarming motility by GacA, RsmA, as a downstream element of the Gac/Rsm cascade, positively controls the swarming ability of P. aeruginosa PAO1 (55). The GacS/GacA TCS in P. fluorescens F113 represses swimming motility via the KinB/AlgB TCS (56). Interestingly, the M18 transcriptomic data suggested that the transcript level of the response regulator gene algB, not the sensor gene kinB, was significantly downregulated by the gacA mutation. However, it remains unknown whether the Gac system represses motility through KinB/AlgB in M18. In contrast to the Gac repression of F113 motility, the gacA mutant almost entirely impairs the swarming motility but does not obviously influence the swimming motility of the rhizobacterium P. protegens Pf-5 (23). In addition, the swarming motility of P. aeruginosa requires flagella and pili. It is also dependent on the Rhl QS system and is positively regulated by the Las QS system (57). In short, bacterial motility shows apparent strain specificity and is subject to complex regulation of genetic and environmental factors. Therefore, further research is needed to clarify the molecular regulatory mechanism of the Gac system on the motility of P. aeruginosa M18.
In conclusion, the GacS/GacA two-component system of P. aeruginosa M18 globally controls multiple sets of diverse biological processes, including secondary metabolism, primary metabolism, bacterial secretion, and motility, which contribute to ecological fitness or competence, ion homeostasis and nutritional competence, biocontrol or pathogenesis, etc. The P. aeruginosa PAK Gac system exerts direct transcriptional control only on the expression of sRNAs RsmYZ and thus posttranscriptionally regulates many relevant target genes or gene clusters. In P. aeruginosa M18, rsmY expression was almost entirely inhibited by the gacA mutation, while the gacA mutation led to only a partial decrease in rsmZ expression (unpublished data). However, in M18, it remains unknown whether all GacA regulons involved in structural and regulatory functions are exclusively regulated via the Gac/Rsm cascade, composed of three different components, the GacS/GacA two-component system, the sRNAs RsmY and RsmZ, and the RNA-binding repressor RsmA. Identification and characterization of more direct target genes of each component in the Gac/Rsm system and their downstream key regulators should be conducted through the use of high-throughput technologies, such as chromatin immunoprecipitation with microarray technology, RNA sequencing, and interactomic techniques, to distinguish the Gac/Rsm-derived direct regulatory pathways from indirect pathways. Therefore, further research is needed to understand the complex regulatory pathways and networks that are commonly made up from the Gac/Rsm cascade and other global or pathway-specific regulatory systems or factors, such as other TCSs, QSs, CSSs, sRNAs, transcriptional or posttranscriptional regulators of different families, etc.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (31270083), the National Key Basic Research Program (973 Program) of China (2009CB118906, 2012CB721005), the National High-Tech Research and Development Program (863 Program) of China (2007AA02Z215, 2012AA02Z107), and the Shanghai Leading Academic Discipline Project (B203).
Footnotes
Published ahead of print 24 May 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00214-13.
REFERENCES
- 1. Huang X, Zhu D, Ge Y, Hu H, Zhang X, Xu Y. 2004. Identification and characterization of pltZ, a gene involved in the repression of pyoluteorin biosynthesis in Pseudomonas sp. M18. FEMS Microbiol. Lett. 232: 197– 202 [DOI] [PubMed] [Google Scholar]
- 2. Wu D, Ye J, Ou H, Wei X, Huang X, He Y, Xu Y. 2011. Genomic analysis and temperature-dependent transcriptome profiles of the rhizosphere originating strain Pseudomonas aeruginosa M18. BMC Genomics 12: 438. 10.1186/1471-2164-12-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee X, Fox A, Sufrin J, Henry H, Majcherczyk P, Haas D, Reimmann C. 2010. Identification of the biosynthetic gene cluster for the Pseudomonas aeruginosa antimetabolite l-2-amino-4-methoxy-trans-3-butenoic acid. J. Bacteriol. 192:4251– 4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Y, Du X, Lu ZJ, Wu D, Zhao Y, Ren B, Huang J, Huang X, Xu Y. 2011. Regulatory feedback loop of two phz gene clusters through 5′-untranslated regions in Pseudomonas sp. M18. PLoS One 6: e19413. 10.1371/journal.pone.0019413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang X, Yan A, Zhang X, Xu Y. 2006. Identification and characterization of a putative ABC transporter PltHIJKN required for pyoluteorin production in Pseudomonas sp. M18. Gene 376:68– 78 [DOI] [PubMed] [Google Scholar]
- 6. Nowak-Thompson B, Chaney N, Wing JS, Gould SJ, Loper JE. 1999. Characterization of the pyoluteorin biosynthetic gene cluster of Pseudomonas fluorescens Pf-5. J. Bacteriol. 181: 2166– 2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brodhagen M, Paulsen I, Loper JE. 2005. Reciprocal regulation of pyoluteorin production with membrane transporter gene expression in Pseudomonas fluorescens Pf-5. Appl. Environ. Microbiol. 71: 6900– 6909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winstanley C, Langille MG, Fothergill JL, Kukavica-Ibrulj I, Paradis-Bleau C, Sanschagrin F, Thomson NR, Winsor GL, Quail MA, Lennard N, Bignell A, Clarke L, Seeger K, Saunders D, Harris D, Parkhill J, Hancock RE, Brinkman FS, Levesque RC. 2009. Newly introduced genomic prophage islands are critical determinants of in vivo competitiveness in the Liverpool epidemic strain of Pseudomonas aeruginosa. Genome Res. 19: 12– 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kidarsa TA, Goebel NC, Zabriskie TM, Loper JE. 2011. Phloroglucinol mediates cross-talk between the pyoluteorin and 2,4-diacetylphloroglucinol biosynthetic pathways in Pseudomonas fluorescens Pf-5. Mol. Microbiol. 81: 395– 414 [DOI] [PubMed] [Google Scholar]
- 10. Ge Y, Huang X, Wang S, Zhang X, Xu Y. 2004. Phenazine-1-carboxylic acid is negatively regulated and pyoluteorin positively regulated by gacA in Pseudomonas sp. M18. FEMS Microbiol. Lett. 237: 41– 47 [DOI] [PubMed] [Google Scholar]
- 11. Yan A, Huang X, Liu H, Dong D, Zhang D, Zhang X, Xu Y. 2007. An rhl-like quorum-sensing system negatively regulates pyoluteorin production in Pseudomonas sp. M18. Microbiology 153: 16– 28 [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Wang X, Huang X, Zhang X, Xu Y. 2008. Las-like quorum-sensing system negatively regulates both pyoluteorin and phenazine-1-carboxylic acid production in Pseudomonas sp. M18. Sci. China C Life Sci. 51: 174– 181 [DOI] [PubMed] [Google Scholar]
- 13. Huang X, Zhang X, Xu Y. 2008. PltR expression modulated by the global regulators GacA, RsmA, LasI and RhlI in Pseudomonas sp. M18. Res. Microbiol. 159: 128– 136 [DOI] [PubMed] [Google Scholar]
- 14. Huang J, Xu Y, Zhang H, Li Y, Huang X, Ren B, Zhang X. 2009. Temperature-dependent expression of phzM and its regulatory genes lasI and ptsP in rhizosphere isolate Pseudomonas sp. strain M18. Appl. Environ. Microbiol. 75: 6568– 6580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu J, Huang X, Li K, Li S, Zhang M, Wang Y, Jiang H, Xu Y. 2009. LysR family transcriptional regulator PqsR as repressor of pyoluteorin biosynthesis and activator of phenazine-1-carboxylic acid biosynthesis in Pseudomonas sp. M18. J. Biotechnol. 143: 1– 9 [DOI] [PubMed] [Google Scholar]
- 16. Wang G, Huang X, Li S, Huang J, Wei X, Li Y, Xu Y. 2012. The RNA chaperone Hfq regulates antibiotic biosynthesis in the rhizobacterium Pseudomonas aeruginosa M18. J. Bacteriol. 194: 2443– 2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang X, Wang S, Geng H, Ge Y, Huang X, Hu H, Xu Y. 2005. Differential regulation of rsmA gene on biosynthesis of pyoluteorin and phenazine-1-carboxylic acid in Pseudomonas sp. M18. W. J. Microbiol. Biotechnol. 21: 883– 889 [Google Scholar]
- 18. Blumer C, Heeb S, Pessi G, Haas D. 1999. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc. Natl. Acad. Sci. U. S. A. 96: 14073– 14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haas D, Keel C. 2003. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 41: 117– 153 [DOI] [PubMed] [Google Scholar]
- 20. Schubert M, Lapouge K, Duss O, Oberstrass FC, Jelesarov I, Haas D, Allain FH. 2007. Molecular basis of messenger RNA recognition by the specific bacterial repressing clamp RsmA/CsrA. Nat. Struct. Mol. Biol. 14: 807– 813 [DOI] [PubMed] [Google Scholar]
- 21. Yan A, Wang X, Zhang X, Xu Y. 2007. LysR family factor PltR positively regulates pyoluteorin production in a pathway-specific manner in Pseudomonas sp. M18. Sci. China Ser. C Life Sci. 37: 325– 332 [DOI] [PubMed] [Google Scholar]
- 22. Li S, Huang X, Wang G, Xu Y. 2012. Transcriptional activation of pyoluteorin operon mediated by the LysR-type regulator PltR bound at a 22 bp lys box in Pseudomonas aeruginosa M18. PLoS One 7: e39538. 10.1371/journal.pone.0039538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hassan KA, Johnson A, Shaffer BT, Ren Q, Kidarsa TA, Elbourne LD, Hartney S, Duboy R, Goebel NC, Zabriskie TM, Paulsen IT, Loper JE. 2010. Inactivation of the GacA response regulator in Pseudomonas fluorescens Pf-5 has far-reaching transcriptomic consequences. Environ. Microbiol. 12:899– 915 [DOI] [PubMed] [Google Scholar]
- 24. Brencic A, McFarland KA, McManus HR, Castang S, Mogno I, Dove SL, Lory S. 2009. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol. Microbiol. 73: 434– 445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brencic A, Lory S. 2009. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol. Microbiol. 72: 612– 632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44: 301– 307 [PubMed] [Google Scholar]
- 27. Levitch ME, Stadtman ER. 1964. Study of the biosynthesis of phenazine-1-carboxylic acid. Arch. Biochem. Biophys. 106: 194– 199 [DOI] [PubMed] [Google Scholar]
- 28. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Press Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 29. Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. 2003. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31: e15. 10.1093/nar/gng015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 31. Bakker AW, Schipper B. 1987. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas SPP-mediated plant growth-stimulation. Soil Biol. Biochem. 19:451– 457 [Google Scholar]
- 32. Schwyn B, Neilands JB. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160: 47– 56 [DOI] [PubMed] [Google Scholar]
- 33. Gebhard S, Tran SL, Cook GM. 2006. The Phn system of Mycobacterium smegmatis: a second high-affinity ABC-transporter for phosphate. Microbiology 152: 3453– 3465 [DOI] [PubMed] [Google Scholar]
- 34. Kertesz MA, Leisinger T, Cook AM. 1993. Proteins induced by sulfate limitation in Escherichia coli, Pseudomonas putida, or Staphylococcus aureus. J. Bacteriol. 175: 1187– 1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rashid MH, Kornberg A. 2000. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 97: 4885– 4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lamarche MG, Wanner BL, Crepin S, Harel J. 2008. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 32: 461– 473 [DOI] [PubMed] [Google Scholar]
- 37. Lapouge K, Sineva E, Lindell M, Starke K, Baker CS, Babitzke P, Haas D. 2007. Mechanism of hcnA mRNA recognition in the Gac/Rsm signal transduction pathway of Pseudomonas fluorescens. Mol. Microbiol. 66: 341– 356 [DOI] [PubMed] [Google Scholar]
- 38. Paulsen IT, Press CM, Ravel J, Kobayashi DY, Myers GS, Mavrodi DV, DeBoy RT, Seshadri R, Ren Q, Madupu R, Dodson RJ, Durkin AS, Brinkac LM, Daugherty SC, Sullivan SA, Rosovitz MJ, Gwinn ML, Zhou L, Schneider DJ, Cartinhour SW, Nelson WC, Weidman J, Watkins K, Tran K, Khouri H, Pierson EA, Pierson LS, III, Thomashow LS, Loper JE. 2005. Complete genome sequence of the plant commensal Pseudomonas fluorescens Pf-5. Nat. Biotechnol. 23: 873– 878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406: 959– 964 [DOI] [PubMed] [Google Scholar]
- 40. Gross H, Loper JE. 2009. Genomics of secondary metabolite production by Pseudomonas spp. Nat. Prod. Rep. 26: 1408– 1446 [DOI] [PubMed] [Google Scholar]
- 41. Sacherer P, Defago G, Haas D. 1994. Extracellular protease and phospholipase C are controlled by the global regulatory gene gacA in the biocontrol strain Pseudomonas fluorescens CHA0. FEMS Microbiol. Lett. 116: 155– 160 [DOI] [PubMed] [Google Scholar]
- 42. Barker AP, Vasil AI, Filloux A, Ball G, Wilderman PJ, Vasil ML. 2004. A novel extracellular phospholipase C of Pseudomonas aeruginosa is required for phospholipid chemotaxis. Mol. Microbiol. 53: 1089– 1098 [DOI] [PubMed] [Google Scholar]
- 43. Dalbey RE, Kuhn A. 2012. Protein traffic in Gram-negative bacteria—how exported and secreted proteins find their way. FEMS Microbiol. Rev. 36: 1023– 1045 [DOI] [PubMed] [Google Scholar]
- 44. Filloux A. 2011. Protein secretion systems in Pseudomonas aeruginosa: an essay on diversity, evolution, and function. Front. Microbiol. 2: 155. 10.3389/fmicb.2011.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Silverman JM, Brunet YR, Cascales E, Mougous JD. 2012. Structure and regulation of the type VI secretion system. Annu. Rev. Microbiol. 66:453– 472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Llamas MA, van der Sar A, Chu BC, Sparrius M, Vogel HJ, Bitter W. 2009. A novel extracytoplasmic function (ECF) sigma factor regulates virulence in Pseudomonas aeruginosa. PLoS Pathog. 5: e1000572. 10.1371/journal.ppat.1000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burrows LL. 2012. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol. 66:493– 520 [DOI] [PubMed] [Google Scholar]
- 48. Sola-Landa A, Moura RS, Martin JF. 2003. The two-component PhoR-PhoP system controls both primary metabolism and secondary metabolite biosynthesis in Streptomyces lividans. Proc. Natl. Acad. Sci. U. S. A. 100: 6133– 6138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giraud C, Bernard CS, Calderon V, Yang L, Filloux A, Molin S, Fichant G, Bordi C, de Bentzmann S. 2011. The PprA-PprB two-component system activates CupE, the first non-archetypal Pseudomonas aeruginosa chaperone-usher pathway system assembling fimbriae. Environ. Microbiol. 13: 666– 683 [DOI] [PubMed] [Google Scholar]
- 50. Bernard CS, Bordi C, Termine E, Filloux A, de Bentzmann S. 2009. Organization and PprB-dependent control of the Pseudomonas aeruginosa tad locus, involved in Flp pilus biology. J. Bacteriol. 191: 1961– 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goodman AL, Kulasekara B, Rietsch A, Boyd D, Smith RS, Lory S. 2004. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7: 745– 754 [DOI] [PubMed] [Google Scholar]
- 52. Mettrick KA, Lamont IL. 2009. Different roles for anti-sigma factors in siderophore signalling pathways of Pseudomonas aeruginosa. Mol. Microbiol. 74: 1257– 1271 [DOI] [PubMed] [Google Scholar]
- 53. Lapouge K, Schubert M, Allain FHT, Haas D. 2008. Gac/Rsm signal transduction pathway of gamma-proteobacteria: from RNA recognition to regulation of social behaviour. Mol. Microbiol. 67: 241– 253 [DOI] [PubMed] [Google Scholar]
- 54. Hong CS, Shitashiro M, Kuroda A, Ikeda T, Takiguchi N, Ohtake H, Kato J. 2004. Chemotaxis proteins and transducers for aerotaxis in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 231: 247– 252 [DOI] [PubMed] [Google Scholar]
- 55. Heurlier K, Williams F, Heeb S, Dormond C, Pessi G, Singer D, Camara M, Williams P, Haas D. 2004. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J. Bacteriol. 186: 2936– 2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barahona E, Navazo A, Martinez-Granero F, Zea-Bonilla T, Perez-Jimenez RM, Martin M, Rivilla R. 2011. Pseudomonas fluorescens F113 mutant with enhanced competitive colonization ability and improved biocontrol activity against fungal root pathogens. Appl. Environ. Microbiol. 77: 5412– 5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kohler T, Curty LK, Barja F, van Delden C, Pechere JC. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182: 5990– 5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.