Abstract
Escherichia coli heat-stable toxin b (STb) causes diarrhea in animals. STb binds to sulfatide, its receptor, and is then internalized. In the cytoplasm, through a cascade of events, STb triggers the opening of ion channels, allowing ion secretion and water loss and leading to diarrhea. Tight junctions (TJs) are well known for controlling paracellular traffic of ions and water by forming a physical intercellular barrier in epithelial cells, and some bacterial toxins are known to affect adversely TJs. The present study aimed at determining the effect of STb on TJs. T84 cells were treated for 24 h with purified STb and a nontoxic STb mutant (D30V). Transepithelial resistance (TER), paracellular flux marker, and confocal microscopy were used to analyze the effect of STb on TJs. Purified STb caused a significant reduction of TER parallel to an increase in paracellular permeability compared to the results seen in untreated cells or mutant D30V. The increased paracellular permeability was associated with a marked alteration of F-actin stress fibers. F-actin filament dissolution and condensation were accompanied by redistribution and/or fragmentation of ZO-1, claudin-1, and occludin. These changes were also observed following treatment of T84 cells with an 8-amino-acid peptide found in the STb sequence corresponding to a consensus sequence of Vibrio cholerae Zot toxin. These effects were not observed with a scrambled peptide or mutant D30V. Our findings indicate that STb induces epithelial barrier dysfunction through changes in TJ proteins that could contribute to diarrhea.
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is a major cause of severe diarrhea in newborn animals (1) and diarrhea in young children in developing countries as well as in travelers worldwide (2). ETEC express virulence factors, including adhesins that mediate the attachment of bacteria to the intestinal epithelial cells and enterotoxins that disrupt intestinal fluid homeostasis, causing diarrhea (3, 4). In addition to adhesive and enterotoxic virulence factors, pathogenesis involves host factors among which, most importantly, are the adhesin and enterotoxin receptors. The characteristics and mechanisms of action of many adhesive and enterotoxic virulence factors are well known, but others are partially understood or completely unknown.
Heat-stable toxin b (STb), a 48-amino-acid peptide of 5.2 kDa, is one of the thermostable enterotoxins secreted by ETEC that is responsible for intestinal fluid secretion resulting in diarrhea in many animal species (4, 5). STb acts by binding to cells through its receptor, sulfatide (6). Then, STb is internalized and activates a pertussis toxin-sensitive G protein (Gαi3), resulting in calcium ion entry through a ligand-gated calcium ion channel (7). The elevated intracellular Ca2+ concentration activates protein kinase C (PKC), which phosphorylates the cystic fibrosis transmembrane regulator (CFTR), resulting in secretion of Cl−. This Ca2+ increase also stimulates arachidonic acid release from membrane phospholipids through phospholipase C activity and subsequent production of prostaglandin E2 (PGE2) and 5-hydroxytryptamine (5-HT) (8, 9). These compounds mediate transport of water and electrolytes such as HCO3−, Na+, and Cl− into the intestinal lumen, resulting in watery diarrhea (4).
The intestinal lumen contains a single layer of epithelial cells. This is an integral and essential part of the host mucosal defense system, acting as a physical barrier (10). This barrier is maintained by connections between adjoining epithelial cells consisting of specialized intercellular junctions: tight junctions (TJs) and adherens junctions. TJs seal the intercellular spaces between adjacent cells and are essential for the integrity of barrier function by restricting paracellular permeability (11).
TJs are mainly composed of a protein complex that includes occludin and the claudin family of transmembrane proteins known to associate with the apical perijunction of F-actin ring via cytoplasmic plaque proteins such as ZO-1, ZO-2, and ZO-3 (12). TJs limit the diffusion of solutes through the intercellular spaces of intestinal epithelial cells and provide a barrier for the entry of pathogens and harmful antigens (13). Furthermore, the establishment and maintenance of TJs are crucial to both the development and normal functioning of epithelia (14). For example, when TJs of epithelial cells that cover the biliary tree and gastrointestinal tract become disorganized, jaundice and diarrhea, respectively, occur (15). However, some pathogenic bacteria such as enteropathogenic E. coli, serotype O127:H6 (EPEC) (16, 17), and enterohemorrhagic E. coli, serotype O157:H7 (EHEC) (18), and bacterial toxins such as Clostridium difficile toxins A and B (19), Vibrio cholerae Zonula occludens toxin (Zot) (20), secreted autotransporter toxin (SAT) (21), and Shiga toxin (22) target and disrupt TJs. This can be measured by a decrease in transepithelial resistance (TER), an increase in paracellular flux, changes in the distribution of tight junction protein complexes, and reorganization of F-actin, leading to disruption of homeostasis.
TJ disruption may result either from direct modification of tight junction proteins (ZO-1, occludin, and claudins) or by direct binding to TJ components (e.g., Clostridium perfringens enterotoxin) and/or by alteration of the cell cytoskeleton. Zot can, after interacting with a mammalian cell receptor, induce modifications of the cytoskeleton organization that lead to the opening of TJs. This occurs through transmembrane phospholipase C activity and subsequent protein kinase C-α-dependent polymerization of actin filaments strategically localized to regulate the paracellular pathway (23).
Interestingly, a sequence comparison between STb and Zot revealed an 8-amino-acid shared sequence in which 5 of the 8 amino acids are identical (GXLXVXDG). Binding of Zot and STb to their respective receptors involves this shared sequence, and for Zot toxin it has been shown to be involved in TJ disassembly. The aim of the present study was to examine the effects of STb on TJ structure and function in T84 intestinal epithelial cells, a cell line commonly used to study bacterial enterotoxin secretory processes (24, 25, 26, 27, 28, 29).
MATERIALS AND METHODS
Culture media, antibodies, and reagents.
Dulbecco's modified Eagle medium (DMEM), Ham's F-12 nutrient mixture (F-12), phosphate-buffered saline (PBS; pH 7.4, without calcium chloride and magnesium chloride), fetal bovine serum (FBS), mouse monoclonal anti-ZO-1, rabbit polyclonal anti-occludin, rabbit polyclonal anti-claudin-1, goat anti-mouse Alexa 568, and goat anti-rabbit Alexa 488 antibodies, and bovine serum albumin-fluorescein isothiocyanate (BSA-FITC) conjugates and DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride) were purchased from Invitrogen, Canada. FITC-phalloidin and STb24-31peptide (GFLGVRDG) and the scrambled peptide (LGRDGGVF) were purchased from Sigma and Biomatik (Canada), respectively. Isopropyl-β-d-thiogalactopyranoside (IPTG) was purchased from Sigma, factor Xa was from Roche, and phenymethanesulfonyl fluoride (PMSF) was from Gibco BRL.
Production and purification of STb.
STb enterotoxin was produced as described previously (30). Briefly, E. coli HB101 strains harboring pMal-STb, a plasmid encoding wild-type STb protein fused to the maltose binding protein, were used. Ampicillin, at a final concentration of 50 μg/ml, was used as the selection agent for bacteria carrying the plasmid pMAL-STb. Bacteria were grown in rich medium (10 g tryptone, 5 g yeast extract, 5 g NaCl, 2 g dextrose [per liter, containing 50 μg/ml of ampicillin]) for 18 h in an orbital shaker set at 37°C and 180 rpm. A volume of 5 ml of an overnight bacterial culture was transferred to 500 ml of fresh rich medium and returned to the orbital shaker until the optical density at 600 nm reached 0.5. Then, 0.3 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to induce the synthesis of the fusion protein. The induction was allowed to proceed for 3 h in the orbital shaker. Cells were harvested by centrifugation at 4,000 × g for 15 min at 4°C. The pellet was gently washed in a volume of 250 ml of 30 mM Tris-HCl (pH 8) containing 20% sucrose and 1 mM EDTA. After centrifugation at 8,000 × g for 20 min at 4°C, an osmotic shock of bacteria was induced using a solution of 5 mM MgSO4 containing 0.4 mM PMSF and then centrifuged at 8,000 × g for 20 min at 4°C. The supernatant containing the fusion protein (MBP-STb) was filter sterilized using a 0.22-μm-pore-size tangential flow filter (VacuCap; Pall Life Sciences). The fusion protein was purified using an amylose chromatography affinity column (New England BioLabs) (30-ml column bed volume) at a flow rate of 0.2 ml/min at 4°C. A solution of 10 mM maltose was used to elute the fusion protein. The eluted proteins were dialyzed against MilliQ water using a 12,000- to 14,000-Da membrane (Spectrum, Dominguez, CA).
Dialyzed material was then concentrated and cleaved using factor Xa enzyme (Roche) in a cleavage buffer consisting of 100 mM NaCl, 50 mM Tris-HCl, and 1 mM CaCl2 (pH 8). Using an AKTA-10 purifier system (GE Healthcare), the cleaved material was loaded onto a C8 reverse-phase column (PerkinElmer, Montreal, Canada) and eluted with a linear gradient of acetonitrile in water solution containing 0.1% trifluoroacetic acid. Using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific), a standard curve of various concentrations of aprotinin (molecular weight [MW], ∼6,500) with absorbance at 214 nm was prepared. STb preparations were quantified using the generated standard curve and kept at −20°C until use. Mutated STb (D30V) binding to sulfatide but for which no biological activity was observed in vivo was purified using the same method (31).
Intestinal cell culture and treatments.
T84 human colon intestinal epithelial cells, used as a model to study the effects of enterotoxins on TJ proteins (24, 25, 26, 27, 28, 29), were obtained from the American Type Culture Collection. Cells (passage 5 to 18) were maintained in equal volumes of Dulbecco's modified eagle medium (DMEM) and F-12 supplemented with 5% (vol/vol) fetal bovine serum (FBS) (Invitrogen, Canada). Cells were cultured in T-75 culture flasks (Sarstedt, Canada) and were kept at 37°C in a 5% CO2/95% air atmosphere. For maintenance purposes, confluent T84 monolayers were passaged weekly using trypsin-EDTA treatment in phosphate-buffered saline (PBS) without calcium chloride and magnesium chloride. To generate polarized T84 cell monolayers for transepithelial electrical resistance (TER) and permeability assays, a total of 300,000 cells/ml were plated on collagen-coated 12-mm-diameter polycarbonate Transwell permeable support cell culture inserts (Costar) (0.4 pore size) and grown for 6 to 8 days, during which cells were fed daily. After this period, electrically tight monolayers were formed. For immunofluorescence experiments, T84 cells were seeded at a density of 150,000 cells/ml on LabTek 8-well chamber slides (Fisher Scientific, Canada) and used after 2 days. One hour before treatment with purified STb or a nontoxic STb mutant (D30V) that can bind to sulfatide, STb24-31 peptide corresponding to the consensus sequence of Zot toxin, or the scrambled peptide, cell monolayers were washed using PBS and the medium was changed to medium without FBS.
Purified STb (4 nmol) and STb24-31 peptide (12 nmol) were applied to the apical surfaces of T84 monolayers during 24 h for TER and paracellular permeability assays. An amount of 200 μg of protease inhibitors (soybean trypsin inhibitor; Boehringer Mannheim GmbH, Germany) was added to both treated and control cell monolayers before treatment.
TER measurements.
Electrical resistance is a measure of the barrier property of the epithelium with respect to passive ion movement, where decreased resistance indicates an increase in permeability (16). T84 cells were seeded onto collagen-coated 12-mm-diameter polycarbonate Transwell permeable support cell culture inserts (Costar) (0.4 pore size). A time course study of transepithelial electrical resistance (TER) variation was done for STb. A TER reduction was noted after 4 h, but as the most consistent and reproducible changes were observed after 24 h, we retained this time interval for our experiments. Prior to infection and 24 h after intoxication or infection, TER was measured to check the integrity of the monolayers using an Endhom-12 volt-ohm meter (World Precision Instruments, Inc.). Polarized confluent T84 cell monolayers were formed when resistance was between 300 and 600 Ωcm2. The background of a free control filter was substracted from the sample readings. Experiments were performed in triplicate, and the data are expressed as percentages of preinfection TER and TER measurements obtained at 24 h posttreatment.
Intestinal cell monolayer permeability assays.
The paracellular permeability through T84 cell monolayers was determined by measuring the passage of BSA-FITC (66 kDa) from the apical to basolateral compartments of collagen-coated 12-mm-diameter polycarbonate Transwell permeable support cell culture inserts. T84 cell monolayers were treated in triplicate as described above.
To determine the flux from the apical to basolateral side, PBS containing FITC-BSA (Sigma) at 5 mg/ml was added to the apical side of cell monolayers, which were then incubated at 37°C for 24 h. After this incubation period, samples were taken from the basolateral compartment and read using a fluorescence microplate reader (Biotek, Winooski, VT). The fluorescence due to FITC-BSA was determined using the Gen5 program at an excitation wavelength of 485 nm and an emission wavelength of 527 nm. Values are expressed as fluorescence arbitrary units (a.u).
Confocal microscopy of actin cytoskeleton and tight junction proteins.
At 24 h after treatment, the cell monolayers were washed with PBS and fixed for 15 min at room temperature in 4% paraformaldehyde–PBS. For staining of F-actin filaments, cells were washed three times with PBS, permeabilized with 0.1% Triton X-100 for 10 min at room temperature, and blocked with 2% BSA–PBS for 45 min before incubation with fluorescein-phalloidin for 45 min at room temperature. The cell monolayers were rewashed three times with PBS using a 1/1,000 DAPI dilution in the first wash in order to visualize nuclei. For TJ protein staining, cells were fixed with 100% ethanol at −20°C for 20 min and permeabilized with 1% Triton X-100 for 10 min at room temperature. Then, cells were blocked using 5% BSA–PBS for 30 min at room temperature. All cells were incubated in 1% BSA–PBS, and primary antibodies were added for 1 h at room temperature. Rabbit anti-claudin-1 (1:25), rabbit anti-occludin (1:100), and mouse anti-ZO-1 (1:100) antibodies were detected using secondary antibodies (goat anti-mouse Alexa 568 and goat anti-rabbit Alexa 488) at a 1:300 dilution in 1% BSA–PBS reacted for 1 h at room temperature. Cover slides were added to cell monolayers with Dabco fluorescence mounting medium (Sigma). The slides were observed with a confocal microscope (Olympus FV1000 IX81) at ×40 and ×100 magnifications for TJ proteins and F-actin, respectively.
Statistical analysis.
All data are presented as the means ± the standard deviations. The statistical significance for resistance data analysis, paracellular permeability, and calculation of means was determined by one-way analysis of variance (ANOVA) followed by multiple pairwise comparisons with Tukey's post hoc tests. Data were transformed using the arcsine of square root transformation. Levene's test was used to examine homogeneity of variances. Differences were considered statistically significant at P < 0.05.
RESULTS
Determination of assay parameters.
A wide range of purified STb concentrations was tested on the T84 cell line in a time-response manner (data not shown). Purified STb was used at 4 nmol and STb24-31 peptide at 12 nmol for the assays because they gave us robust, reliable, and reproducible results after 24 h without being cytotoxic. The mutated STb (D30V) and scrambled peptide were tested at 4 nmol and 12 nmol, respectively. STb24-31 was also tested at 4 nmol, and the effect on TJ proteins was similar to what is reported for 12 nmol (data not shown). The T84 cell line was used in our study because it was used for other E. coli enterotoxins (LT and STa) as well as V. cholerae and Yersinia enterocolitica enterotoxins although these act not in the colon but in the small intestine (24, 25, 26, 27, 28, 29). This cell line exhibits tight junctions and desmosomes and maintains electrolyte vectorial transport; thus, T84 cells represent a reference for the work we had undertaken. Before starting our study, we made sure that sulfatide was present on these cells (using anti-sulfatide monoclonal antibodies) (32) (data not shown). This cell line was used before for STb research (26).
STb induces a significant reduction in TER of T84 cell monolayers.
Changes in TER were investigated in T84 cell monolayers to characterize the effects of STb on the intestinal barrier function. Purified STb was added to the apical side of the cell monolayers, and TER was measured after 24 h. Controls consisted of cell monolayers that were left untreated or treated with STb mutant D30V. After 24 h of treatment with STb, the barrier function of T84 cell monolayers was altered as indicated by a 40% reduction in TER (Fig. 1A). The cell monolayers were intact after treatment with STb as observed by light microscopy (data not shown).
Fig 1.
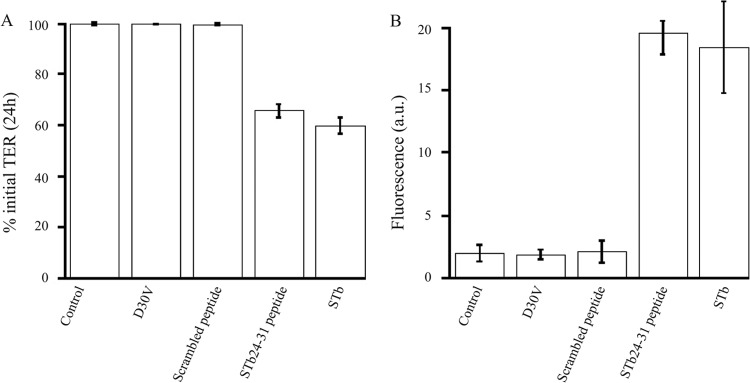
STb and STb24-31 produce a decrease in T84 cell monolayer transepithelial resistance (TER) and an increase in paracellular permeability. (A) Confluent T84 cell monolayers were treated with purified STb mutant D30V, the scrambled peptide, STb24-31, and STb. TER was measured after 24 h and compared to that seen with untreated cell monolayers (control). Purified STb and STb24-31 caused a significant reduction in TER compared to the control (P < 0.0001). Mutant D30V and the scrambled peptide did not affect TER compared to the control. (B) T84 cell monolayers were treated as described above, and levels of BSA-FITC flux (fluorescence in arbitrary units [a.u.]) from apical to basolateral chambers were measured after 24 h. STb and STb24-31 produced an increase in the paracellular flux (P < 0.0001) compared to the control. Mutant D30V and the scrambled peptide did not affect the paracellular permeability. Optical microscopic examination revealed that the cell monolayers were intact after treatment with STb. Three independent experiments comprising three replicates of each treatment within each experiment were conducted. The data are reported as the mean percentages of initial TER ± standard deviations.
There was no significant difference in TER between untreated cell monolayers and cell monolayers treated with D30V mutant. At 24 h postincubation with purified STb, the reduction of TER was statistically significant compared to the results seen with the untreated control (P < 0.0001).
STb enhances paracellular permeability of T84 epithelial cell monolayers.
A reduction in TER may result either from disruption of TJ integrity or from physiological modification of ion flux (33). To determine if the drop in TER in infected cell monolayers was related to paracellular permeability, changes across T84 cell monolayers were measured by adding FITC-conjugated BSA to the apical side of cell monolayers. The capacity of the fluorescent marker to cross the paracellular space was assessed by measuring the fluorescence in the basolateral chamber after 24 h at 37°C. As show in Fig. 1B, T84 cell monolayers treated with purified STb exhibited a significant increase in the paracellular permeability (18 a.u.) compared with either untreated cell monolayers or monolayers treated with D30V STb mutant (P < 0.0001).
An 8-amino-acid peptide corresponding to a consensus sequence of Vibrio cholerae Zot toxin is sufficient to disrupt the epithelial barrier.
The consensus sequence of V. cholerae Zot toxin, after interacting with a specific cell surface receptor, is responsible for the permeabilizing action on epithelial intestinal TJs. STb24-31, a synthetic octapeptide (GFLGVRDG) sharing a sequence with Zot toxin, was synthesized and tested on T84 cell monolayers. Following incubation with the synthetic octapeptide, TER decreased by 35% within 24 h (Fig. 1A), indicating that the integrity of TJ protein complexes was affected to a level comparable to that seen with STb. Nontreated and scrambled peptide-treated cell monolayers showed no change in TER over the same period of time. Incubation of cells with STb24-31 resulted in a flux of FITC-BSA from the apical to basolateral chamber (20 a.u.) compared to the control (Fig. 1B). Changes in TER and in the paracellular permeability were statistically significant (P < 0.0001).
F-actin organization is altered by STb.
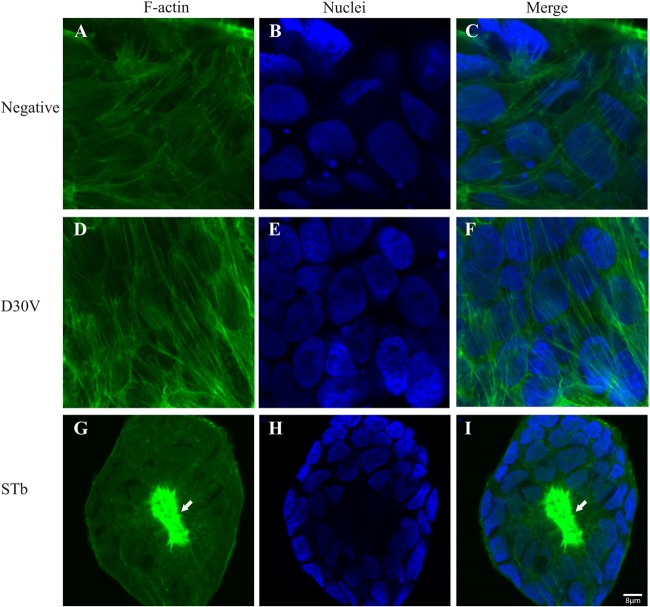
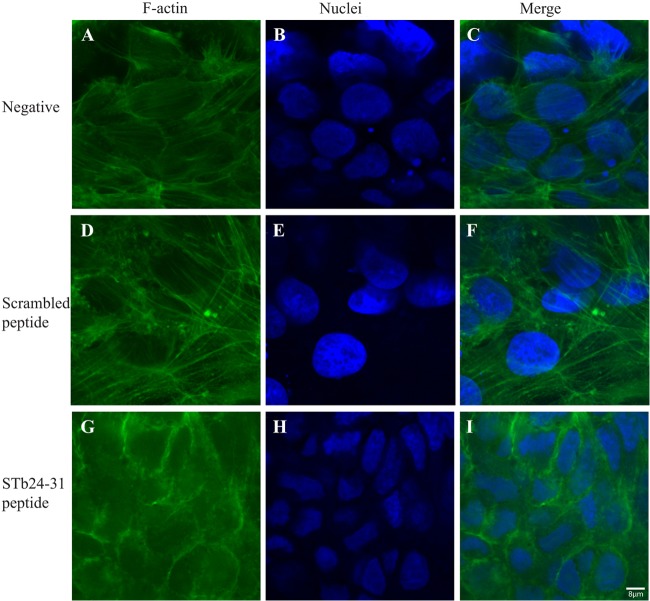
Following treatment for 24 h with STb and the D30V mutant, STb24-31, and the scrambled peptide, T84 cell monolayers were fixed, permeabilized, and stained with FITC-phalloidin to highlight the F-actin organization. Control untreated T84 cell monolayers showed well-organized F-actin filaments and stress fibers (Fig. 2A and C), while STb-treated cell monolayers exhibited disruption of stress fibers with condensation of F-actin filaments (Fig. 2G and F). STb24-31 also altered F-actin organization but to a lesser extent. Stress fibers were lost, but no actin condensation was observed (Fig. 3G and I). Mutated STb (D30V) and the scrambled peptide had no effect on F-actin filaments (Fig. 2D and F and Fig. 3D and F, respectively). Nucleus staining with DAPI indicated changes in morphology and size for both STb- and STb24-31-treated cells (Fig. 2H and 3H, respectively) compared to the negative control (Fig. 2B and E and Fig. 3B and E). These structural changes are characteristic of preapoptotic alterations consisting of chromatin condensation followed by DNA fragmentation (30).
Fig 2.
STb enterotoxin alters F-actin organization. After 24 h of treatment, T84 cells were fixed, permeabilized, and stained with FITC-phalloidin to visualize F-actin filaments. (A and C) F-actin in control (untreated) cells showed well-organized stress fibers with regular cell boundaries. (G and I) In STb-treated cells, disruption of stress fibers accompanied by condensation of F-actin was observed (arrows). (D and F) No change in actin organization was observed for mutated STb (D30V). (B and H) Nucleus staining with DAPI indicated changes in morphology and size (H) compared to the control (B). Three independent experiments were conducted, and the images shown are representative of the results of each treatment. The same magnification (×100) was used for all treatments whose results are shown in Fig. 2 and 3.
Fig 3.
STb24-31 peptide alters F-actin organization. After 24 h of treatment, T84 cells were fixed, permeabilized, and stained with FITC-phalloidin to visualize F-actin filaments. (A and C) F-actin in control (untreated) cells showed well-organized F-actin stress fibers with regular cell boundaries. (G and I) STb24-31 caused dissolution of stress fibers, but no actin condensation was observed. (D and F) No change in actin organization was observed for the scrambled peptide. (B and H) Nucleus staining with DAPI indicated morphology and size changes (H) compared to the control (B). Three independent experiments were conducted, and the images shown are representative of the results of each treatment. The same magnification (×100) was used for all treatments whose results are shown in Fig. 2 and 3.
STb causes disassembly of TJ proteins and cellular morphology.
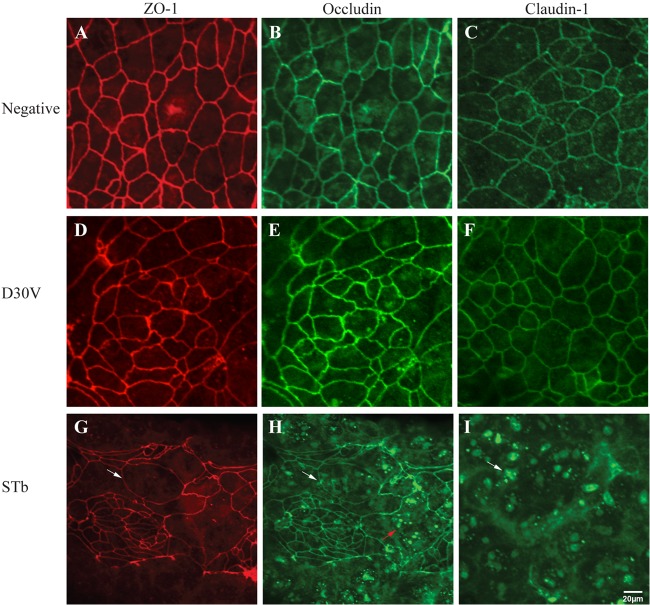
Confocal microscopy investigations of TJ transmembrane and cytoplasmic proteins were performed. Control untreated monolayers stained for ZO-1, occludin, and claudin-1 showed a characteristic brightly stained continuous band around each cell (Fig. 4A, B, and C). After 24 h of treatment with STb, the distribution of TJ proteins was altered. Cells labeled for ZO-1 and occludin appeared enlarged and elongated (Fig. 4G and H, respectively), with occludin redistributed from the lateral membrane to an intracellular location (Fig. 4H). Reorganization of claudin-1 was observed as a complete fragmentation of cell boundaries and focus formation (Fig. 4I).
Fig 4.
Effects of STb on tight junction structural proteins. After 24 h of treatment, T84 cells were fixed, permeabilized, and incubated with antibodies against ZO-1, occludin, or claudin-1. Secondary antibodies coupled to Alexa 488 (green, occludin and claudin-1) and Alexa 586 (red, ZO-1) were then added. (A, B, and C) Control (untreated) cells stained for ZO-1, occludin, and claudin-1 showed a continuous band around each cell. (G and H) Cells treated with STb and labeled for ZO-1 and occludin appeared enlarged and elongated (white arrow). (H) Occludin was redistributed from the lateral membrane to an intracellular location (red arrow). (I) For claudin-1, we observed fragmentation of the cell boundaries and focus formation (white arrow). (D, E, and F) No change in TJ proteins was observed for mutated STb (D30V). Three independent experiments were conducted, and the images shown are representative of the results of each treatment. The same magnification (×40) was used for all treatments whose results are shown in Fig. 4 and 5.
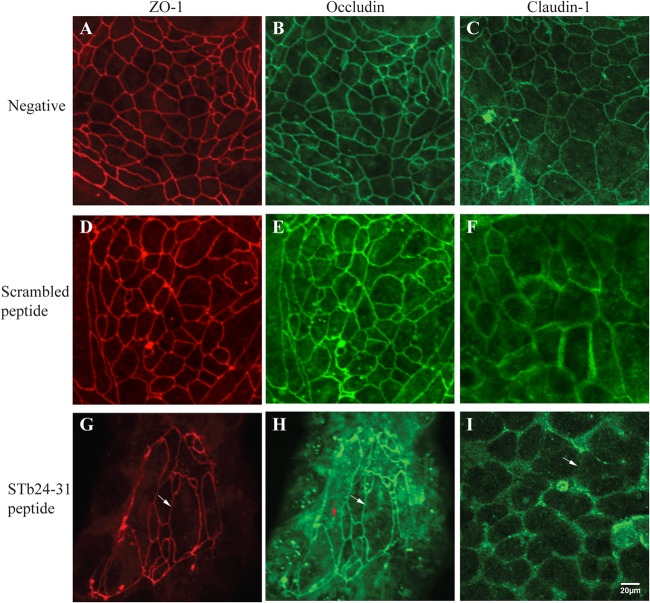
Treatment with STb24-31 resulted in similar effects on ZO-1 (Fig. 5G) and occludin (Fig. 5H), whereas claudin-1 was lost from cell-cell contact sites (Fig. 5I). This change could be observed as the absence of the brightly stained cell boundaries, indicating a loss and/or redistribution of claudin-1. In contrast, no change in TJ proteins was seen with mutated STb (D30V) and the scrambled peptide (Fig. 4D, E, and F and Fig. 5D, E, and F, respectively).
Fig 5.
Effects of STb24-31 peptide on tight junction structural proteins. After 24 h of treatment, T84 cells were fixed, permeabilized, and incubated with antibodies against ZO-1, occludin, or claudin-1. Secondary antibodies coupled to Alexa 488 (green, occludin and claudin-1) and Alexa 586 (red, ZO-1) were then added. (A, B, and C) Control (untreated) cells stained for ZO-1, occludin, and claudin-1 showed a continuous line at cell boundaries. (G and H) Cells labeled for ZO-1 and occludin appeared enlarged and elongated (white arrow). (H) Occludin was redistributed from the lateral membrane to an intracellular location (red arrow). (I) Claudin-1 was lost from cell-cell contact sites in STb24-31-treated cells (white arrow). (D, E, and F) No change in TJ proteins was observed for the scrambled peptide. Three independent experiments were conducted, and the images shown are representative of the results of each treatment. The same magnification (×40) was used for all treatments whose results are shown in Fig. 4 and 5.
DISCUSSION
A variety of enteric pathogens have developed strategies to disrupt epithelial integrity (34, 35). Several studies have shown that toxins often have the ability to dilate TJs and increase paracellular permeability (36, 37). These effects may result from direct modification of TJ proteins (occludin, claudins, ZO-1, ZO-2, ZO-3, etc. …) or by direct binding to a TJ component and/or by alteration of the perijunctional actin filaments. In this study, we demonstrated for the first time that STb induces barrier dysfunction of T84 cell monolayers. Our data indicate that incubation of T84 monolayers with purified STb as well as STb24-31 corresponding to a consensus sequence of V. cholerae Zot toxin induces a significant reduction in TER and a parallel increase in paracellular permeability. The observed effect of STb on TER is likely to contribute to the pathogenesis of ETEC by allowing the passage of electrolytes and water through the paracellular space. In the same way, LT and STa toxin were recently shown to cause a reduction in TER and passage of dextran-FITC by disrupting TJs of T84 cells (27, 29).
These changes were associated with a marked alteration in F-actin filament organization for STb- and STb24-31-treated cells. However, STb24-31 did not show actin condensation as severe as that observed for the whole toxin. We hypothesize that a region of STb not present in the tested octapeptide is required to produce the dramatic effect observed in Fig. 2. Confocal microscopy analysis showed changes and reorganization of TJ proteins ZO-1 and occludin for STb- and STb24-31-treated cells compared to the control for which a morphologically normal pattern of “chicken wire” was observed. Similar changes for ZO-1 and occludin have been observed in T84 cells infected with enteroaggregative E. coli strain 042 (28). Similar to what Chen et al. observed with Caco-2 cell monolayers infected with Campylobacter jejuni (38), STb-treated T84 cell monolayers resulted in the redistribution of occludin from a lateral to an intracellular location. While STb24-31-treated cells exhibited loss and redistribution of claudin-1 from cell-cell contact sites, as seen with Caco-2 cells treated with Zot (20), we also observed an important disruption of the claudin-1 arrangement in STb-treated cells. A similar change in T84 cells exposed to C. difficile toxin A was previously reported (39).
Our data showed that STb24-31 produces changes in TER and paracellular permeability comparable to those seen with the whole STb. STb and Zot do not share the same receptor. For Zot toxin, a putative protein receptor of 66 kDa showing homology with human alpha-1-chimaerin has been proposed (40). The observation of STb24-31 altering the biological characteristics of TJs presumes that binding to sulfatide is occurring, as it is required for STb biological activity. It was shown that STb has to be internalized to be enterotoxic (41), and we can thus assume that STb24-31 is taken up by T84 cells. The STb24-31 sequence comprises a glycine-rich loop that was previously shown to be important for binding to the sulfatide receptor and/or for enterotoxicity expression (5, 31). Interestingly, STb mutant D30V with a single amino acid change did not cause changes in TJs, as TER and paracellular flux variations, in T84 cell monolayers. As the mutated aspartic acid 30 is present in the octapeptide tested in our study and is also found in Zot, it supports the idea that this region of STb and/or this specific amino acid plays an important role in the changes observed in TJs.
It is known that claudins represent a large family of proteins which are the major gatekeepers of the paracellular pathway (42). Thus, the loss of claudin-1 from TJs in response to STb may explain the effect observed on paracellular permeability and barrier function. Several pathogens and their toxins, including E. coli strain C25, C. difficile toxin A, and V. cholerae Zot toxin, cause redistribution of claudin-1 in parallel to an increase in paracellular permeability (20, 39, 43).
In general, the effects of STb on the cytoskeletal structure and TJ proteins were somewhat similar to the modifications reported to be induced by C. difficile toxin A (44). Although a similar effect was observed for Zot, major differences were noticed. In fact, Zot produces a reversible effect (2 to 3 h) on cytoskeletal rearrangement (45) and TJ protein disruption, while STb causes changes that seem to be irreversible during the time period over which our experiments were conducted (24 h). This long-lasting effect was also observed for LT-positive and STa ETEC strains (27, 29).
TJ structure and function are regulated by the perijunctional actinomyosin ring (46), and actin stress fibers have been shown to be involved in regulation of TJ assembly and function (33, 47). Interestingly, an increase in permeability with a significant drop of TER caused by STb was associated with disruption of stress fibers and condensation of F-actin filaments. The effects of STb on the cytoskeleton and on TJ proteins are related to STb toxicity as expressed in an animal model; i.e., no change was observed with STb mutant (D30V) showing no toxicity in vivo and the scrambled peptide.
TJ protein alterations caused by STb may be due to actin rearrangement, as a loss of organization in the perijunctional F-actin ring has been identified to be a critical event in the mechanism controlling TER and paracellular permeability (19). The mechanisms underlying tight junctional physiology are incompletely understood. Protein kinases are thought to be involved in the regulation of TJ assembly and paracellular permeability changes. For example, both tyrosine (48) and serine/threonine (49, 50) protein phosphorylation changes are known to correlate with permeability changes in existing TJs as well as with their assembly. Several independent reports have been published on the role of protein phosphorylation in cytoskeletal rearrangement (46).
Like most TJ proteins, occludin is also a phosphoprotein known to have an important role in the TJ function, and in vivo it is likely phosphorylated on serine and threonine residues (49, 51). Occludin dephosphorylation and ZO-1 phosphorylation are associated with stimulus-induced TJ disassembly and paracellular permeability changes (52). Activated PKC with TPA (12-O-tetradecanoylphorbol-13-acetate) stimulates the activity of serine/threonine phosphatase, which in turn dephosphorylates occludin, resulting in a rapid increase in paracellular permeability evidenced by a decrease in TER, suggesting that dephosphorylation of occludin could be an important step in TJ regulation (53).
A previous study has shown that binding of STb to its receptor leads to the uptake of Ca2+ into the cell, activating PKC, which through phosphorylation activates CFTR (8). Based on the fact that STb activates PKC and its involvement in cytoskeletal rearrangement and TJ regulation, we can hypothesize that the PKC signaling pathway could be involved in TJ dysfunction as well as the cytoskeletal changes and TJ protein alterations observed. As many secretagogues and signaling pathways have been reported to be involved in STb secretion, we believe that this enterotoxin could affect intestinal cells through altered electrolyte transport via transapical cell membrane secretion in combination with tight junction alterations. Changes in TJs could be reversible, as observed for Zot toxin, thus explaining the reversible secretory action observed for STb enterotoxin (4, 45). STb is a relatively fast-acting toxin (e.g., 3 to 6 h), and this rapid secretion could be due to the transapical secretion whereas TJ modifications could be responsible for the more lasting effect (12 to 24 h).
Exposure of swine jejunum to crude culture filtrates containing STb induced alterations in cell morphology (54, 55, 56). Changes in villous epithelium from columnar to cuboidal or squamous cell types were noted. Overall, alterations resulting from STb action on TJs, observed in our study, could relate to the microscopic histological alterations already reported.
In this study, T84 epithelial monolayers were reconfirmed as a useful in vitro model for detection of TJ effectors. Further studies are required to understand the pathways involved in STb-mediated alteration of TER and TJ protein modulation. However, as TER reflects the integrity of a cell monolayer, it is reasonable to report that STb induces the opening of TJs as indicated by the significant decrease in TER and a parallel increase of paracellular permeability to a high-molecular-weight marker, correlated by redistribution of TJ proteins and rearrangement of the actin cytoskeleton.
ACKNOWLEDGMENTS
This work was supported by a Discovery grant from the National Research Council of Canada (no. 139070) to J.D.D. C.N.M. was recipient of various grants from “Centre de recherche en infectiologie porcine” and the Faculty of Veterinary Medicine (Université de Montréal).
Footnotes
Published ahead of print 28 May 2013
REFERENCES
- 1. Nagy B, Fekete PZ. 2005. Enterotoxigenic Escherichia coli in veterinary medicine. Int. J. Med. Microbiol. 295:443–454 [DOI] [PubMed] [Google Scholar]
- 2. Kosek M, Bern C, Guerrant RL. 2003. The global burden of diarrhoeal disease as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 81:197–204 [PMC free article] [PubMed] [Google Scholar]
- 3. Nagy B, Fekete PZ. 1999. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet. Res. 30:259–284 [PubMed] [Google Scholar]
- 4. Dubreuil JD. 2008. Escherichia coli STb toxin and colibacillosis: knowing is half the battle. FEMS Microbiol. Lett. 278:137–145 [DOI] [PubMed] [Google Scholar]
- 5. Sukumar M, Rizo J, Wall M, Dreyfu LA, Kupersztoch YM, Gierasch LM. 1995. The structure of Escherichia coli heat-stable enterotoxin by nuclear magnetic resonance and circular dichroism. Protein Sci. 4:1718–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rousset E, Harel J, Dubreuil JD. 1998. Sulfatide from the pig jejunum brush border epithelial cell surface is involved in binding of Escherichia coli enterotoxin b. Infect. Immun. 66:5650–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dreyfus LA, Harville B, Howard DE, Shaban R, Beatty DM, Morris SJ. 1993. Calcium influx mediated by the Escherichia coli heat-stable enterotoxin B (STB). Proc. Natl. Acad. Sci. U. S. A. 90:3202–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harville BA, Dreyfus LA. 1995. Involvement of 5-hydroxytryptamine and prostaglandin E2 in the intestinal secretory action of Escherichia coli heat-stable enterotoxin B. Infect. Immun. 63:745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harville BA, Dreyfus LA. 1996. Release of serotonin from RBL-2H3 cells by the Escherichia coli peptide toxin STb. Peptides 17:363–366 [DOI] [PubMed] [Google Scholar]
- 10. Berkes J, Viswanathan VK, Savkovic SD, Hecht G. 2003. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52:439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balkovetz DF, Katz J. 2003. Bacterial invasion by a paracellular route: divide and conquer. Microbes Infect. 5:613–619 [DOI] [PubMed] [Google Scholar]
- 12. Puthenedam M, Williams PH, Lakshmi BS, Balakrishnan A. 2007. Modulation of tight junction barrier function by outer membrane proteins of enteropathogenic Escherichia coli: role of F-actin and junctional adhesion molecule-1. Cell Biol. Int. 31:836–844 [DOI] [PubMed] [Google Scholar]
- 13. Roselli M, Finamore A, Britti MS, Konstantinov SR, Smidt H, de Vos WM, Mengheri E. 2007. The novel porcine Lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage. J. Nutr. 137:2709–2716 [DOI] [PubMed] [Google Scholar]
- 14. Anderson JM, Balda MS, Fanning AS. 1993. The structure and regulation of tight junctions. Curr. Opin. Cell Biol. 5:772–778 [DOI] [PubMed] [Google Scholar]
- 15. Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H. 2003. Tight junctions and human diseases. Med. Electron Microsc. 36:147–156 [DOI] [PubMed] [Google Scholar]
- 16. Philpott DJ, Mckay DM, Sherman PM, Perdue MH. 1996. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am. J. Physiol. 270:G634–645 [DOI] [PubMed] [Google Scholar]
- 17. Yuhan R, Koutsouris A, Savkovic SD, Hecht G. 1997. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 113:1873–1882 [DOI] [PubMed] [Google Scholar]
- 18. Philpott DJ, McKay DM, Mak W, Perdue MH, Sherman PM. 1998. Signal transduction pathways involved in enterohemorrhagic Escherichia coli-induced alterations in T84 epithelial permeability. Infect. Immun. 66:1680–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nusrat A, Von Eichel-Streiber C, Turner J. 2001. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect. Immun. 69:1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmidt E, Kelly SM, van der Walle CF. 2007. Tight junction modulation and biochemical characterisation of the zonula occludens toxin C- and N-termini. FEBS Lett. 581:2974–2980 [DOI] [PubMed] [Google Scholar]
- 21. Guignot J, Chaplais C, Coconnier-Polter MH, Servin AL. 2007. The secreted autotransporter toxin, Sat, functions as a virulence factor in Afa/Dr diffusely adhering Escherichia coli by promoting lesions in tight junction of polarized epithelial cells. Cell. Microbiol. 9:204–221 [DOI] [PubMed] [Google Scholar]
- 22. Li Z, Elliott E, Payne J, Isaacs J, Gunning P, O'loughlin EV. 1999. Shiga toxin-producing Escherichia coli can impair T84 cell structure and function without inducing attaching/effacing lesions. Infect. Immun. 67:5938–5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Pierro M, Lu R, Uzzau S, Wang W, Margaretten K, Pazzani C, Maimone F, Fasano A. 2001. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 276:19160–19165 [DOI] [PubMed] [Google Scholar]
- 24. Toriano R, Kierbel A, Ramirez MA, Malnic G, Parisi M. 2001. Spontaneous water secretion in T84 cells: effects of STa enterotoxin, bumetanide, VIP, forskolin, and A-23187. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G816–G822 [DOI] [PubMed] [Google Scholar]
- 25. Visweswariah SS, Shanti G, Balganesh TS. 1992. Interaction of heat-stable enterotoxins with human colonic (T84) cells: modulation of the activation of guanylyl cyclase. Microb. Pathog. 12:209–218 [DOI] [PubMed] [Google Scholar]
- 26. Chao KL, Dreyfua LA. 1997. Interaction of Escherichia coli heat-stable enterotoxin B with cultured human intestinal epithelial cells. Infect. Immun. 65:3209–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakashima R, Kamata Y, Nishikawa Y. 2013. Effect of Escherichia coli heat-stable enterotoxin and guanylin on the barrier integrity of intestinal epithelial T84 cells. Vet. Immunol. Immunopathol. 152:78–81 [DOI] [PubMed] [Google Scholar]
- 28. Strauman MC, Harper JM, Harrington SM, Boll EJ, Nataro JP. 2010. Enteroaggregative Escherichia coli disrupts epithelial cell tight junctions. Infect. Immun. 78:4958–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kreisberg RB, Harper J, Strauman MC, Marohn M, Clements JD, Nataro JP. 2011. Induction of increased permeability of polarized enterocyte monolayers by enterotoxigenic Escherichia coli heat-labile enterotoxin. Am. J. Trop. Med. Hyg. 84:451–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Syed HC, Dubreuil JD. 2012. Escherichia coli STb toxin induces apoptosis in intestinal epithelial cell lines. Microb. Pathog. 53:147–153 [DOI] [PubMed] [Google Scholar]
- 31. Labrie V, Beausoleil H-E, Harel J, Dubreuil JD. 2001. Binding to sulfatide and enterotoxicity of various Escherichia coli STb mutants. Microbiology 147:3141–3148 [DOI] [PubMed] [Google Scholar]
- 32. Fredman P, Mattsson L, Andersson K, Davidsson P, Ishizuka I, Jeansson S, Mansson JE, Svennerholm L. 1988. Characterization of the binding epitope of a monoclonal antibody to sulphatide. Biochem. J. 251:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schneeberger EE, Lynch RD. 2004. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 286:C1213–C1228 [DOI] [PubMed] [Google Scholar]
- 34. Bonazzi M, Cossart P. 2011. Impenetrable barriers or entry portals? The role of cell-cell adhesion during infection. J. Cell Biol. 195:348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guttman JA, Finlay BB. 2009. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 1788:832–841 [DOI] [PubMed] [Google Scholar]
- 36. Hofman P. 2003. Pathological interactions of bacteria and toxins with the gastrointestinal epithelial tight junctions and/or the zonula adherens; an update. Cell. Mol. Biol. 49:65–75 [PubMed] [Google Scholar]
- 37. Soong G, Parker D, Magargee M, Prince AS. 2008. The type III toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J. Bacteriol. 190:2814–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen ML, Ge Z, Fox JG, Schauer DB. 2006. Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni. Infect. Immun. 74:6581–6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen M, Pothoulakis C, Lamont T. 2002. Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to Clostridium difficile toxin A. J. Biol. Chem. 277:4247–4254 [DOI] [PubMed] [Google Scholar]
- 40. Uzzau S, Lu R, Wang W, Fiore C, Fasano A. 2001. Purification and preliminary characterization of the zonula occludens toxin receptor from human (CaCo2) and murine (IEC6) intestinal cell lines. FEMS Microbiol. Lett. 194:1–5 [DOI] [PubMed] [Google Scholar]
- 41. Labrie V, Harel J, Dubreuil JD. 2002. Escherichia coli heat-stable enterotoxin b (STb) in vivo internalization in rat intestinal epithelial cells. Vet. Res. 33:223–228 [DOI] [PubMed] [Google Scholar]
- 42. Abuazza G, Becker A, Williams SS, Chakravarty S, Truong HT, Lin F, Baum M. 2006. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am. J. Physiol. Renal Physiol. 291:F1132–F1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zareie M, Riff J, Donato K, McKay DM, Perdue MH, Soderholm JD, Karmali M, Cohen MB, Hawkins J, Sherman PM. 2005. Novel effects of the prototype translocating Escherichia coli, strain C25 on the intestinal epithelial structure and barrier function. Cell Microbiol. 7:1782–1797 [DOI] [PubMed] [Google Scholar]
- 44. Fiorentini C, Thelestam M. 1991. Clostridium difficile toxin A and its effects on cells. Toxicon. 29:543–567 [DOI] [PubMed] [Google Scholar]
- 45. Karyekar CS, Fasano A, Raje S, Lu R, Dowling TC, Eddington ND. 2003. Zonula occludens toxin increases the permeability of the moleculer weight markers and chemotherapeutic agents across the bovine brain microvessel endothelial cells. J. Pharm. Sci. 92:414–423 [DOI] [PubMed] [Google Scholar]
- 46. Turner JR. 2000. ‘Putting the squeeze’ on the tight junction: understanding cytoskeletal regulation. Semin. Cell Dev. Biol. 11:301–308 [DOI] [PubMed] [Google Scholar]
- 47. Rajasekaran AK, Rajasekaran SA. 2003. Role of Na-K-ATPase in the assembly of tight junctions. Am. J. Physiol. Renal Physiol. 285:F388–F396 [DOI] [PubMed] [Google Scholar]
- 48. Stuart RO, Nigam SK. 1995. Regulated assembly of tight junctions by protein kinase C. Proc. Natl. Acad. Sci. U. S. A. 92:6072–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. 1997. Possible involvement of phosphorylation of occludin in tight junction formation. J. Cell Biol. 137:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Citi S, Denisenko N. 1995. Phosphorylation of the tight junction protein cingulin and the effects of protein kinase inhibitors and activators in MDCK epithelial cells. J. Cell Sci. 108:2917–2926 [DOI] [PubMed] [Google Scholar]
- 51. Cordenonsi M, Mazzon E, De Rigo L, Baraldo S, Meggio F, Citi S. 1997. Occludin dephosphorylation in early development of Xenopus laevis. J. Cell Sci. 110:3131–3139 [DOI] [PubMed] [Google Scholar]
- 52. Sheth P, Basuroy S, Li C, Naren AP, Rao RK. 2003. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J. Biol. Chem. 278:49239–49245 [DOI] [PubMed] [Google Scholar]
- 53. Clarke H, Soler AP, Mullin JM. 2000. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J. Cell Sci. 113:3187–3196 [DOI] [PubMed] [Google Scholar]
- 54. Whipp SC, Moon HW, Kemeny LJ, Argenzio RA. 1985. Effect of virus-induced destruction of villous epithelium on intestinal secretion induced by heat-stable Escherichia coli enterotoxins and prostaglandin E1 in swine. Am. J. Vet. Res. 46:637–642 [PubMed] [Google Scholar]
- 55. Whipp SC, Moseley SL, Moon HW. 1986. Microscopic alterations in jejunal epithelium of 3-week-old pigs induced by pig-specific, mouse-negative, heat-stable Escherichia coli enterotoxin. Am. J. Vet. Res. 47:615–618 [PubMed] [Google Scholar]
- 56. Whipp SC, Kokue E, Morgan RW, Rose R, Moon HW. 1987. Functional significance of histologic alterations induced by Escherichia coli pig-specific, mouse-negative, heat-stable enterotoxin (STb). Vet. Res. Commun. 11:41–55 [DOI] [PubMed] [Google Scholar]