Abstract
Although typhoid fever has been intensively studied, chronic typhoid carriage still represents a problem for the transmission and persistence of the disease in areas of endemicity. This chronic state is highly associated with the presence of gallstones in the gallbladder of infected carriers upon which Salmonella can form robust biofilms. However, we hypothesize that in addition to gallstones, the gallbladder epithelium aids in the establishment/maintenance of chronic carriage. In this work, we present evidence of the role of the gallbladder epithelium in chronic carriage by a mechanism involving invasion, intracellular persistence, and biofilm formation. Salmonella was able to adhere to and invade polarized gallbladder epithelial cells apically in the absence and presence of bile in a Salmonella pathogenicity island 1 (SPI-1)-dependent manner. Intracellular replication of Salmonella was also evident at 12 and 24 h postinvasion. A flowthrough system revealed that Salmonella is able to adhere to and form extensive bacterial foci on gallbladder epithelial cells as early as 12 h postinoculation. In vivo experiments using a chronic mouse model of typhoid carriage showed invasion and damage of the gallbladder epithelium and lamina propria up to 2 months after Salmonella infection, with an abundant presence of macrophages, a relative absence of neutrophils, and extrusion of infected epithelial cells. Additionally, microcolonies of Salmonella cells were evident on the surface of the mouse gallbladder epithelia up to 21 days postinfection. These data reveal a second potential mechanism, intracellular persistence and/or bacterial aggregation in/on the gallbladder epithelium with luminal cell extrusion, for Salmonella maintenance in the gallbladder.
INTRODUCTION
Typhoid fever, caused primarily by Salmonella enterica serovar Typhi (S. Typhi), is a human-specific disease that is responsible for an estimated 21 million new infections per year, resulting in approximately 200,000 deaths annually worldwide (1). After contaminated water or food has been ingested, bacteria cross the intestinal epithelial barrier, are phagocytosed by macrophages, and spread systematically, producing acute disease (2). However, 3 to 5% of the population infected with S. Typhi become chronic carriers, with the gallbladder being a site of persistence (3, 4). These carriers serve as a critical reservoir for further spread of the disease through bacterial shedding in feces (5). Chronic infections can persist for decades, and although highly contagious, they are typically asymptomatic (6–8). In addition, chronic carriers have an approximately 8-fold greater risk of developing gallbladder carcinoma than noncarriers (9, 10). This impact on human health combined with the high incidence of typhoid fever in many parts of the world highlights the importance of understanding the mechanisms involved in typhoid carriage.
Clinical observations of carriers with respect to the difficulty of eradicating the bacterium with antibiotics and its confinement to an organ with shedding from this site, coupled with evidence for long-term invasion of the immune response, are consistent with a biofilm-related disease (11). Biofilms are communities of microorganisms that adhere to each other and to inert or live substrates. They are typically encased in an extracellular matrix and are associated with many chronic and acute human infections (12, 13). The carrier state is highly related to the presence of gallstones (6, 14, 15). Indeed, studies in our laboratory have shown that Salmonella can form biofilms on the surface of cholesterol gallstones in vitro and in vivo in the gallbladder of mice and human carriers (16, 17). These reservoirs of bacteria have been demonstrated to be a mechanism of persistence and chronic colonization. To date, removal of the gallbladder (cholecystectomy) is the most common treatment for chronic typhoid carriers and gallbladder abnormalities; however, this treatment is both costly and invasive. Therefore, alternative treatments are needed to eradicate this carriage state.
Despite the fact that gallstones are associated with chronic carriage of Salmonella, patients without gallstones have also been reported as chronic carriers (18, 19). Previous studies have shown that Salmonella can invade mouse gallbladder epithelial cells (20) and can also form biofilms on the surface of various epithelial cells, including HEp-2 cells and chicken intestinal tissue (21). However, it is unknown if Salmonella is able to persist in or on the gallbladder epithelium during long-term carriage. Here, we hypothesized that Salmonella also persists in the gallbladder by invading gallbladder epithelial cells and/or forming biofilms on the surface of gallbladder epithelia. The elucidation of these mechanisms is crucial for the development of new therapies to eradicate typhoid carriage, thus blocking the transmission of typhoid fever.
In this study, we not only demonstrate that Salmonella can persist in and on the gallbladder epithelium by intracellular residence and by forming epithelial cell surface biofilms, but we also show that the gallbladder epithelium responds to chronic Salmonella infection in a manner that differs from acute models of infection.
MATERIALS AND METHODS
Ethics statement.
Mice were housed and used in strict accordance with guidelines established by The Ohio State University (OSU) Institutional Animal Care and Use Committee (IACUC), and all efforts were made to minimize animal suffering. The work performed in this study was approved by the OSU IACUC.
The Ohio State University Animal Care and Use Program is accredited by The Association for the Assessment and Accreditation of Laboratory Animal Care International. The protocol identification number is 2009A0057. All research activities must conform to the statutes of the Animal Welfare Act and the guidelines of the Public Health Service, as issued in the Guide for the Care and Use of Laboratory Animals (revised 1996).
Bacterial strains and growth conditions.
Wild-type and derivatives of S. enterica serovar Typhimurium ATCC 14028 (JSG210) were used in these studies. The entire list of strains used is given in Table 1. All cultures were grown in Luria-Bertani (LB) broth at 37°C with aeration at 225 rpm. Antibiotics, when needed, where used at the following concentrations: chloramphenicol, 25 μg/ml; kanamycin, 45 μg/ml; ampicillin, 100 μg/ml. Construction of complementation strains was not successful for Salmonella pathogenicity island 1 (SPI-1), csgA, and yciE mutants. However, to verify that their phenotypes were not the result of an unlinked gene, mutations were instead back-transduced by P22HTint into a wild-type background.
Table 1.
Bacterial strains and plasmids used in the study
| Strain or plasmid | Genotype or relevant phenotype | Reference and/or source |
|---|---|---|
| Strains | ||
| JSG210 | Wild-type S. Typhimurium ATCC 14028 | ATCC |
| JSG3391 | ΔSPI-1 | Steve Libby |
| JSG3392 | ΔfimAICDHF | Andreas Baumler |
| JSG3132 | ΔcsgA | Michael McClelland lab |
| JSG3125 | ΔyciE | Michael McClelland lab |
| JSG1149 | Wild-type S. Typhimurium ATCC 14028(pFPV25.1) | 17 |
| JSG3535 | ΔSPI-1 pFPV25.1 | This study |
| JSG3534 | ΔfimAICDHF pFPV25.1 | This study |
| JSG3658 | ΔcsgA pFPV25.1 | This study |
| JSG3536 | ΔyciE pFPV25.1 | This study |
| Plasmid | ||
| pFPV25.1 | GFP constitutive vector | Raphael Valdivia and Stanley Falkow |
Growth of gallbladder epithelial cells.
All tissue culture assays were performed with dog gallbladder epithelial cells (DGEC) (donation of Sum P. Lee lab, University of Washington). Cells were grown at 37°C in 5% CO2 using Dulbecco's modified Eagle's medium with a high concentration of glucose (DMEM-high glucose) (Invitrogen, CA) supplemented with 10% fetal bovine serum (FBS), 100 μg/ml streptomycin, 100 IU/ml penicillin, and 1× nonessential amino acids.
Attachment, invasion, and intracellular survival assays.
DGEC were seeded into 24-mm collagen-coated Transwell-COL inserts (Corning, MA). Polarization and differentiation of the cells were verified by transepithelial electrical resistance (TEER) (>700 Ω cm−2) using a Millicell Electrical Resistance System (ERS) (Millipore, MA) and by observation under transmission electron microscopy (TEM). This process generally took 8 to 10 days. One day before infection, the medium was exchanged with serum-free medium with or without 0.3% ox bile (Sigma, MO). This bile concentration was determined based on tolerance assays in which the TEER and viability of DGEC were not altered. Infections of epithelial cells were performed apically for 2 h at a multiplicity of infection (MOI) of 100. Cells were then incubated with serum-free medium with or without gentamicin (50 μg/ml) for 30 min, washed two times with 1× phosphate-buffered saline (PBS), and then lysed with 0.1% Triton X-100 for 10 min at 37°C and 5% CO2. Lysates were then plated on LB medium for bacterial enumeration. These assays were performed four times in triplicate. The number of attached bacteria was obtained by subtraction of total bacteria recovered from wells not treated with gentamicin from the number in wells treated with gentamicin. Intracellular survival assays were performed two times in triplicate and included 6-, 12-, and 24-h postinfection time points. For these assays, gentamicin (10 μg/μl) was used throughout the course of the experiment.
Monitoring of attachment and invasion by microscopy.
DGEC were grown as above, and infections were performed using an MOI of 100 or 10 for 2- and 9-h time points, respectively. The 2-h postinoculation time point allows observation of bacteria initially interacting/invading the epithelium, whereas the 9-h time point is primarily for observation of biofilm/microcolony formation on the epithelium. Cells were then fixed and observed by confocal microscopy, scanning electron microscopy (SEM), and TEM according to the protocols described below.
Flowthrough assays.
Flowthrough plastic chambers (Stovall Life Science, Inc., IA) coated with poly-l-lysine (Sigma) were inoculated with 2 × 106 DGEC and allowed to differentiate and form a confluent monolayer at 37°C and 5% CO2 (4 days total). DGEC were then inoculated with S. Typhimurium harboring pFPV25.1 (constitutively expressing green fluorescent protein [GFP]) (Table 1) at an MOI of 100 and allowed to adhere for 1 h. A flowthrough system was created whereby DMEM-high glucose (Invitrogen) supplemented with 10% FBS, 100 μl/ml ampicillin, and 1× nonessential amino acids with or without 0.3% bile was applied to the infected DGEC for 12 h at 37°C and 5% CO2 at a flow rate of 270 μl/min. Cells were then observed with confocal microscopy or SEM as described below. Biofilm and planktonic cells were also quantified by CFU enumeration. Experiments were performed in triplicate.
Mouse infections.
Considering that typhoid fever is a human-specific disease, modeling of the disease in mice is performed by using S. enterica serovar Typhimurium (S. Typhimurium) since it produces a typhoid fever-like disease. Female 129X/SvJ mice (Jackson Laboratories, ME) were fed a normal diet (Harlan Laboratories, IN) or a normal diet supplemented with 1% cholesterol (Sigma) and 0.5% cholic acid (Sigma) for 9 weeks. Mice fed the cholesterol diet developed gallstones in their gallbladders. Mice were then inoculated intraperitoneally with approximately 104 bacteria and sacrificed at 7, 14, 21, and 60 days postinfection (dpi). Feces, liver, gallbladder, bile, and gallstones were collected, homogenized, and/or diluted for bacterial enumeration and plating on Salmonella-Shigella agar (Becton Dickenson, NJ). The gallbladder tissues of some mice were also analyzed by histology, immunohistochemistry (IHC), and electron microscopy. Data were gathered and combined from two independent experiments.
IHC.
Mouse tissues were fixed in 10% buffered formalin phosphate (Fisher Scientific, MA) for 72 h. Tissues were processed by routine methods, paraffin embedded, cut in sections (thickness, 4 μm), and stained for IHC using the avidin-biotin complex (ABC) method of the OSU Comparative Pathology and Mouse Phenotyping Shared Resource. The primary antibodies included the macrophage marker anti-F4/80 (1/200 dilution), the neutrophil marker anti-Ly6G (1/200 dilution), and anti-Salmonella lipopolysaccharide (LPS) (1/500 dilution). All antibodies were from Novus Biologicals (Littleton, CO). Briefly, sections were treated with target retrieval solution (for use with F4/80 and Ly6G antibodies) or proteinase K (for use with Salmonella antibodies) for 5 min. Sections were treated with hydrogen peroxide for 10 min, rinsed, blocked with serum-free protein for 10 min, and incubated with primary antibody for 30 min. They were again rinsed and then incubated with mouse-adsorbed biotinylated rabbit anti-mouse antibody (1/200 dilution for F4/80 and Ly6G; 1/1,000 dilution for anti-Salmonella antibody) for 30 min. Samples were incubated with Vector RTU ABC Elite complex for 30 min, rinsed, incubated with chromogen (diaminobenzidine [DAB]) for 5 min, counterstained with hematoxylin, rinsed, treated with 1% ammonium hydroxide, dehydrated in ethanol, cleared in xylene, and mounted on coverslips. Histological scoring of inflammatory cell recruitment was performed as follows: 0, absent; 1, mild; 2, mild/moderate; 3, moderate; 4, moderate to marked; and 5, marked.
Confocal microscopy.
DGEC (grown on transwells or flow chambers) were stained before infection with 5 M Cell Tracker Red CMPTX (Invitrogen) according to the manufacturer's directions. After the respective postinfection time points, DGEC were fixed with 4% paraformaldehyde in 0.1 M sodium phosphate, pH 7.4, for 15 min at room temperature, rinsed, and observed using an Olympus Fluoview FV10.1 instrument.
Electron microscopy.
For SEM observations, samples were fixed overnight at 4°C with 2.5% glutaraldehyde in 0.1 M phosphate buffer–0.1 M sucrose (pH 7.4), rinsed twice with 0.1 M phosphate buffer, and dehydrated by addition of solutions of ethanol in a graded series, as follows: 35%, 50%, 70%, 80%, 95%, 100%. Samples were chemically dried with consecutive washes of 25%, 50%, 75%, and 100% hexamethyldisilazane (Ted Pella, CA). Samples were then dried overnight in a fume hood, mounted on aluminum stubs, and sputter coated with gold for observation using an FEI NOVA nanoSEM at the OSU Campus Microscopy and Imaging Facility (CMIF).
For TEM observations, transwells or mouse tissues were fixed overnight at 4°C with 1.5% paraformaldehyde–1.5% glutaraldehyde in 0.1 M cacodylate buffer, rinsed, postfixed for 1 h with 1% osmium tetroxide in 0.1 M cacodylate buffer, rinsed again, and stained en bloc in 2% uranyl acetate with 10% ethanol. Consecutive washes with increasing concentrations of ethanol were performed as described above before the application of propylene oxide. Samples were then embedded in Eponate resin and polymerized at 60°C for 16 to 24 h. Sectioning was performed at 80 nm on a Reichtert Ultracut E ultramicrotome before observation using an FEI Technai G2 Spirit transmission electron microscope (CMIF).
RESULTS
S. Typhimurium bacteria attach to the surface of polarized DGEC resembling microcolonies.
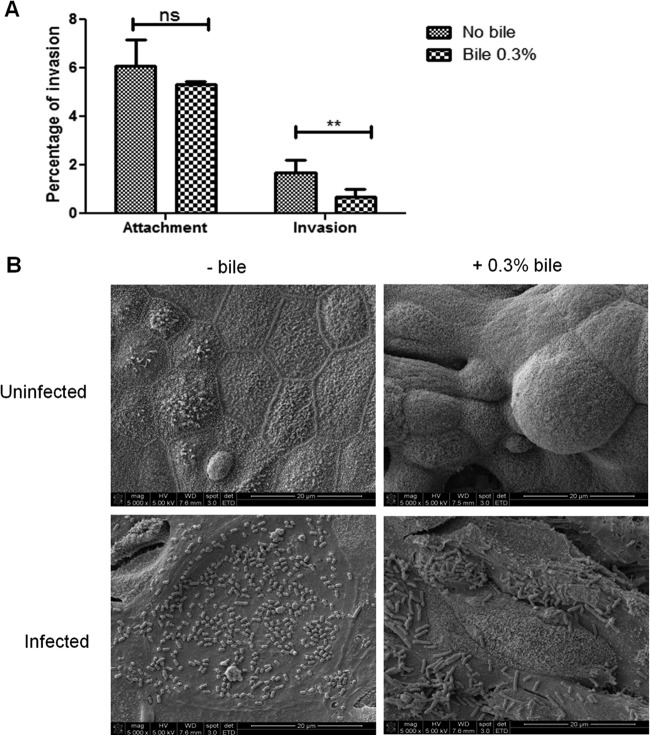
To model the in vivo host-pathogen interface and examine the initial interaction of Salmonella with the gallbladder epithelium, bacteria were added to polarized monolayers of DGEC. Salmonella was able to attach to the surface of DGEC regardless of exposure to bile (Fig. 1A). Interestingly, during this attachment, bacteria-bacteria interactions were observed as early as 2 h postinfection. These early interactions resemble the formation of microcolonies, widely reported as the initial process of biofilm formation (Fig. 1B).
Fig 1.
S. Typhimurium attaches and invades into/on polarized DGEC in vitro. (A) Apical attachment and invasion (mean plus standard deviation) of S. Typhimurium bacteria on/into DGEC previously exposed or not exposed to 0.3% bile. Invasion after 2.5 h is shown as a percentage of the inoculum. Exposure of the epithelial cells to bile significantly decreased bacterial invasion but not attachment. Means between conditions with (+) and without (−) bile treatment were compared by a Student's t test (**, P < 0.01; ns, not significant). Experiments were performed in triplicate and repeated four times. (B) S. Typhimurium attaches to the surface of polarized DGEC, resembling microcolonies. Representative SEM images of uninfected and infected DGEC in the absence and presence of 0.3% bile are shown. Note the differentiation when cells were exposed to bile. Cells were fixed and processed at 2 h postinfection.
S. Typhimurium invades polarized gallbladder epithelial cells in vitro.
Salmonella can invade and replicate within epithelial cells, typically utilizing a type III secretion system to gain entry into these nonphagocytic cells. S. Typhimurium was able to apically invade in vitro polarized gallbladder epithelial cells that had or had not been previously exposed to 0.3% ox bile. However, the exposure of DGEC to bile before infection significantly decreased bacterial invasion (Fig. 1A). TEM imaging demonstrated that epithelial cells exposed to bile possessed mucus granules on their apical sides that were absent in cells untreated with bile (Fig. 2). S. Typhimurium was intracellularly contained in Salmonella-containing vacuoles (SCV) evident at 2 h postinfection (Fig. 2). Invasion assays were also performed from the basolateral side, but the number of recovered CFU was observed to be extremely low (data not shown). Invasion of DGEC was SPI-1 dependent since a ΔSPI-1 strain was significantly impaired in the ability to invade these cells (Fig. 3). This was also supported by SEM observation of membrane ruffling after 2 h of infection (data not shown), which is a host cell response known to be mediated by SPI-1 (22).
Fig 2.
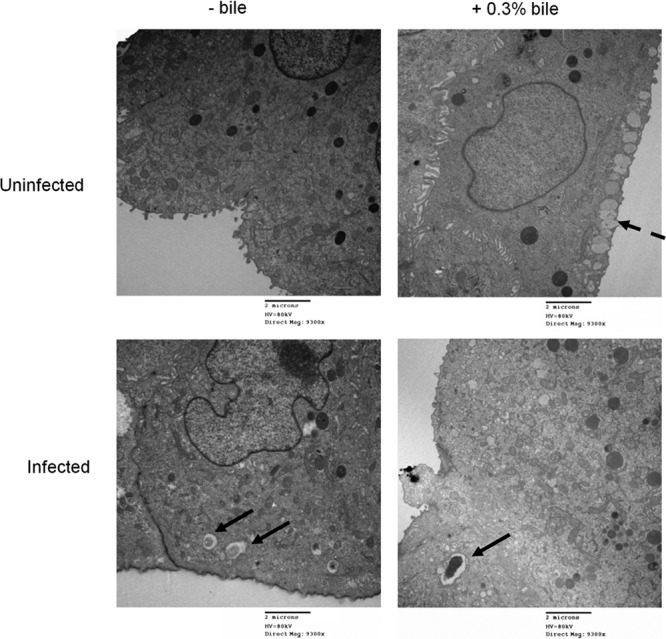
Exposure of DGEC to bile decreases invasion by S. Typhimurium. Representative TEM images showing uninfected or S. Typhimurium-infected DGEC in the absence or presence of bile are shown. Solid arrows, Salmonella-containing vacuoles; dashed arrows, mucus granules that were only present in DGEC exposed to bile. Cells were fixed and processed at 2 h postinfection.
Fig 3.
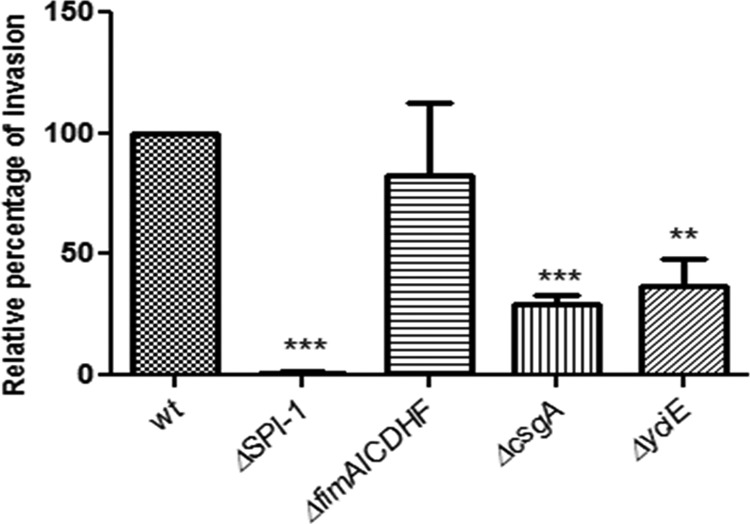
SPI-1, curli fimbriae, and yciE but not type 1 fimbria mutants decrease invasion into DGEC in vitro. Invasion relative to the wild-type (wt) level (mean plus standard deviation) of S. Typhimurium mutants into DGEC exposed to 0.3% bile at 2.5 h postinfection. Means of wild-type and mutant values were compared by a Student t test (**, P < 0.01; ***, P < 0.001). Assays were performed in triplicate on two separate occasions.
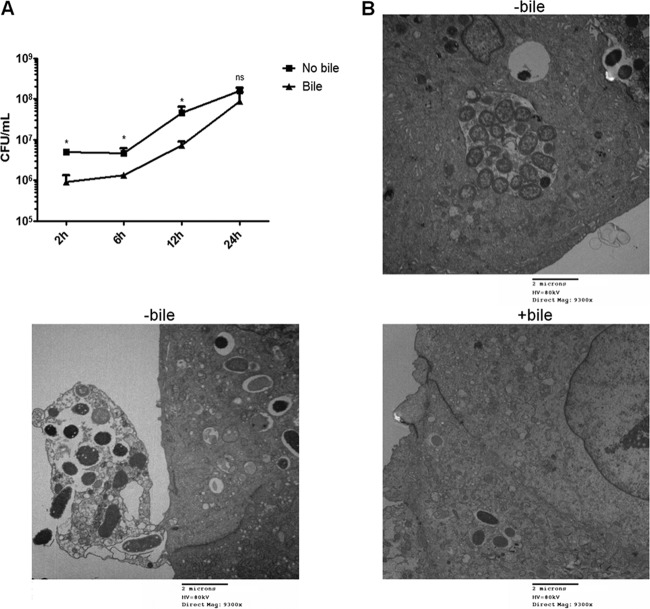
S. Typhimurium can replicate intracellularly in DGEC regardless of previous exposure of the epithelium to bile.
Replication of bacteria that had invaded the epithelium would aid in creating an intracellular niche. Intracellular survival assays showed that S. Typhimurium replicates inside DGEC, thus representing a good in vitro model to study host-pathogen interactions in the gallbladder. Up to 12 h postinfection, intracellular replication was significantly different between DGEC that were untreated and those that were exposed to bile, with bile-exposed DGEC harboring fewer bacteria. However, this difference was not significant at 24 h postinfection (Fig. 4A). Monitoring of the intracellular behavior of S. Typhimurium by TEM revealed that after 9 h postinfection, Salmonella bacteria were observed actively replicating inside an SCV. In accordance with the CFU results, the bacterial burden appeared higher in the absence of bile. Interestingly, when these cells were highly loaded with bacteria, some epithelial cells were observed to be translocated to the extracellular environment, in agreement with a process observed by Knodler et al. (23) (Fig. 4B). Impaired microvillus architecture was also evident. These phenotypes were not observed in a ΔSPI-1 strain (data not shown).
Fig 4.
S. Typhimurium can replicate intracellularly in DGEC. (A) Intracellular replication of S. Typhimurium in DGEC cultured with or without bile measured as CFU/ml. There is statistical significance between no bile and bile treatments at all time points except 24 h postinfection (*, P < 0.05, by a Student's t test). (B) Representative TEM images showing infected DGEC at 9 h postinfection with S. Typhimurium in the absence or presence of bile. In the absence of bile, bacteria were seen replicating inside SCV in higher numbers than in cells exposed to bile. Cell/cell debris being released to the extracellular environment was also observed.
S. Typhimurium forms extensive cellular aggregations/biofilms on the surface of DGEC.
In addition to bacterial invasion of the gallbladder epithelium, the growth and biofilm formation on the surface of the epithelium would aid in chronic colonization. Flowthrough experiments verified the ability of Salmonella to adhere to and form extensive bacterial foci on the surface of DGEC at 12 h postinoculation (Fig. 5). Extrapolymeric substance production comprising the biofilm matrix was evident, as observed by electron microscopy. Biofilm formation occurred regardless of the presence of bile in the tissue culture medium during the length of the experiment. However, CFU enumeration revealed that in the presence of bile, the number of bacteria recovered from biofilms was increased (8 × 108 ± 2.1 × 107 CFU/ml) compared to the number from biofilms on DGEC in the absence of bile (8.5 × 108 ± 1.4 × 108 CFU/ml). This difference was statistically significant by a Student's t test (P < 0.05).
Fig 5.
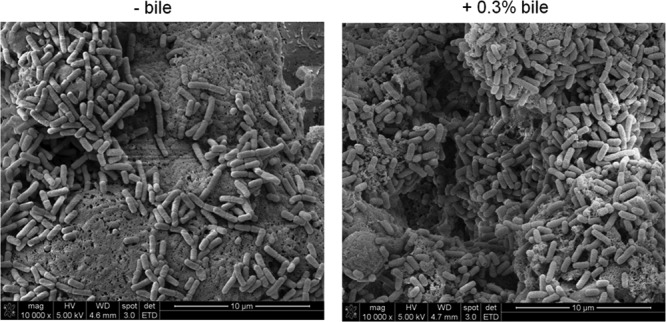
S. Typhimurium forms extensive cellular aggregations/biofilms on the surface of DGEC. Representative SEM images of S. Typhimurium biofilms on DGEC at 12 h postinoculation in a flow chamber in the absence or presence of 0.3% bile are shown. The presence of bile modestly increased biofilm formation.
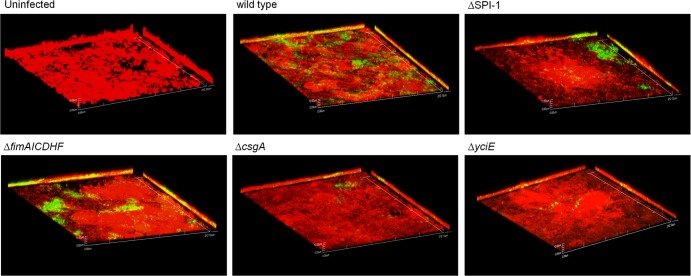
Curli and yciE affect invasion into and biofilm formation on DGEC.
Considering the observed phenotypes, we tested bacterial factors already reported or highly suggested to affect Salmonella invasion and biofilm formation. A ΔSPI-1 mutant was used as an invasion-deficient strain, and strains lacking type 1 fimbria structural subunits (ΔfimAICDHF strain), the curli fimbria main subunit (ΔcsgA strain), and the stress-related gene yciE (shown in a related study to be highly upregulated during biofilm formation on glass and cholesterol surfaces) (data not shown) were tested as potential strains defective in attachment/biofilm formation. Experiments were performed with DGEC previously exposed to 0.3% bile.
In addition to the already mentioned effect on invasion, a ΔSPI-1 strain also showed impaired attachment compared with that of the wild type (data not shown). However, ΔSPI-1 bacteria could form bacterial foci after 9 h of infection in a manner similar to that of the wild-type strain (Fig. 6). Thus, impaired invasion did not result in altered microcolony/biofilm formation on DGEC. The ΔfimAICDHF strain did not show a significant difference in its invasion or biofilm formation abilities compared with the wild type (Fig. 3 and 6). However, the ΔcsgA and ΔyciE strains showed no difference in initial attachment (data not shown) but demonstrated a significant decrease in invasion into DGEC and impaired microcolony formation on DGEC (Fig. 3 and 6). Thus, SPI-1 and type I fimbriae are not required for biofilm formation on DGEC, but csgA and yciE are important for both invasion and biofilm formation. These results were also verified by SEM imaging (data not shown).
Fig 6.
Curli and yciE affect biofilm formation on DGEC. Representative confocal images are shown of DGEC uninfected or infected with wild-type S. Typhimurium and mutants at 9 h postinfection in the presence of bile. All bacterial strains harbor the plasmid pFPV25.1 constitutively expressing GFP. DGEC are stained with Cell Tracker Red CMPTX. All images have a magnification of ×60.
In vivo assays recapitulate the invasion, intracellular survival, extrusion, and microcolony formation shown in vitro.
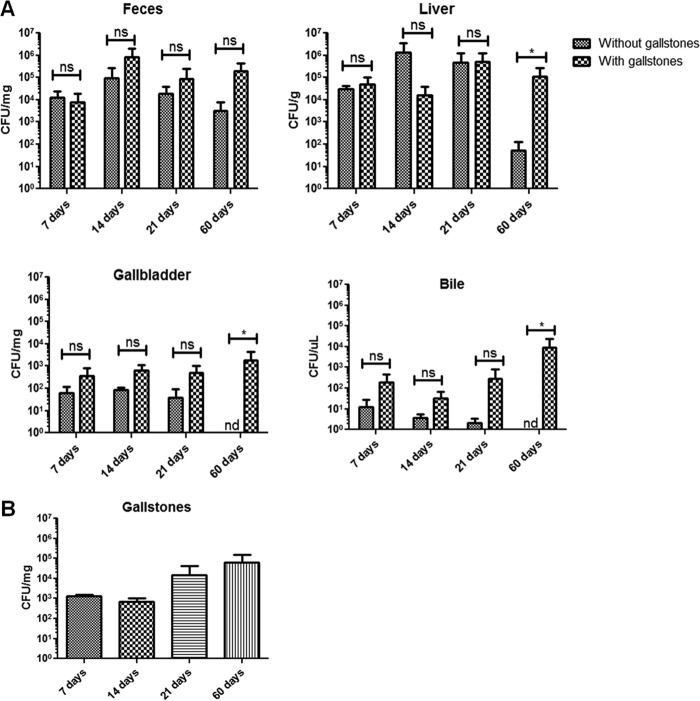
By using wild-type (NRAMP+/+, where NRAMP is natural-resistance-associated macrophage protein) mice with gallstones, we have developed a mouse model of chronic gallbladder infection that more appropriately mimics human infection than the commonly used acute infection mouse models (16). In the current study, infecting bacteria could be recovered from feces, liver, spleen, pancreas, gallbladder tissue, and gallbladder bile. Bacterial loads in the liver, gallbladder tissue, and gallbladder bile were always higher in mice harboring gallstones, especially at later time points such as 21 and 60 dpi (Fig. 7A). Bacteria were also isolated from gallstones at all time points, with higher numbers at later time points such as 21 and 60 dpi (Fig. 7B).
Fig 7.
The presence of gallstones enhances Salmonella colonization at 60 days postinfection. (A) S. Typhimurium enumeration in different mouse sites at 7, 14, 21, and 60 days postinfection. Although all sites showed more bacteria in mice harboring gallstones, the difference was only significant by a Student's t test at 60 dpi in the liver, gallbladder tissue, and bile (*, P < 0.05). (B) S. Typhimurium enumeration on gallstones at different days postinfection. Bacteria were recovered in higher numbers from gallstones at later time points (21 and 60 dpi), but this difference was not significant (analysis of variance test) from amounts at earlier time points (7 and 14 dpi). Data were gathered from two different experiments. ns, not significant. nd, not detected.
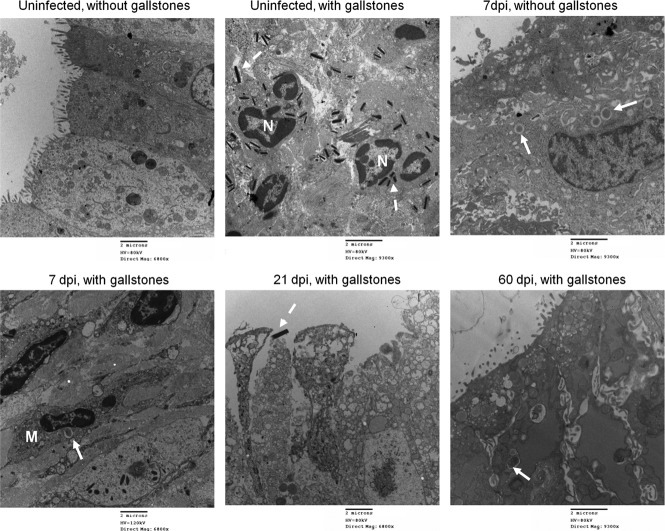
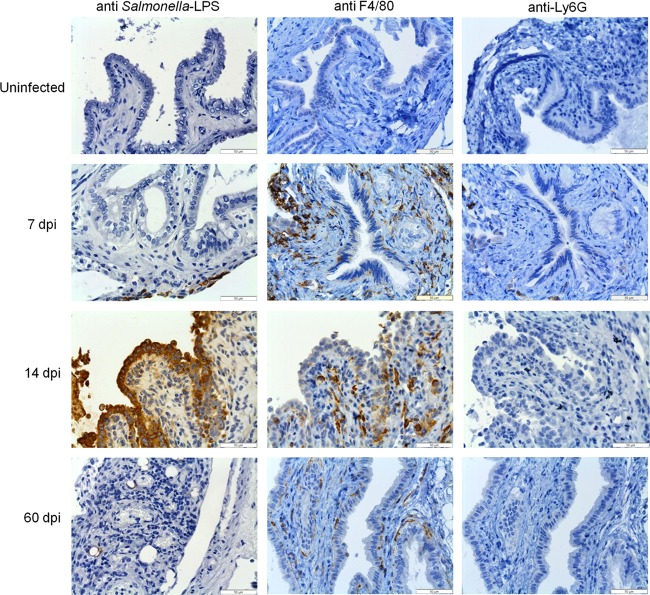
In vivo experiments using our chronic model of typhoid carriage corroborated the in vitro findings of invasion and intracellular replication of Salmonella into gallbladder epithelial cells (Fig. 8). In addition to the enumeration of Salmonella in mouse tissues, we were interested in monitoring the precise localization of Salmonella in the gallbladder as well as the gallbladder response during the transition from acute to chronic infection (e.g., 7 to 14 dpi) and during chronic stage (e.g., 21 to 60 dpi) in the absence and presence of gallstones. For this purpose, we monitored Salmonella infection at 7, 14, 21, and 60 dpi by TEM and IHC. TEM imaging showed damage of the gallbladder epithelium in infected mice up to 21 dpi, clearly affecting the microvilli and the integrity of the tight junctions (Fig. 8). The presence of gallstones per se also caused these phenotypes but to a lesser extent. In contrast to in vitro observations, the SCV were always seen containing only one or two bacteria. Macrophages were actively recruited and seen engulfing Salmonella bacteria (Fig. 8). IHC detection of Salmonella also showed the bacterium colonizing the lamina propria (Fig. 9).
Fig 8.
In vivo assays recapitulate invasion, intracellular survival, and extrusion. Representative TEM images of the mouse gallbladder epithelium in the absence and the presence of gallstones are shown. Damage of the gallbladder epithelium is evident at all days postinfection including in uninfected mice with gallstones. SCV are indicated with solid arrows and were observed containing only one or two bacteria. Dashed arrows indicate cholesterol crystals. Macrophages (M) were sometimes observed harboring SCV. Neutrophils (N) were actively seen engulfing cholesterol crystals. The release of epithelial cells was commonly seen, especially at 21 dpi. Regeneration of tight junctions was evident especially by 60 dpi. However, Salmonella was still seen intracellularly in mice harboring gallstones.
Fig 9.
Inflammation of the gallbladder is mostly macrophage related during infection in the absence of gallstones. Images show immunohistochemical analysis of representative sections of the gallbladder epithelium in the absence of gallstones from uninfected or infected mice at 7, 14, and 60 dpi by using anti-Salmonella LPS, anti-F4/80 (macrophage marker), and anti-Ly6G (neutrophil marker) antibodies. Brown areas are positive. The recruitment of macrophages was the hallmark throughout the course of infection.
As observed in vitro, at 21 dpi extrusion of infected epithelial cells was also evident, further suggesting an alternative mechanism for gallbladder persistence and transmission (Fig. 8). At 60 dpi, we were still able to observe SCV in the gallbladder epithelium, which corroborates the intracellular persistence of Salmonella in this niche (Fig. 8). Interestingly, the gallbladder epithelia tended to recover at 60 dpi, which was evident by recovery of tight junction and microvillus normal architecture (Fig. 8). In the absence of gallstones, this was concomitant with low bacterial burden (Fig. 7 and 9). In contrast, in the presence of gallstones, recovery of the epithelium was also evident but along with a moderate bacterial burden (Fig. 7).
Bacteria were seen extracellularly attached to the surface of the gallbladder epithelium, forming microcolonies at 21 dpi. This phenotype was not seen earlier, and it was present only in mice harboring gallstones (Fig. 10). Bacteria were still seen at the surface of the epithelium after 2 months of infection although they were sometimes difficult to observe because of extensive mucus production due to the presence of gallstones and/or the inflammatory response.
Fig 10.

S. Typhimurium can form microcolonies on the surface of the mouse gallbladder epithelium. Representative SEM images of uninfected/infected mouse gallbladder epithelium in the absence or presence of gallstones are shown. Salmonella microcolonies were observed on the epithelium only of mice harboring gallstones and only at 21 dpi.
Inflammation of the gallbladder is primarily macrophage related during chronic salmonellosis.
The decreased Salmonella colonization in the gallbladder of mice without gallstones led us to the hypothesis that an effective immune response is different in mice with and without gallstones. Thus, as an initial indicator, we monitored by IHC the presence of macrophages and neutrophils. Examination of mouse gallbladder tissues showed that in the absence of gallstones, macrophages and not neutrophils comprise the primary cellular infiltrate throughout the course of infection (Fig. 9 and Table 2).These patterns seem to be specific for gallbladder tissue since histopathological analysis of liver sections revealed that neutrophils and macrophages were actively present at all time points, with histological scoring (median) for macrophage recruitment as follows: at 7 days, 3; 14 days, 3; 21 days, 2; 60 days, 3. Scoring for neutrophil recruitment was as follows: at 7 days, 3; 14 days 2; 21 days, 2; and 60 days, 2. These data provide evidence that in the absence of gallstones, the immune response against chronic Salmonella infection in the gallbladder is mostly macrophage related.
Table 2.
Histological scoring of macrophage and neutrophil recruitment in mouse gallbladder tissue at different days postinfection
| Time point (dpi) | Histological score by and condition and cell typea |
|||
|---|---|---|---|---|
| − Gallstones |
+ Gallstones |
|||
| Macrophages | Neutrophils | Macrophages | Neutrophils | |
| Uninfected | 0 | 0 | 2 | 2 |
| 7 | 2 | 1 | 2 | 1 |
| 14 | 3 | 1 | 2 | 2 |
| 21 | 2 | 1 | 3 | 3 |
| 60 | 1 | 0 | 3 | 3 |
The absence (−) or presence (+) of gallstones is indicated.
In the presence of gallstones, macrophages and neutrophils were also observed at all time points in both infected and uninfected mice (likely due solely to the presence of gallstones). Although the recruitment of macrophages was observed at all time points, neutrophils were highly recruited at 21 and 60 dpi (Table 2). In addition, histopathology analysis also showed epithelial hyperplasia and hyalinosis. These data provide evidence that when gallstones are present, chronic inflammation is mediated by both macrophages and neutrophils, which along with epithelial changes (e.g., hyperplasia) may contribute to Salmonella carriage.
DISCUSSION
These studies describe the in vitro and in vivo interactions of S. Typhimurium with gallbladder epithelial cells. These assays uncovered two potential (non-gallstone-associated) mechanisms by which Salmonella can establish a persistent infection in the gallbladder during chronic carriage: biofilm formation on the epithelium and intracellular persistence within the epithelium, coupled with cell extrusion. We demonstrated that in vitro, Salmonella infection of polarized gallbladder epithelial cells corroborates in vivo mouse model findings and thus represents a good model for host-pathogen interaction studies. These DGEC have been intensively used for physiological studies, which supported their use as a model for human gallbladder epithelial cells (24–29). The percentage of S. Typhimurium invasion obtained in this study is relatively lower than results of other studies with polarized epithelial cells such as the dog kidney epithelial cell line (MDCK) and T84 cells, where approximately 4 to 5% of the inoculum could invade after 25 min of infection for MDCK cells or after 1 to 2 h of infection for T84 cells (30–32).
It is noteworthy that DGEC were exposed to bile during at least part of all assays to mimic closely the gallbladder environment. It is known that bile exposure increases mucus production, which contributes to bile sterility (27, 33). We observed that DGEC exposed to bile had increased numbers of apical mucus granules, which could explain the decreased invasion we obtained when these cells were pretreated with bile. It has also been shown that LPS increases mucus production (34). Thus, mucus induction represents an innate response of the gallbladder for initial protection against pathogens. Regarding the pathogen, bile is an environmental signal that alters expression of many Salmonella genes (35, 36), including those that decrease invasion of epithelial cells by downregulating SPI-1 gene expression (37). However, bile was removed from the DGEC prior to bacterial addition in invasion assays, so SPI-1 regulation is likely not directly responsible for the observed invasion defects seen in Fig. 1. Bile is also known to increase biofilm formation (38). This was also observed in our in vitro studies of biofilm formation on DGEC, although not dramatically, where cells exposed to bile had more bacteria attached to their surfaces after 12 h of flowthrough.
Corroborating in vitro observations, the mouse model demonstrated that Salmonella was able to attach to, invade, and replicate in the gallbladder epithelium. We demonstrated by TEM and IHC analysis that Salmonella is able to persist in the gallbladder epithelium up to 2 months postinfection. The presence of gallstones increased the bacterial loads in the liver, gallbladder, and bile. This is in accordance with previous findings in our laboratory (16) where we monitored Salmonella carriage for a shorter time period. Although we did not detect Salmonella by CFU counts in the absence of gallstones at 60 dpi, we were able to detect the bacteria in the epithelial layer by TEM and IHC. It is unclear why there was this discrepancy. In addition, mice without gallstones still possessed bacteria in the liver, thus putting the gallbladder at risk for transient or permanent colonization by Salmonella. In support of this observation, it was common to recover bacteria from bile but not from the gallbladder tissue in the same mouse.
We also observed Salmonella-containing cells being extruded from the epithelium both in vitro and in vivo, further implicating the gallbladder epithelium as a facilitator of the innate defense response. This phenomenon has been reported to be a pyroptosis-related, caspase-1 event triggered by flagellin of intracellular Salmonella. Signaling events result in proinflammatory cell death, releasing bacteria to the extracellular environment (23, 39). We believe that the liberation of Salmonella to the gallbladder lumen may play an important role in chronic carriage and dissemination by releasing bacteria that can recolonize the gallbladder epithelium or gallstones or that can be shed into the intestine for release.
Biofilm formation on gallstone surfaces has been shown to be the primary mechanism of Salmonella persistence in the gallbladder (16). Here, we showed both in vitro and in vivo that Salmonella is also able to form cell aggregates that appear to be biofilms on the gallbladder epithelium. It is unknown if these in vivo bacteria come from the gallstone surfaces or from the intracellular niche to colonize the epithelium, but biofilm formation on the gallbladder wall might represent an adaptation occurring during the chronic state of the disease. In support of this, there are epidemiological data showing that human chronic typhoid carriers from areas of endemicity possess bacterial aggregates attached to the surface of the gallbladder epithelium (S. Baker, personal communication). In the absence of gallstones in vivo, however, epithelial surface biofilms do not seem to be the primary mechanism of persistence, as we only detected biofilms on the epithelial surface of infected mice harboring gallstones in our model. This suggests that in the absence of gallstones, invasion of the gallbladder epithelium may be the primary mechanism of persistence. In the presence of gallstones, all three mechanisms (gallstone biofilms, epithelial cell biofilms, and cell invasion with cell sloughing) may be active.
Considering the potential role of invasion and biofilm formation in perpetuating Salmonella carriage in the gallbladder, we studied bacterial factors that could be involved in these phenotypes. We hypothesized that invasion-deficient mutants may build up on the epithelial cell surface, resulting in an increase in microcolonies/biofilms. We found that this was not the case because an invasion-deficient mutant (ΔSPI-1) attached and formed microcolonies on the surface of the epithelium in vitro. In support of this, SPI-1 mutants still firmly attach to the surface of the mouse cecum epithelia in resistant mouse strains (40).
Type 1 fimbriae are reported to be important for biofilm formation on HEp-2 tissue culture cells and in vivo on mouse and chicken intestinal epithelial cells (41–43). In contrast to this, it has been previously noted that different fimbrial types are required to colonize specific organs and that type 1 fimbriae are not required for persistence in mouse intestines (44, 45). Here, we show that genes encoding the structural components of the fimbriae shaft (fimAICDHF) are not required for attachment/biofilm on or invasion into gallbladder epithelial cells. In addition, results in our laboratory have also showed that a hyperfimbriate strain does not demonstrate increased attachment to gallstone surfaces (46). We have also shown, in our model of chronic carriage, that type 1 fimbria mutants colonize the mouse gallbladder in a manner similar to the wild-type strain (data not shown). Thus, while this could be a niche-specific phenotype, our evidence points to the lack of a role for type 1 fimbriae in gallbladder epithelial cell biofilms.
Curli fibers are widely reported to be an important component of the extrapolymeric substance that comprises the Salmonella biofilm matrix upon interaction with surfaces, including epithelial cells (43, 47–51). Although our results show that strains lacking csgA (encoding the main subunit of curli) are still able to attach to DGEC, they were not able to form microcolonies/biofilms on the surface of the gallbladder epithelium. This phenotype is similar to the csgA mutant phenotype observed on chicken intestinal tissue (21). It is also in accordance with studies on plant surfaces, where csgA and csgB genes were not required for initial attachment but for extrapolymeric substance production that characterizes mature biofilm (52).
The yciE gene belongs to the operon yciGFE-katN, which is RpoS dependent, and it is upregulated in the presence of bile (36, 53–55). In addition, this gene was found to be 51-fold upregulated in biofilms on glass and cholesterol surfaces in a bile-containing medium (data not shown). The precise physiological role of most of the genes within this operon is unknown, but since yciE encodes a putative cytoplasmic protein and is upregulated during biofilm formation in the presence of bile, we hypothesized that this gene might be important for DGEC attachment/biofilms and/or invasion. In fact, a yciE mutant was highly impaired in not only biofilm formation but also invasion. This suggests that yciE may be important in allowing bacteria to endure a bile-containing environment, and thus its absence could impair colonization of the gallbladder epithelium. Supporting this, we have previously shown that RpoS is necessary for biofilm formation on glass and gallstone surfaces (56).
During gallbladder colonization, Salmonella has to overcome the gallbladder innate immune response, including the observed recruitment of macrophages and neutrophils in our in vivo model. In acute models of typhoid fever, it has been demonstrated that neutrophils are actively recruited to the gallbladder lumen as a result of Salmonella invasion (20). Here in our model of chronic carriage, we show that the less bactericidal macrophages, not neutrophils, comprise the primary infiltrating cells in the gallbladder tissue, which may facilitate the development of chronic carriage. However, in the presence of gallstones, in addition to macrophages, neutrophils were also highly recruited at 21 and 60 dpi, which suggests an exacerbated inflammatory environment that, along with hyperplasia of the gallbladder epithelium, can also contribute to Salmonella persistence.
Based on these observations, we propose a model for chronic carriage in the absence or presence of gallstones. In the absence of gallstones and during acute stages of infection, Salmonella reaches the gallbladder from the liver and encounters mucus, surface IgA (sIgA), and other innate immune components present in bile and in or on the gallbladder epithelium that likely decrease bacterial load. Bile will limit Salmonella invasion into the epithelium, but the bacteria may escape bile when it is within the mucus layer surrounding the epithelium. Some bacteria will invade and, once inside the gallbladder epithelium, actively replicate and in the long term remain intracellularly with a relatively low bacterial load, decreased active inflammation, and intact epithelia. Some cells with replicating bacteria will likely be extruded via pyroptosis. Other bacteria reaching the gallbladder lumen may attach to the surface or be cleared by phagocytic cells or shed into the intestine. Although biofilm formation on the gallbladder epithelium does not appear to be a frequent phenomenon in our in vivo model, evidence from human carriers does suggest that bacterial foci on the epithelial cell surface occur (S. Baker, personal communication).
In addition to the factors mentioned above, in the presence of gallstones Salmonella encounters an already inflamed gallbladder (mediated by both neutrophils and macrophages). Also as a result of the presence of gallstones, there is increased epithelial proliferation (hyperplasia), mucus production, and altered bile composition (57–59). This environment may benefit Salmonella not only in colonization of the compromised gallbladder epithelium but also in attachment to gallstone surfaces, resulting in prolonged carriage of bacteria in all gallbladder niches.
There are still many questions to answer, including how Salmonella is able to persist in the gallbladder in the presence of an immune response while the host remains primarily asymptomatic. It is likely that both the bacterium and host respond to the milieu and one another, resulting in a permissive environment responsible for this chronic infection. The findings of future studies related to this unique host-pathogen interface in the gallbladder will provide more insights about how chronic carriage occurs and persists as well as new treatment/preventive strategies.
ACKNOWLEDGMENTS
We thank Sum P. Lee and Christopher Savard from the Division of Gastroenterology at the University of Washington for their donation of the primary dog gallbladder epithelial cells used in this study. We also thank staff members from the OSU Campus Microscopy and Imaging Facility and the OSU Comparative Pathology and Mouse Phenotyping Shared Resource for their support and contributions of electron microscopy and histology/IHC, respectively. In addition, we also thank Steve Libby (University of Washington), Andreas Baumler (University of California, Davis), and Michael McClelland (San Diego Institute for Biological Research) for their strain donations.
This work was supported by a grant from the U.S. National Institutes of Health (AI066208).
Footnotes
Published ahead of print 3 June 2013
REFERENCES
- 1. Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull. World Health Organ. 82:346–353 [PMC free article] [PubMed] [Google Scholar]
- 2. Vazquez-Torres A, Jones-Carson J, Baumler AJ, Falkow S, Valdivia R, Brown W, Le M, Berggren R, Parks WT, Fang FC. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804–808 [DOI] [PubMed] [Google Scholar]
- 3. Levine MM, Black RE, Lanata C. 1982. Precise estimation of the numbers of chronic carriers of Salmonella Typhi in Santiago, Chile, an endemic area. J. Infect. Dis. 146:724–726 [DOI] [PubMed] [Google Scholar]
- 4. Merselis JG, Jr, Kaye D, Connolly CS, Hook EW. 1964. Quantitative bacteriology of the typhoid carrier state. Am. J. Trop. Med. Hyg. 13:425–429 [DOI] [PubMed] [Google Scholar]
- 5. Vogelsang TM, Boe J. 1948. Temporary and chronic carriers of Salmonella Typhi and Salmonella Paratyphi B. J. Hyg. (Lond.) 46:252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dongol S, Thompson CN, Clare S, Nga TV, Duy PT, Karkey A, Arjyal A, Koirala S, Khatri NS, Maskey P, Poudel S, Jaiswal VK, Vaidya S, Dougan G, Farrar JJ, Dolecek C, Basnyat B, Baker S. 2012. The microbiological and clinical characteristics of invasive Salmonella in gallbladders from cholecystectomy patients in Kathmandu, Nepal. PLoS One 7:e47342. 10.1371/journal.pone.0047342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinnott CR, Teall AJ. 1987. Persistent gallbladder carriage of Salmonella Typhi. Lancet i:976. [DOI] [PubMed] [Google Scholar]
- 8. Shpargel JS, Berardi RS, Lenz D. 1985. Salmonella Typhi carrier state 52 years after illness with typhoid fever: a case study. Am. J. Infect. Control 13:122–123 [DOI] [PubMed] [Google Scholar]
- 9. Caygill CP, Hill MJ, Braddick M, Sharp JC. 1994. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 343:83–84 [DOI] [PubMed] [Google Scholar]
- 10. Shukla VK, Singh H, Pandey M, Upadhyay SK, Nath G. 2000. Carcinoma of the gallbladder—is it a sequel of typhoid? Dig. Dis. Sci. 45:900–903 [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez-Escobedo G, Marshall JM, Gunn JS. 2011. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat. Rev. Microbiol. 9:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monds RD, O'Toole GA. 2009. The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol. 17:73–87 [DOI] [PubMed] [Google Scholar]
- 13. Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 14. Schioler H, Christiansen ED, Hoybye G, Rasmussen SN, Greibe J. 1983. Biliary calculi in chronic Salmonella carriers and healthy controls: a controlled study. Scand. J. Infect. Dis. 15:17–19 [DOI] [PubMed] [Google Scholar]
- 15. Karaki K, Matsubara Y. 1984. Surgical treatment of chronic biliary typhoid and paratyphoid carriers. Nihon Shokakibyo Gakkai Zasshi. 81:2978–2985 . (In Japanese.) [PubMed] [Google Scholar]
- 16. Crawford RW, Rosales-Reyes R, Ramirez-Aguilar Mde L, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. 2010. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc. Natl. Acad. Sci. U. S. A. 107:4353–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prouty AM, Schwesinger WH, Gunn JS. 2002. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect. Immun. 70:2640–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharma V, Chauhan VS, Nath G, Kumar A, Shukla VK. 2007. Role of bile bacteria in gallbladder carcinoma. Hepatogastroenterology 54:1622–1625 [PubMed] [Google Scholar]
- 19. Vaishnavi C, Singh S, Kochhar R, Bhasin D, Singh G, Singh K. 2005. Prevalence of Salmonella enterica serovar Typhi in bile and stool of patients with biliary diseases and those requiring biliary drainage for other purposes. Jpn. J. Infect. Dis. 58:363–365 [PubMed] [Google Scholar]
- 20. Menendez A, Arena ET, Guttman JA, Thorson L, Vallance BA, Vogl W, Finlay BB. 2009. Salmonella infection of gallbladder epithelial cells drives local inflammation and injury in a model of acute typhoid fever. J. Infect. Dis. 200:1703–1713 [DOI] [PubMed] [Google Scholar]
- 21. Ledeboer NA, Jones BD. 2005. Exopolysaccharide sugars contribute to biofilm formation by Salmonella enterica serovar Typhimurium on HEp-2 cells and chicken intestinal epithelium. J. Bacteriol. 187:3214–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Finlay BB, Ruschkowski S, Dedhar S. 1991. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J. Cell Sci. 99:283–296 [DOI] [PubMed] [Google Scholar]
- 23. Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, Steele-Mortimer O. 2010. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl. Acad. Sci. U. S. A. 107:17733–17738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuver R, Savard C, Oda D, Lee SP. 1994. PGE generates intracellular cAMP and accelerates mucin secretion by cultured dog gallbladder epithelial cells. Am. J. Physiol. 267:G998–1003 [DOI] [PubMed] [Google Scholar]
- 25. Klinkspoor JH, Kuver R, Savard CE, Oda D, Azzouz H, Tytgat GN, Groen AK, Lee SP. 1995. Model bile and bile salts accelerate mucin secretion by cultured dog gallbladder epithelial cells. Gastroenterology 109:264–274 [DOI] [PubMed] [Google Scholar]
- 26. Yoshida T, Klinkspoor JH, Kuver R, Wrenn SP, Kaler EW, Lee SP. 2000. Cholestan-3β,5α,6β-triol, but not 7-ketocholesterol, suppresses taurocholate-induced mucin secretion by cultured dog gallbladder epithelial cells. FEBS Lett. 478:113–118 [DOI] [PubMed] [Google Scholar]
- 27. Klinkspoor JH, Yoshida T, Lee SP. 1998. Bile salts stimulate mucin secretion by cultured dog gallbladder epithelial cells independent of their detergent effect. Biochem. J. 332:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Choi J, Klinkspoor JH, Yoshida T, Lee SP. 1999. Lipopolysaccharide from Escherichia coli stimulates mucin secretion by cultured dog gallbladder epithelial cells. Hepatology 29:1352–1357 [DOI] [PubMed] [Google Scholar]
- 29. Malik R, Lee SK, Savard CE, Oda D, Wong WS, Chan BY, Lee SP. 1997. The effect of N-methyl-N′-nitro-N-nitrosoguanidine on cultured dog gallbladder epithelial cells. Hepatology 26:1296–1302 [DOI] [PubMed] [Google Scholar]
- 30. Wagner C, Polke M, Gerlach RG, Linke D, Stierhof YD, Schwarz H, Hensel M. 2011. Functional dissection of SiiE, a giant non-fimbrial adhesin of Salmonella enterica. Cell. Microbiol. 13:1286–1301 [DOI] [PubMed] [Google Scholar]
- 31. Bishop A, House D, Perkins T, Baker S, Kingsley RA, Dougan G. 2008. Interaction of Salmonella enterica serovar Typhi with cultured epithelial cells: roles of surface structures in adhesion and invasion. Microbiology 154:1914–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raffatellu M, Wilson RP, Chessa D, Andrews-Polymenis H, Tran QT, Lawhon S, Khare S, Adams LG, Baumler AJ. 2005. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype Typhimurium invasion of epithelial cells. Infect. Immun. 73:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Begley M, Gahan CG, Hill C. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29:625–651 [DOI] [PubMed] [Google Scholar]
- 34. Zen Y, Harada K, Sasaki M, Tsuneyama K, Katayanagi K, Yamamoto Y, Nakanuma Y. 2002. Lipopolysaccharide induces overexpression of MUC2 and MUC5AC in cultured biliary epithelial cells: possible key phenomenon of hepatolithiasis. Am. J. Pathol. 161:1475–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prouty AM, Brodsky IE, Falkow S, Gunn JS. 2004. Bile-salt-mediated induction of antimicrobial and bile resistance in Salmonella Typhimurium. Microbiology 150:775–783 [DOI] [PubMed] [Google Scholar]
- 36. Prouty AM, Brodsky IE, Manos J, Belas R, Falkow S, Gunn JS. 2004. Transcriptional regulation of Salmonella enterica serovar Typhimurium genes by bile. FEMS Immunol. Med. Microbiol. 41:177–185 [DOI] [PubMed] [Google Scholar]
- 37. Prouty AM, Gunn JS. 2000. Salmonella enterica serovar Typhimurium invasion is repressed in the presence of bile. Infect. Immun. 68:6763–6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crawford RW, Gibson DL, Kay WW, Gunn JS. 2008. Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect. Immun. 76:5341–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Jong HK, Parry CM, van der Poll T, Wiersinga WJ. 2012. Host-pathogen interaction in invasive salmonellosis. PLoS Pathog. 8:e1002933. 10.1371/journal.ppat.1002933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sivula CP, Bogomolnaya LM, Andrews-Polymenis HL. 2008. A comparison of cecal colonization of Salmonella enterica serotype Typhimurium in white leghorn chicks and Salmonella-resistant mice. BMC Microbiol. 8:182. 10.1186/1471-2180-8-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boddicker JD, Ledeboer NA, Jagnow J, Jones BD, Clegg S. 2002. Differential binding to and biofilm formation on, HEp-2 cells by Salmonella enterica serovar Typhimurium is dependent upon allelic variation in the fimH gene of the fim gene cluster. Mol. Microbiol. 45:1255–1265 [DOI] [PubMed] [Google Scholar]
- 42. Dwyer BE, Newton KL, Kisiela D, Sokurenko EV, Clegg S. 2011. Single nucleotide polymorphisms of fimH associated with adherence and biofilm formation by serovars of Salmonella enterica. Microbiology 157:3162–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ledeboer NA, Frye JG, McClelland M, Jones BD. 2006. Salmonella enterica serovar Typhimurium requires the Lpf, Pef, and Tafi fimbriae for biofilm formation on HEp-2 tissue culture cells and chicken intestinal epithelium. Infect. Immun. 74:3156–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Norris TL, Kingsley RA, Bumler AJ. 1998. Expression and transcriptional control of the Salmonella Typhimurium lpf fimbrial operon by phase variation. Mol. Microbiol. 29:311–320 [DOI] [PubMed] [Google Scholar]
- 45. Weening EH, Barker JD, Laarakker MC, Humphries AD, Tsolis RM, Baumler AJ. 2005. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in mice. Infect. Immun. 73:3358–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crawford RW, Reeve KE, Gunn JS. 2010. Flagellated but not hyperfimbriated Salmonella enterica serovar Typhimurium attaches to and forms biofilms on cholesterol-coated surfaces. J. Bacteriol. 192:2981–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jonas K, Tomenius H, Kader A, Normark S, Romling U, Belova LM, Melefors O. 2007. Roles of curli, cellulose and BapA in Salmonella biofilm morphology studied by atomic force microscopy. BMC Microbiol. 7:70. 10.1186/1471-2180-7-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jain S, Chen J. 2007. Attachment and biofilm formation by various serotypes of Salmonella as influenced by cellulose production and thin aggregative fimbriae biosynthesis. J. Food Prot. 70:2473–2479 [DOI] [PubMed] [Google Scholar]
- 49. Austin JW, Sanders G, Kay WW, Collinson SK. 1998. Thin aggregative fimbriae enhance Salmonella enteritidis biofilm formation. FEMS Microbiol. Lett. 162:295–301 [DOI] [PubMed] [Google Scholar]
- 50. Romling U, Sierralta WD, Eriksson K, Normark S. 1998. Multicellular and aggregative behaviour of Salmonella Typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28:249–264 [DOI] [PubMed] [Google Scholar]
- 51. Sukupolvi S, Lorenz RG, Gordon JI, Bian Z, Pfeifer JD, Normark SJ, Rhen M. 1997. Expression of thin aggregative fimbriae promotes interaction of Salmonella Typhimurium SR-11 with mouse small intestinal epithelial cells. Infect. Immun. 65:5320–5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barak JD, Gorski L, Naraghi-Arani P, Charkowski AO. 2005. Salmonella enterica virulence genes are required for bacterial attachment to plant tissue. Appl. Environ. Microbiol. 71:5685–5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ibanez-Ruiz M, Robbe-Saule V, Hermant D, Labrude S, Norel F. 2000. Identification of RpoS (σS)-regulated genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:5749–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robbe-Saule V, Coynault C, Ibanez-Ruiz M, Hermant D, Norel F. 2001. Identification of a non-haem catalase in Salmonella and its regulation by RpoS (σS). Mol. Microbiol. 39:1533–1545 [DOI] [PubMed] [Google Scholar]
- 55. Beraud M, Kolb A, Monteil V, D'Alayer J, Norel F. 2010. A proteomic analysis reveals differential regulation of the σS-dependent yciGFE(katN) locus by YncC and H-NS in Salmonella and Escherichia coli K-12. Mol. Cell. Proteomics 9:2601–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prouty AM, Gunn JS. 2003. Comparative analysis of Salmonella enterica serovar Typhimurium biofilm formation on gallstones and on glass. Infect. Immun. 71:7154–7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Finzi L, Barbu V, Burgel PR, Mergey M, Kirkwood KS, Wick EC, Scoazec JY, Peschaud F, Paye F, Nadel JA, Housset C. 2006. MUC5AC, a gel-forming mucin accumulating in gallstone disease, is overproduced via an epidermal growth factor receptor pathway in the human gallbladder. Am. J. Pathol. 169:2031–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chang HJ, Suh JI, Kwon SY. 1999. Gallstone formation and gallbladder mucosal changes in mice fed a lithogenic diet. J. Korean Med. Sci. 14:286–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mathur SK, Duhan A, Singh S, Aggarwal M, Aggarwal G, Sen R, Singh S, Garg S. 2012. Correlation of gallstone characteristics with mucosal changes in gall bladder. Trop. Gastroenterol. 33:39–44 [DOI] [PubMed] [Google Scholar]