Abstract
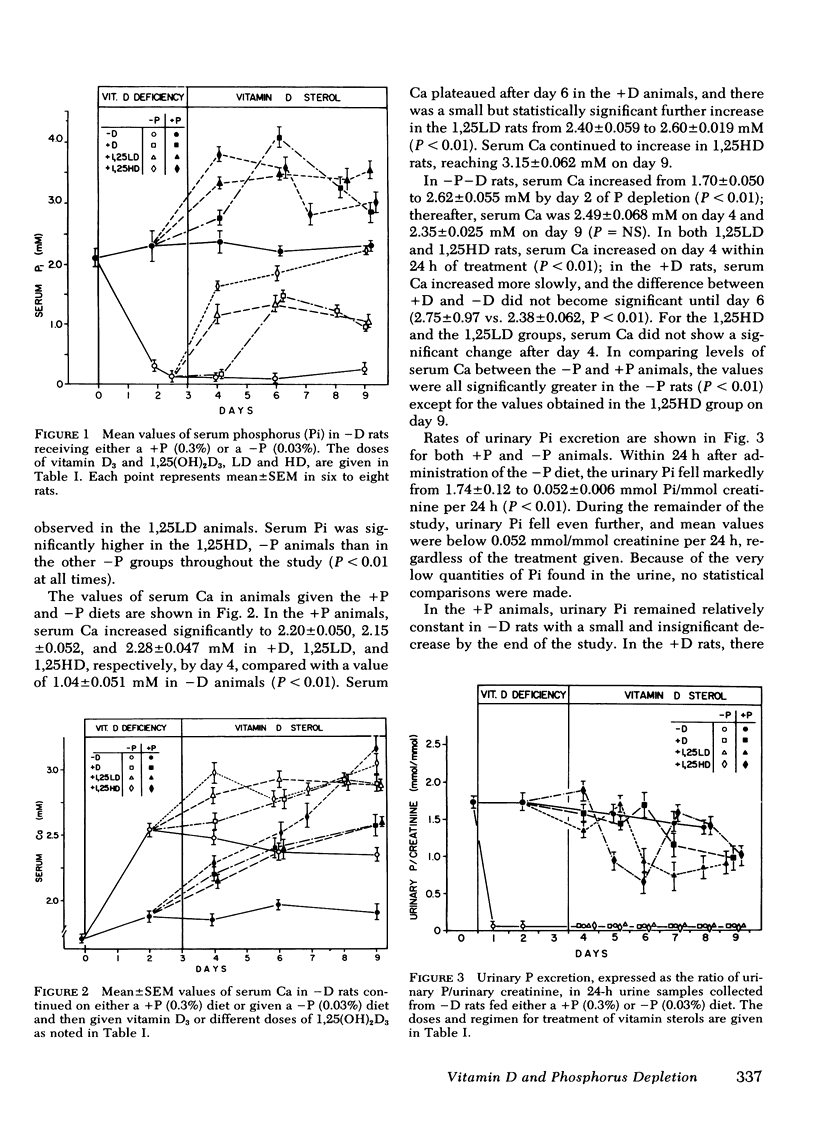
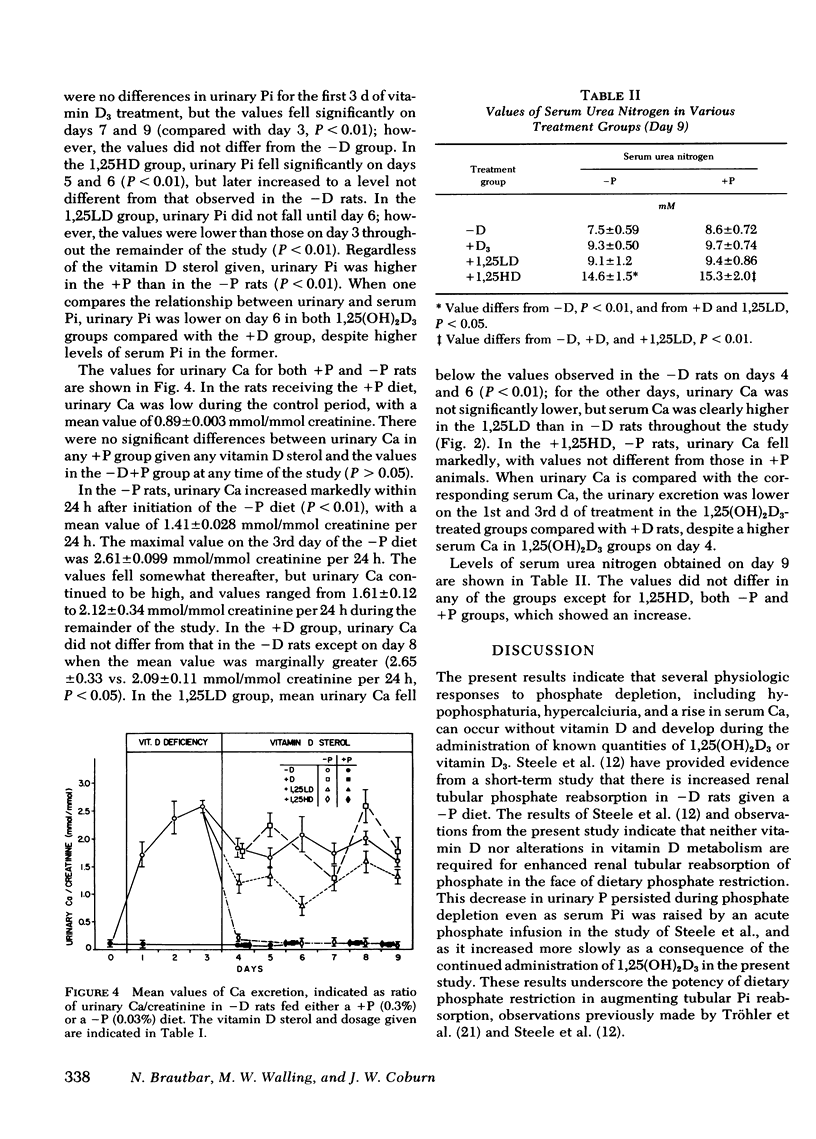
To evaluate the role of vitamin D in the physiologic response to phosphorus depletion (P depleton) and the response to vitamin D administration in P depletion, we studied vitamin D-deficient (−D) rats, fed either a normal or low phosphorus diet and then injected intraperitoneally on alternate days with replacement vitamin D3, 1.25 μg qod (D3); 1.25-dihydroxy-vitamin D3[1,25(OH)2D3] in physiologic, 54 ng qod (LD), and pharmacologic doses, 400 ng qod (HD); or vehicle alone (−D). The following results were obtained: (a) With P depletion, urinary excretion of inorganic phosphorus (Pi) fell to almost undetectable levels in −D rats, and two physiologic features of P depletion a calcemic effect and hypercalciuria, ensued. (b) With administration of vitamin D3 or 1,25(OH)2D3 in either doses to P-depleted rats, the renal retention of Pi was unaltered despite a significant elevation of serum Pi. (c) The calcemic response to P depletion was accentuated by vitamin D sterols, and the hypercalciuria of P depletion was reduced by 1,25(OH)2D3, HD > LD > D3. (d) In −D animals receiving normal Pi (+P), D3, and 1,25(OH)2D3, both LD and HD produced a significant calcemic and phosphatemic effect. (e) Urinary Pi excretion in +P animals was reduced slightly by vitamin D3 whereas 1,25(OH)2D3, both LD and HD, lowered urinary Pi markedly despite an increased serum Pi. (f) The serial values of serum Ca and Pi and urinary Ca in PD rats and the sequential values for urinary and serum Pi in +P rats indicated more rapid effects of 1,25(OH)2D3, both HD and LD, compared with D3. We conclude that: (a) The renal adaptation and physiologic response to PD does not require the presence of vitamin D. (b) 1,25(OH)2D3 may directly enhance the renal tubular reabsorption of Pi even as serum Pi rises. (c) A hypocalciuric action of 1,25(OH)2D3 in rats on low phosphorus diet could be direct or occur as a consequence of an increase in serum Pi produced by 1,25(OH)2D3. The different sequential renal response to D3 compared with 1,25-(OH)2D3 raises the possibility that other natural forms of vitamin D3 [i.e., 25(OH)D3, 24,25(OH)2D3, etc.] which may be present in vitamin D-fed rats but not those given only 1,25(OH)2D3, could modify the actions of 1,25(OH)2D3.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylink D., Wergedal J., Stauffer M. Formation, mineralization, and resorption of bone in hypophosphatemic rats. J Clin Invest. 1971 Dec;50(12):2519–2530. doi: 10.1172/JCI106752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonjour J. P., Preston C., Fleisch H. Effect of 1,25-dihydroxyvitamin D3 on the renal handling of Pi in thyroparathyroidectomized rats. J Clin Invest. 1977 Dec;60(6):1419–1428. doi: 10.1172/JCI108903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle I. T., Gray R. W., DeLuca H. F. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman A. S., Coburn J. W., Massry S. G., Norman A. W. 1,25 Dihydroxy-vitamin D3 in normal man and patients with renal failure. Ann Intern Med. 1974 Feb;80(2):161–168. doi: 10.7326/0003-4819-80-2-161. [DOI] [PubMed] [Google Scholar]
- Brickman A. S., Hartenbower D. L., Norman A. W., Coburn J. W. Actions of 1 alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on mineral metabolism in man. I. Effects on net absorption of phosphorus. Am J Clin Nutr. 1977 Jul;30(7):1064–1069. doi: 10.1093/ajcn/30.7.1064. [DOI] [PubMed] [Google Scholar]
- Castillo L., Tanaka Y., DeLuca H. F. The mobilization of bone mineral by 1,25-dihydroxyvitamin D3 in hypophosphatemic rats. Endocrinology. 1975 Oct;97(4):995–999. doi: 10.1210/endo-97-4-995. [DOI] [PubMed] [Google Scholar]
- Chen T. C., Castillo L., Korycka-Dahl M., DeLuca H. F. Role of vitamin D metabolites in phosphate transport of rat intestine. J Nutr. 1974 Aug;104(8):1056–1060. doi: 10.1093/jn/104.8.1056. [DOI] [PubMed] [Google Scholar]
- Coburn J. W., Massry S. G. Changes in serum and urinary calcium during phosphate depletion: studies on mechanisms. J Clin Invest. 1970 Jun;49(6):1073–1087. doi: 10.1172/JCI106323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo L. S., Sheehe P. R., Weiner I. M. Renal actions of vitamin D in D-deficient rats. Am J Physiol. 1974 Jun;226(6):1490–1495. doi: 10.1152/ajplegacy.1974.226.6.1490. [DOI] [PubMed] [Google Scholar]
- Cuisnier-Gleizes P., Thomasset M., Sainteny-Debove F., Mathieu H. Phosphorus deficiency, parathyroid hormone and bone resorption in the growing rat. Calcif Tissue Res. 1976 Jun 14;20(3):235–249. doi: 10.1007/BF02546412. [DOI] [PubMed] [Google Scholar]
- Dominguez J. H., Gray R. W., Lemann J., Jr Dietary phosphate deprivation in women and men: effects on mineral and acid balances, parathyroid hormone and the metabolism of 25-OH-vitamin D. J Clin Endocrinol Metab. 1976 Nov;43(5):1056–1068. doi: 10.1210/jcem-43-5-1056. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Pezant E., Miravet L., Fellot C., Balsan S. 1,25-Dihydroxycholecalciferol effect on serum phosphorus homeostasis in rats. Endocrinology. 1976 Mar;98(3):794–799. doi: 10.1210/endo-98-3-794. [DOI] [PubMed] [Google Scholar]
- Henry H. L., Taylor A. N., Norman A. W. Response of chick parathyroid glands to the vitamin D metabolites, 1,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol. J Nutr. 1977 Oct;107(10):1918–1926. doi: 10.1093/jn/107.10.1918. [DOI] [PubMed] [Google Scholar]
- Hohenwallner W., Wimmer E. The Malachite green micromethod for the determination of inorganic phosphate. Clin Chim Acta. 1973 Apr 30;45(2):169–175. doi: 10.1016/0009-8981(73)90406-3. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Brumbaugh P. F., Hussler M. R., Wergedal J. E., Baylink D. J. Regulation of serum 1alpha,25-dihydroxyvitamin D3 by calcium and phosphate in the rat. Science. 1975 Nov 7;190(4214):578–580. doi: 10.1126/science.1188357. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Russell J., Avioli L. V. 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol: conversion impaired by systemic metabolic acidosis. Science. 1977 Mar 11;195(4282):994–996. doi: 10.1126/science.841324. [DOI] [PubMed] [Google Scholar]
- Llach F., Coburn J. W., Brickman A. S., Kurokawa K., Norman A. W., Canterbury J. M., Reiss E. Acute actions of 1,25-dihydroxy-vitamin D3 in normal man: effect on calcium and parathyroid status. J Clin Endocrinol Metab. 1977 Jun;44(6):1054–1060. doi: 10.1210/jcem-44-6-1054. [DOI] [PubMed] [Google Scholar]
- Lotz M., Zisman E., Bartter F. C. Evidence for a phosphorus-depletion syndrome in man. N Engl J Med. 1968 Feb 22;278(8):409–415. doi: 10.1056/NEJM196802222780802. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Wong R. G. Biological activity of the vitamin D metabolite 1,25-dihydroxycholecalciferol in chickens and rats. J Nutr. 1972 Dec;102(12):1709–1718. doi: 10.1093/jn/102.12.1709. [DOI] [PubMed] [Google Scholar]
- Popovtzer M. M., Robinette J. B., DeLuca H. F., Holick M. F. The acute effect of 25-hydroxycholecalciferol on renal handling of phosphorus. Evidence for a parathyroid hormone-dependent mechanism. J Clin Invest. 1974 Mar;53(3):913–921. doi: 10.1172/JCI107632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschett J. B., Beck W. S., Jr Parathyroid hormone and 25-hydroxy vitamin D3: synergistic and antagonistic effects on renal phosphate transport. Science. 1975 Oct 31;190(4213):473–475. doi: 10.1126/science.1166316. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Niemann I. Effect of phosphate, calcium and magnesium on bone resorption and hormonal responses in tissue culture. Endocrinology. 1969 Sep;85(3):446–452. doi: 10.1210/endo-85-3-446. [DOI] [PubMed] [Google Scholar]
- Ribovich M. L., DeLuca H. F. The influence of dietary calcium and phosphorus on intestinal calcium transport in rats given vitamin D metabolites. Arch Biochem Biophys. 1975 Oct;170(2):529–535. doi: 10.1016/0003-9861(75)90148-4. [DOI] [PubMed] [Google Scholar]
- STEENBOCK H., HERTING D. C. Vitamin D and growth. J Nutr. 1955 Dec 10;57(4):449–468. doi: 10.1093/jn/57.4.449. [DOI] [PubMed] [Google Scholar]
- Siest G., Vigneron C. Etude du dosage de l'urée et de la citrulline en milieu protéique. Méthode manuelle et automatique à la diacetylmonoxime. Clin Chim Acta. 1968;20(3):373–379. doi: 10.1016/0009-8981(68)90291-x. [DOI] [PubMed] [Google Scholar]
- Steele T. H., DeLuca H. F. Influence of dietary phosphorus on renal phosphate reabsorption in the parathyroidectomized rat. J Clin Invest. 1976 Apr;57(4):867–874. doi: 10.1172/JCI108363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele T. H., Engle J. E., Tanaka Y., Lorenc R. S., Dudgeon K. L., DeLuca H. F. Phosphatemic action of 1,25-dihydroxyvitamin D3. Am J Physiol. 1975 Aug;229(2):489–495. doi: 10.1152/ajplegacy.1975.229.2.489. [DOI] [PubMed] [Google Scholar]
- Trohler U., Bonjour J. P., Fleisch H. Renal tubular adaptation to dietary phosphorus. Nature. 1976 May 13;261(5556):145–146. doi: 10.1038/261145a0. [DOI] [PubMed] [Google Scholar]
- Tröhler U., Bonjour J. P., Fleisch H. Inorganic phosphate homeostasis. Renal adaptation to the dietary intake in intact and thyroparathyroidectomized rats. J Clin Invest. 1976 Feb;57(2):264–273. doi: 10.1172/JCI108277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling M. W., Hartenbower D. L., Coburn J. W., Norman A. W. Effects of 1 alpha,25-, 24R,25-, and 1 alpha,24R,25-hydroxylated metabolites of vitamin D3 on calcium and phosphate absorption by duodenum from intact and nephrectomized rats. Arch Biochem Biophys. 1977 Jul;182(1):251–257. doi: 10.1016/0003-9861(77)90305-8. [DOI] [PubMed] [Google Scholar]
- Walling M. W., Rothman S. S. Phosphate-independent, carrier-mediated active transport of calcium by rat intestine. Am J Physiol. 1969 Oct;217(4):1144–1148. doi: 10.1152/ajplegacy.1969.217.4.1144. [DOI] [PubMed] [Google Scholar]


